Abstract
In this paper, three cellulose/metal sulfide (CL@HgS, CL@FeS and CL@PbS) nanocomposites were synthesized in situ and characterized by FT-IR, XRD, EDS-SEM, DSC and TGA. All results showed the permanent embedding of single phase of HgS, FeS and PbS nanoparticles on the cellulose polymer. The presence of HgS, FeS and PbS nanoparticles on the surface of cellulose can be clearly seen in the SEM images. This is also confirmed by the results of EDS elemental analysis and XRD patterns. The intensity of peaks in as-prepared nanocomposite is lower than pure chitosan, predicted that the low crystallinity of prepared compounds. Thermogravimetric results (TGA) confirmed the better thermal stability of CL@FeS and CL@PbS than CL@HgS. The residue weight at the temperature of 800 °C is the highest for CL@FeS nanocomposite and is about 66%. DSC thermogram of CL@FeS is completely different from the CL@PbS than CL@HgS, because it shows a sharp and strong peak related to the oxidation of Fe(II) to Fe(III) at a temperature of 276.1 °C. Finally, antibacterial activities of the as-prepared nanocomposites showed the more effective of CL@HgS nanocomposites against S. aureus and E. coli than CL@FeS and CL@PbS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiphase solid materials consisting of a polymer (synthetic or natural) as continuous matrices and inorganic nanoparticles (ZnO, MnO2, Cr2O3, TiO2) are known as nanocomposites (Li et al. 2021; Wahid et al. 2019; Zong et al. 2021; Lu et al. 2020; Wang et al. 2021; He et al. 2020; Gupta et al. 2017). In recent years, the interest in polymer nanocomposites has been increased because of their promising application in various fields such as adsorption, chromium and phosphate removal, and antibacterial activity (Li et al. 2021; Wahid et al. 2019; Zong et al. 2021; Lu et al. 2020; Wang et al. 2021; He et al. 2020; Gupta et al. 2017; Syed et al. 2021; Kumar et al. 2019; Zhang et al. 2019). Nowadays, the nanocomposites prepared using renewable natural polymers such as cellulose and chitosan are very interesting (Li et al. 2021; Wahid et al. 2019; Zong et al. 2021; Gupta et al. 2017; Zhang et al. 2019). Cellulose (Scheme 1), the most abundant polymer on earth, is made up of repeating glucose residues (Aziz et al. 2022; French 2017) is natural, biodegradable, environmentally friendly, low-cost, renewable, sustainable and non-toxic, has excellent mechanical strength and also easy functionalization (Li et al. 2021; Zong et al. 2021; Noremylia et al. 2022; Naz et al. 2019). However, it has poor reactivity and slow removal rate (Jawhid et al. 2021). Cellulose can be produced in plants by photosynthesis using CO2 (Zong et al. 2021), has high specific surface area due to its fibrous structure (Batmaz et al. 2014; Carpenter et al. 2015), cotton is an inexpensive source (Najaflou et al. 2021) and known as a good candidate for the preparation of other functionalized materials due to, its abundant hydroxyl (OH) groups and also the inherent abundance naturally (Tao et al. 2020; Varshney et al. 2021). To prepare cellulose derivatives, grafting, esterification, acylation, and etherification have all been used (Seddiqi et al. 2021; Aziz et al. 2022; Kumar et al. 2019; Najaflou et al. 2021; Jawhid et al. 2021). Recently, preparation of different modification and nanocomposites based cellulose are interesting due to their photocatalysis (Wahid et al. 2019; Lu et al. 2020), antioxidant application (Lu et al. 2020), and heavy metal removal (Hu et al. 2021; Ampiaw and Lee 2020; Najaflou et al. 2021; Jawhid et al. 2021; Li et al. 2019), phosphate removal (Zong et al. 2021), antioxidant (Ayoub et al. 2020; Johnston and Nilsson 2011), biosensors (Zhao et al. 2019; Wang et al. 2011) and antibacterial activities (Li et al. 2021; Wahid et al. 2019; Zong et al. 2021). For example, Zong et al. (2021) reported antibacterial activities of TiO2/cellulose nanocomposites. Amirmahani et al. (2020), prepared cellulose membranes with gold-coating as solar cells semiconductors and used them in the water filtration method. A green substrate for flexible top emission organic light-emitting diodes (TE-OLEDS) was prepared by Miikkulainen et al. (2013) using SiO2-cellulose paper with Au anodes. Nanoparticles containing metal such as oxides and sulfides and the composites obtained from them have good biological properties (Lei et al. 2022; Lin et al. 2022; Bahadoran et al. 2022; Xiao et al. 2021; Long et al. 2022; Mao et al. 2022; Lai et al. 2022), but in most cases, nanocomposite prepared from them with a cellulose natural polymer shows better biological properties (Zheng et al. 2022; Peng et al. 2023; Bao et al. 2018; Xie et al. 2019; Caschera et al. 2020; Ahmad 2021). For example, Li et al. (2021) fabricated ZnO-embedded cellulose nanocomposite films and studied the antibacterial activities. They exhibited excellent antibacterial activity against S. aureus and E. coli. Wahid et al. (2019) studied on preparation bacterial cellulose/ZnO nanocomposite films for antibacterial activities against S. aureus, B. subtilis, P. aeruginosa and E. coli. The combination of metal oxides and sulfides with natural polymers such as cellulose and chitosan, imparts remarkable antibacterial functions to as-prepared nanocmposites (Li et al. 2021; Lu et al. 2020; Zong et al. 2021; Syed et al. 2021; Wahid et al. 2019). In recent years, chalcogenide nanomaterials such as FeS, PbS, FeSe and HgS have attracted much attention due to their useful physical and chemical properties (Bagade et al. 2016; Rajashree et al. 2015; Suganya and Balu 2017; Govindaraj et al. 2015; Dutta et al. 2012). Nanostructures of metal sulfides might possess improved chemical and physical properties compared to that of their bulk materials, which makes them efficient antimicrobial agents (Suganya and Balu 2017) and biosensing of H2O2 (Dutta et al. 2012).
In this paper, three cellulose/metal sulfide (CL@HgS, CL@FeS and CL@PbS) nanocomposites were in situ synthesized (Eq. 1) and characterized. In addition, antibacterial activities of them studied against S. aureus, and E. coli. To the best of author’s knowledge there is no similar reported technique for preparation cellulose/metal sulfide nanocomposite and also their antibacterial studies.
Experimental
Materials and methods
Cellulose powder, HgCl2 (≥ 98%), FeCl2 (98%), PbCl2 (99.99%) and Na2S∙9H2O (≥ 98%) used in this paper were purchases from Sigma-Aldrich company and used without further purification. FT-IR spectra were recorded on a Perkin-Elmer spectrometer, using KBr pellets in the range of 4000–400 cm−1. The XRD patterns were identified via X-ray powder diffraction using a Bruker Advance D8 diffractometer (using monochromatic Cu Kα radiation, λ = 1.5418 Å, current 40 mA and voltage 40 kV). The morphology samples were recorded by scanning electron microscopy (SEM, JEOL-JSM 7600 F) which was operated at speeding up the voltage at 10 kV. TGA/DTA were analyzed by SETARAM TGA 92 under air atmosphere from 25 to 850 °C at a heating rate of 20 °C/min and DSC curves were recorded by DSC analyzer Model 60A, Shimadzu, under air atmosphere from 25 to 350 °C at a heating rate of 20 °C/min.
Synthesis of cellulose/metal sulfide nanocomposites
1 g of cellulose and 0.25 g of MCl2, M = Hg, Fe and Pb, were mixed in 50 mL water/ethanol (50:50 v/v) and stirred for about 30 min at room temperature. After that, about 0.25 Na2S was added under vigorous stirring and the mixture was stirred for 2 h at 80 °C. The gray precipitates of CL@HgS, CL@FeS and CL@PbS nanocomposites were filtered, washed, dried and characterized using different techniques.
Antibacterial studies
The antibacterial activities of the as-prepared cellulose/metal sulfide nanocomposites were studied against E. coli, and S. aureus bacterial strains using agar-well diffusion method according to the previous described method (Wahid et al. 2019). All compounds, solution and bacterial strains were incubated at 37 °C for 24 h.
Results and discussion
FT-IR spectra
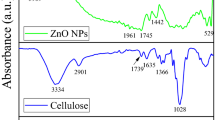
FT-IR spectra of pure cellulose, and the as-prepared cellulose/metal sulfide nanocomposites CL@HgS, CL@FeS and CL@PbS are shown in Fig. 1. The broad peak at about 3349 cm−1 is assigned to the hydroxyl (OH) groups, confirming hydrogen bonding of cellulose, while the weak band at about 2898 cm−1 is attributed to the C–H stretching (Li et al. 2021; Wahid et al. 2019; Zong et al. 2021). The band at 1650 cm−1 is attributed to the adsorbed water molecules. The multiple peaks observed between 1300 to 1450 cm−1 can be assigned to the C–O and C–H stretching of cellulose (Li et al. 2021; Wahid et al. 2019; Zong et al. 2021). The peaks at 1050 and 1175 cm−1 were ascribed to C–O–C and C–OH. All peaks in the as-prepared cellulose/metal sulfide nanocomposites shifted to slight higher wavenumber exhibited the interaction of cellulose and metal sulfides (Wahid et al. 2019). Finally, new bands at about 500–700 cm−1 corresponded to bending and stretching vibration of M–S bonds (Kumar et al. 2019), confirming that metal sulfides have been grown on the cellulose structure (Zong et al. 2021). These results supported by other characterization results such as DSC, TGA, SEM/EDS and XRD (Figs. 2, 3, 4, 5 and 6).
DSC curves
DSC thermograms of CL, CL@HgS, CL@FeS and CL@PbS nanocomposites are shown in Fig. 2. Cellulose shows a weak endothermic peak at 89.7 °C corresponding to the evaporation of water molecules adsorbed on the its surface and a sharp exothermic peak at 339.5 °C corresponding to the thermal decomposition of polymeric chain of cellulose. CL@HgS shows a strong endothermic peak at 99.2 °C, CL@FeS shows a weak endothermic peak at 97.1 °C and in the DSC thermogram of CL@PbS there is no any peak for water molecules evaporation. Also, in DSC thermograms of CL@HgS, CL@FeS and CL@PbS nanocomposites, there is an exothermic peak shifted to slight lower temperature and appeared at ≈329.1, 322.3, 332.2 °C, respectively, predicted that the interaction of functional groups on the surface of cellulose and metal sulfides. However, in the DSC thermogram of CL@FeS a sharp exothermic peak was observed at 276.1 °C, which is not seen in the other compounds, may be assigned to the oxidation of Fe(II) to Fe(III).
TGA curves
The weight loss and thermal degradation of CL, and the as-prepared nanocomposites CL@HgS, CL@FeS and CL@PbS were carried out and shown in Fig. 3. The pure CL, CL@FeS and CL@PbS nanocomposites are thermally stable up to about 320 °C (Li et al. 2021; Zong et al. 2021). After that, in main decomposition stage, CL, CL@FeS and CL@PbS losses about 82%, 34% and 50% of theirs weight, respectively. While, TGA curve of CL@HgS is different and shows three different decomposition stages. At first stage, it loses about 28% of its weight from 50 to 135 °C, corresponding to the water molecules adsorbed on the surface of CL@HgS nanocomposite. At the second and third stages, losses about 14% and 22%, are respectively assigned to the flexible functional groups on the surface of cellulose and main C–O–C decomposition of the polymeric chains. Finally, at the end of thermal decomposition at 850 °C, the residual weight of 18%, 36%, 66%, and 50% respectively, for CL, CL@HgS, CL@FeS and CL@PbS, which confirmed the presence of metal sulfide on the cellulose structure (Zong et al. 2021). Therefore, TGA results predicted that CL@FeS and CL@PbS are more thermally stable than CL and CL@HgS.
SEM images
SEM images of CL, CL@HgS, CL@FeS and CL@PbS are shown in Fig. 4. The SEM results showed that the surface of cellulose (CL) is smooth and layers with many porous. While for as-prepared cellulose nanocomposites (CL@HgS, CL@FeS and CL@PbS), nanoparticles of metal sulfide (HgS, FeS and PbS) (marked by red color in Fig. 4) with different sizes were observed on the surface of cellulose (Wahid et al. 2019; Zong et al. 2021; Lu et al. 2020), therefore surface of nanocomposite become rough.
EDS spectrograms
EDS spectrograms of pure cellulose (CL), and its nanocomposites (CL@HgS, CL@FeS and CL@PbS) are shown in Fig. 5. EDS results predicted that the metal sulfides (HgS, FeS, and PbS) nanoparticles were successfully dispersed in the matrix of cellulose polymer, because cellulose had only oxygen and nitrogen elements with the mass percentage of 52.40 W% and 47.60 W%, respectively (Zong et al. 2021). While in the as-prepared nanocomposite the new elements of Hg, Fe, Pb and S were observed (Table 1), confirming the preparation of CL@HgS, CL@FeS and CL@PbS nanocomposite using loaded of metal sulfides on the surface of cellulose. These results confirmed by SEM images (Fig. 4).
XRD patterns
XRD patterns of CL, and as-prepared CL@HgS, CL@FeS and CL@PbS nanocomposites are shown in Fig. 6. This results provide suitable evidence for the successful synthesis of CL@HgS, CL@FeS and CL@PbS nanocomposite. The commercial cellulose (CL) displayed three peaks at about 15.26°, 22.79° and 34.77°, which were ascribed to pure cellulose (Zong et al. 2021). In the as-prepared nanocomposites, these peaks are seen with slightly shifted. In the XRD patterns of CL@HgS, CL@FeS and CL@PbS nanocomposite, new diffraction peaks at different 2θ values can be indexed to the (111), (200), (220), and (311) planes of cubic phase of HgS (JCPDS card no: 73-1593) (Marimutha et al. 2015), (110), (002), (111), (200), and (201) planes of FeS (JCPDS card no: 86-0389) (Dutta et al. 2012) and (111), (200), (220), and (311) planes of galena PbS (JCPDS card no: 652935) (Suganya et al. 2017) were identified.
Antibacterial activity
In this paper, the antibacterial activity of CL and its nanocomposite CL@HgS, CL@FeS and CL@PbS was investigated by inhibition zone tests using S. aureus and E. coli as the model of Gram-positive and Gram-negative bacteria (Li et al. 2021; Wahid et al. 2019; Zong et al. 2021) and the results are shown in Fig. 7. Only, CL@HgS exhibit inhibition against both bacteria (S. aureus and E. coli) and other compounds did not exhibit any inhibition. Also, the antibacterial property of CL@HgS exhibited the equal property against both bacterial strains.
Conclusion
Summary, three cellulose/metal sulfide (CL@HgS, CL@FeS and CL@PbS) nanocomposites were in situ synthesized and characterized. Thermogravimetric studies confirmed the thermal stabilities of CL@FeS and CL@PbS were better than CL and CL@HgS. XRD and EDS-SEM results confirmed the presence of metal sulfide nanoparticles. In addition, antibacterial activities exhibited greater activity of CL@HgS than those for CL@FeS and CL@PbS.
Data and materials availability
All data are presented and explained in the article.
References
Ahmad H (2021) Celluloses as support materials for antibacterial agents: a review. Cellulose 28:2715–2761
Amirmahani N, Rashidi M, Mahmoodi NO (2020) Synthetic application of gold complexes on magnetic supports. Appl Organomet Chem 34:e5626
Ampiaw RE, Lee W (2020) Persimmon tannins as biosorbents for precious and heavy metal adsorption in wastewater: a review. Int J Environ Sci Technol 17:3835–3846
Ayoub A, Treasure T, Hansen L, Nypelö T, Jameel H, Khan S, Chang HM, Hubbe MA, Venditti RA (2020) Effect of plasticizers and polymer blends for processing softwood kraft lignin as carbon fiber precursors. Cellulose 28:1039–1053
Aziz T, Farid A, Haq F, Kiran M, Ullah A, Zhang K, Li C, Ghazanfar S, Sun H, Ullah R, Ali A, Muzammal M, Shah M, Akhtar N, Selim S, Hagagy N, Samy M, Al Jaoun SK (2022) A review on the modification of cellulose and its applications. Polymers 14:3206
Bagade CS, Ghanwat VB, Knot KV, Bhosale PN (2016) Efficient improvement of photoelectrochemical performance of CdSe thin film deposited via arrested precipitation technique. Mater Lett 164:52–55
Bahadoran A, Baghbadorani NB, De Lile JR, Masudy-Panah S, Sadeghi B, Li J, Ramakrishna S, Liu Q, JushiJanani B, Fakhri A (2022) Ag doped Sn3O4 nanostructure and immobilized on hyperbranched polypyrrole for visible light sensitized photocatalytic, antibacterial agent and microbial detection process. J Photochem Photobiol B 228:112393
Bao Y, Zhang H, Luan Q, Zheng M, Tang H, Huang F (2018) Fabrication of cellulose nanowhiskers reinforced chitosan-xylan nanocomposite films with antibacterial and antioxidant activities. Carbohydr Polym 184:66–73
Batmaz R, Mohammed N, Zaman M, Minhas G, Berry RM, Tam KC (2014) Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 21:1655–1665
Carpenter AW, De Lannoy CF, Wiesner MR (2015) Cellulose nanomaterials in water treatment technologies. Environ Sci Technol 49:5277–5287
Caschera D, Grazia Toro R, Federici F, Montanari R, de Caro T, Al-Shemy MT, Adel AM (2020) Green approach for the fabrication of silver-oxidized cellulose nanocomposite with antibacterial properties. Cellulose 27:8059–8073
Dutta AK, Maji SK, Mondal A, Karmakar B, Biswas P, Adhikary B (2012) Iron selenide thin film: peroxidase-like behavior, glucose detection and amperometric sensing of hydrogen peroxide. Sens Actuators B 173:724–731
French AD (2017) Glucose, not cellobiose, is the repeating unit of cellulose and why that is important. Cellulose 24:4605–4609
Govindaraj M, Arivanandhan M (2015) Vedhi C (2015) Chemical vapor deposition of β-HgS nanoparticles from a precursor, bis(cinnamylpiperazinedithiocarbamato) mercury(II). Synth React Inorg, Met-Org, Nano-Met Chem 45:217–224
Gupta VK, Fakhri A, Agarwal S, Sadeghi N (2017) Synthesis of MnO2/cellulose fiber nanocomposites for rapid adsorption of insecticide compound and optimization by response surface methodology. Int J Biol Macromol 102:840–846
He X, Gan J, Fakhri A, Faraji Dizaji B, Hasani Azarbaijan M, Hosseini M (2020) Preparation of ceric oxide and cobalt sulfide-ceric oxide/cellulose-chitosan nanocomposites as a novel catalyst for efficient photocatalysis and antimicrobial study. Int J Biol Macromol 143:952–957
Hu T, Hu X, Tang C, Liu D (2021) Adsorbent grafted on cellulose by in situ synthesis of EDTA-like groups and its properties of metal ion adsorption from aqueous solution. Cellulose 29:941–952
Jawhid O, Seyedi N, Zohuri GH, Ramezanian N (2021) Cellulose Schiff base as a bio-based polymer ligand: extraction, modification and metal adsorption study. J Polym Environ 29:1860–1868
Johnston JH, Nilsson TW (2011) Nanogold and nanosilver composites with lignin-containing cellulose fibres. J Mater Sci 47:1103–1112
Kumar S, Krishnakumar B, Sobral AJFN, Koh J (2019) Bio-based (chitosan/PVA/ZnO) nanocomposite film: thermally stable and photoluminescence material for removal of organic dye. Carbohydr Polym 205:559–564
Lai Y, Fakhri A, Jushi Janani B (2022) Synergistic activities of silver indium sulfide/nickel molybdenum sulfide nanostructures anchored on clay mineral for light-driven bactericidal performance, and detection of uric acid from gout patient serum. J Photocehm Photobiol B 234:112526
Lei C, Sun N, Wu H, Zhao Y, Yu C, JushiJanani B, Fakhri A (2022) Bio-photoelectrochemical degradation, and photocatalysis process by the fabrication of copper oxide/zinc cadmium sulfide heterojunction nanocomposites: mechanism, microbial community and antifungal analysis. Chemosphere 308:136375
Li Y, Guo C, Shi R, Zhang H, Dai L (2019) Chitosan/nanofibrillated cellulose aerogel with highly oriented microchannel structure for rapid removal of Pb(II) ions from aqueous solution. Carbohydr Polym 223:115048
Li X, Li H, Wang X, Xu D, You T, Wu Y, Xu F (2021) Facile in situ fabrication of ZnO-embedded cellulose nanocomposite films with antibacterial properties and enhanced mechanical strength via hydrogen bonding interactions. Int J Biol Macromol 183:760–771
Lin H, Li T, JushiJanani B, Fakhri A (2022) Fabrication of Cu2MoS4 decorated WO3 nano heterojunction embedded on chitosan: Robust photocatalytic efficiency, antibacterial performance, and bacteria detection by peroxidase activity. J Photochem Photobiol B 226:112354
Long W, Ubaid Hamza M, Abdul-Fattah MN, Rheima AM, Ahmed YM, Fahim FS, Altimai US, Abdulaim AKO, Jushi Janani B, Fakhri A (2022) Preparation, photocatalytic and antibacterial studies on novel doped ferrite nanoparticles: characterization and mechanism evaluation. Coll Surf A 650:129468
Lu M, Cui Y, Zhao S, Fakhri A (2020) Cr2O3/cellulose hybrid nanocomposites with unique properties: facile synthesis, photocatalyst, bactericidal and antioxidant application. J Photochem Photobiol B Biol 205:111842
Mao Y, Qiu J, Zhang P, Fei Z, Bian C, Jushi Janani B, Fakhri A (2022) A strategy of silver Ferrite/Bismuth ferrite nano-hybrids synthesis for synergetic white-light photocatalysis, antibacterial systems and peroxidase-like activity. J Photocehm Photobiol A 426:113756
Marimuthu G, Arivanandhan M, Vedhi C (2015) Chemical vapor deposition of β-HgS nanoparticles from a precursor, bis(cinnamylpiperazinedithiocarbamato) mercury(II). Synth React Inorg Metal-Org Nano-Metal Chem 45:217–224
Miikkulainen V, Leskela M, Ritala M, Puurunen RL (2013) Crystallinity of inorganic films grown by atomic layer deposition: overview and general trends. J Appl Phys 113:021301
Najaflou S, Forouzesh Rad M, Bagfhdadi M, Nabi Bidhendi GR (2021) Removal of Pb(II) from contaminated waters using cellulose sulfate/chitosan aerogel: equilibrium, kinetics, and thermodynamic studies. J Envorin Manage 286:112167
Naz S, Ali JS, Zia M (2019) Nanocellulose isolation characterization and applications: a journey from non-remedial to biomedical claims. Bio-Des Manufact 2:187–212
Noremylia MB, Hassan MZ, Ismail Z (2022) Recent advancement in isolation, processing, characterization and applications of emerging nanocellulose: a review. Int J Biol Macromol 206:954–976
Peng Y, Zhou H, Ma Z, Tian L, Zhang R, Tu H, Jiang L (2023) In situ synthesis of Ag/Ag2O–cellulose/chitosan nanocomposites via adjusting KOH concentration for improved photocatalytic and antibacterial applications. Int J Biol Macromol 225:185–197
Rajashree C, Balu AR, Nagarethinam VS (2015) Properties of Cd doped PbS thin films: doping concentration effect. Surf Eng 31:316–321
Seddiqi H, Oliaei E, Honarkar H, Jin J, Geonzon LC, Bacabac RG, Klein-Nulend J (2021) Cellulose and its derivatives: towards biomedical applications. Cellulose 28:1893–1931
Suganya S, Balu AR (2017) PbS nanopowder—synthesis, characterization and antimicrobial activity. Mater Sci Poland 35:322–328
Syed A, Marraiki N, Al-Rashed S, Elgorban AM, Yassin MT (2021) A potent multifunctional MnS/Ag-polyvinylpyrrolidone nanocomposite for enhanced detection of Hg2+ from aqueous sample and its photocatalytic and antibacterial applications. Spectrochim Acta A 244:118844
Tao H, Lavoine N, Jiang F, Tang J, Lin N (2020) Reducing end modification on cellulose nanocrystals: strategy, characterization, applications and challenges. Nanoscale Horiz 5:607–627
Varshney S, Mishra N, Gupta MK (2021) Progress in nanocellulose and its polymer based composites: a review on processing, characterization, and applications. Polym Nanocom 42:3660–3686
Wahid F, Duan YX, Hu XH, Chu LQ, Jia SR, Cui JD, Zhong C (2019) A facile construction of bacterial cellulose/ZnO nanocomposites films and their photocatalytic and antibacterial properties. Int J Biol Macromol 132:692–700
Wang W, Zhang TJ, Zhang DW, Li HY, Ma YR, Qi LM, Zhou YL, Zhang XX (2011) Amperometric hydrogen peroxide biosensor based on the immobilization of heme proteins on gold nanoparticles–bacteria cellulose nanofibers nanocomposite. Talanta 84:71–77
Wang G, Lu T, Zhang X, Feng M, Wang C, Yao W, Zhou S, Zhu Z, Ding W, He M (2021) Structure and properties of cellulose/HAP nanocamposite hydrogels. Int J Biol Macromol 186:377–384
Xiao S, Fakhri A, JushiJanani B (2021) Synthesis of spinel Tin ferrite decorated on Bismuth ferrite nanostructures for synergetic photocatalytic, superior drug delivery, and antibacterial efficiencies. Surf Interfaces 27:101490
Xie WQ, Yu KX, Gong YX (2019) Preparation of fluorescent and antibacterial nanocomposite films based on cellulose nanocrystals/ZnS quantum dots/polyvinyl alcohol. Cellulose 26:2363–2373
Zhang H, Peng L, Chen A, Shang C, Lei M, He K, Luo S, Shao J, Zeng Q (2019) Chitosan-stabilized FeS magnetic composite for chromium removal: characterization, performance, mechanism, and stability. Carbohydr Polym 214:276–285
Zhao Y, Sun B, Wang T, Yang L, Xu X, Chen C, Wei F, Lv W, Zhang L, Sun D (2019) Synthesis of cellulose–silica nanocomposites by in situ biomineralization during fermentation. Cellulose 27:703–712
Zheng H, Li X, Liu L, Bai C, Liu B, Liao H, Yan M, Liu F, Han P, Zhang H, He J (2022) Preparation of nanofiber core-spun yarn based on cellulose nanowhiskers/quaternary ammonium salts nanocomposites for efficient and durable antibacterial textiles. Comp Commun 36:101388
Zong E, Wang C, Yang J, Zhu H, Jiang S, Liu X, Song P (2021) Preparation of TiO2/cellulose nanocomposites as antibacterial bio-adsorbents for effective phosphate removal from aqueous medium. Int J Biol Macromol 182:434–444
Acknowledgements
The authors acknowledge the Golestan University for its financial support.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
P Nazari has conducted the experiments and collected their results. A.D. Khalaji is responsible for the main idea of the article and the analysis and writing of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The authors of the article have contributed equally to this article and have full consent.
Consent for publication
The authors agree to publish the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nazari, P., Khalaji, A.D. Cellulose/metal sulfide (CL@HgS, CL@FeS and CL@PbS) nanocomposites: synthesis, characterization, thermal study and antibacterial activity. Cellulose 30, 8899–8907 (2023). https://doi.org/10.1007/s10570-023-05400-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05400-8