Abstract
Lignocellulose biomass effectively produces chemicals and fuels, which are of importance for the establishment of a sustainable society. The conversion of cellulose, the main component of the biomass, into significant precursors that can be further converted to different chemicals or fuels under gentle conditions is a promising route. Organic acids such as acetic, glycolic and formic acid are significant examples. A novel method to produce important platform chemicals from micro-crystalline cellulose (MCC) was developed. MCC was degraded as a result of oxidation with potassium chlorate by microwave radiation in a one-pot procedure. Efficient reaction conditions such as short reaction time and full conversion of cellulose were identified. The reaction products were analyzed by 1H and 13C NMR, XPS, TGA and XRD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is the most abundant renewable resource of polysaccharides on earth. It is found in almost all plant sources including cotton (90%), corn cobs (45%), wood (40–45%), and bagasse (40%) and on stalks (35%) (Sundarraj and Ranganathan, 2018). Cellulose has broad applications in chemical industry due to its high availability, biocompatibility, biodegradability, and good mechanical properties. It is a linear polysaccharide composed of d-glucopyranosyl units bonded by β (1 → 4) glycosidic linkage. Each d-glucose moiety has hydroxyl (OH) groups connected with C2, C3 and C6, which can create intramolecular hydrogen bonds within and between the cellulose macromolecules. Therefore, it is apparent that these OH groups and their intramolecular hydrogen bonds construct the crystalline structure and the rigid structure of cellulose, making it insoluble in common organic solvents and water and hindering its application (Liu et al. 2021).
The passage from nonrenewable carbon resources (petroleum) to renewable bioresources is inescapable for meeting the growing societal needs. The chemical industry is presently undergoing a paradigm shift from the utilization of fossil fuels to plants (cellulose) for the extraction of fine and bulk chemicals (Lin and Tanaka 2006). The conversion of renewable carbohydrates into important platform chemicals like levulinic, gluconic, lactic, and formic acid attracted much importance in recent years (Deng et al. 2014). These significant organic acids are derived from cellulose, as well as isosorbide, xylitol, erythritol, glycerol, methanol, ethylene glycol, glycolaldehyde, and hydroxymethylfurfural by catalysis, hydrolysis, and oxidative cleavage (Li et al. 2018; Delbecq and Len 2018; Wang et al. 2018b)..These organic acids are used in many commercial industries as food preservative, antibacterial agent in livestock feed, textiles, fuel cells, leather, cosmetics, curing and flavoring agents. Particularly, formic acid (FA) is a value-added chemical that was applied for the production of medicaments, solvents, fragrances, and fibers, as well as for the conservation of food products and forage, and was also used in the pulp and leather industries (Aguilo and Horlenko 1980). FA is a very promising substrate for the synthesis of molecular hydrogen over metal catalysts (Zacharska et al. 2015; Wang et al. 2013a, b; Zhang et al. 2013). The yield of FA from cellulose is as important as its conversion from CO2 owing to the renewable nature of cellulose (Herrick and Lipták 2003).
Micro-crystalline cellulose (MCC) is mostly insoluble in water as well as in the most common organic solvents at gentle temperatures. This is an obstacle for the activation of MCC under mild conditions. Also, there are different types of bonds (C–C and C–O) in MCC macromolecules that create difficulty in its breakdown. Hence, the cleavage or oxidation of C–C and C–O bonds in MCC allows selective production of different compounds (Deng et al. 2014).
Considering the novelty of the current work, it must be pointed out that some of the oxidizing materials used in the current experiments were already attempted towards the degradation of cellulose. For example, potassium persulfate was used by Davaritouchaee and coworkers (Davaritouchaee et al. 2019) in the oxidation of wheat straw and revealed an increase in cellulose degradation temperature and a decrease in activation energy from 259 to 223 kJ/mol compared to the raw material. In another work, potassium permanganate was used to oxidize the cellulose, introducing the carboxyl groups into the oxidized polymer (Milanovic et al. 2021; Zhou et al. 2018). Cotton yarns were oxidized with potassium periodate solution with TEMPO systems. Sodium chlorite was also employed for the same purpose (Toshikj et al. 2019). However, to the best of our knowledge microwave radiation was never used in the oxidation of cellulose.
Here, MCC underwent successful oxidative degradation into important organic acids including FA, acetic acid (AA), and glycolic acid (GA) by the inorganic oxidizing reagent potassium chlorate (KClO3) using a short and facile one-step microwave-assisted method without employing any additives. The duration of microwave operation was optimized. This technique provides a novel chemistry tool that reduces the energy consumption and processing time (Scheme 1).
Materials and methods
Materials
The chemicals used in this study, namely MCC, KClO3 and NaOH, were purchased from Sigma Aldrich (Israel). The oxidizing agents potassium permanganate (KMnO4), ammonium perchlorate (NH4ClO4), potassium persulfate (K2S2O8), potassium chromate (K2CrO4), potassium dichromate (K2Cr2O7), potassium periodate (KIO4), potassium perchlorate (KClO4), and sodium periodate (NaIO4) were also obtained from Sigma Aldrich. The chemicals were used as received without another purification. Deionized water was used in all experiments.
Sample preparation
Dissolution of MCC in aqueous NaOH
Cellulose (0.5 g) was suspended in 26.9 mL of water, and 2.5 g of NaOH was added to the suspension. The mixture was shaken to dissolve the NaOH at room temperature. The suspension was cooled and held in a refrigerator until it became a solid frozen mass, which was allowed to thaw at room temperature and was transformed into a gel-like mass. 20.6 mL of water was added, and after gentle shaking, a clear cellulose solution was obtained (Isogai and Atalla 1998).
Oxidation and degradation of MCC
Oxidation of MCC was attempted using different oxidizing reagents with microwave and hydrothermally. The various oxidation agents used in this study are presented in Table 1. It is notable that all experiments were carried out under basic conditions (pH = 12), and the domestic microwave oven (Sharp) was operated for 1, 3 or 5 min at a power of 1100 W. Potassium chlorate (0.1 g) was added to a 250 mL round bottom flask that contained 50 ml of the clear cellulose solution, and the flask was subjected to microwave irradiation for 5 min. The resulting product was centrifuged at 8000 rpm for 30 min. The formation of the gel-like product was allowed for all characterizations.
Table 1 reveals that only KClO3 successfully oxidized the cellulose.
Characterization
The chemical structure of the oxidized MCC was characterized by 1H and 13C nuclear magnetic resonance (NMR) on a 300 MHz spectrometer (Bruker) using NaOH/D2O as the solvent. The elemental composition and chemical bonding were analyzed using X-ray photoelectron spectroscopy (XPS) using a Nexsa (England) spectrometer. The detection and identification of the degradation of MCC were also measured by thermogravimetric analysis (TGA) on a Perkin Elmer Clarus 680/Clarus SQ 8C instrument. The flow rate of nitrogen was 20 mL/min, and the temperature rate was increased at 10 °C/min, the samples (MCC and oxidized MCC) were heated from 30 to 950 °C. The difference in the morphology of the surface and the size of the pure MCC particles and the degraded MCC (DMCC) was characterized by environmental scanning electron microscopy (E-SEM) using a Quanta FEG 250 (FEI) device at 5 kV. The crystalline nature of the MCC and DMCC composites was assessed using an X-ray diffraction (XRD) technique measured by a Bruker (Germany) AXS D8 Advance diffractometer.
Results and discussion
NMR spectrum of the attained product
The NMR spectra of the oxidized MCC in aqueous solution are given in Fig. 1. The evidence for the successful oxidation of MCC was obtained from both 1H (Fig. 1a) and 13C (Fig. 1b) NMR. Significant differences are observed between the initial and final MCC after oxidation in aqueous solution. The 13C NMR spectrum of the initial material was obtained from the literature (Heinze and Koschella 2005). The NMR spectra of the starting compound and product obtained after oxidation of the MCC are compared.
Figure 2 displays the 13C solid-state NMR (SSNMR) spectrum of the cellulose. The spectrum matches very well recently published spectra (Hussin et al. 2018; Okushita et al. 2012; Newman et al. 1996; Wang et al. 2013a, b). The main peaks of the cellulose appear at 65.3, 71.8, 72.6, 75.2, 89.1, and 105.36 ppm are assigned to C6, C2, C3, C5, C4 and C1, respectively (Fig. 2). It is clear from Fig. 1a and b that these peaks disappeared in the product, indicating the successful oxidation and elimination of the cellulose, corroborated by the changes in the peaks of the final product.
Figure 1a illustrates results for the aqueous 1H NMR of the NaOH treated product. Figure 1b presents the corresponding 13C NMR of the solution in D2O of the NaOH solution of the product. The disappearance of the cellulose peaks is the most remarkable feature, indicating the successful oxidation of the cellulose.
Figure 1b shows the peaks of the 13C NMR spectrum located at 24 and 172 ppm which represent the carbons of acetic acid. The two peaks at 62 and 182 ppm represent the carbons of glycolic acid, and the peak at 169 ppm is attributed to formic acid. Moreover, the peaks of the proton NMR spectrum also match the hydrogens of FA, AA and GA, which are found in H (t) at 1.811, 1.837 and 1.847 ppm (AA), 8.111 ppm (FA), and 3.586 ppm (GA) (Table 2).
Moreover, in previous studies, the oxidizing materials that were used for cellulose degradation such as vanadium (V)-containing catalyst, a high-cost material, usually undergo at least two reactions (Lu et al. 2018). Another oxidizing catalyst, Mo-V-P heteropoly acids, have a long catalytic synthesis (Gromov et al. 2016). Carbon electrodes modified by gold nanoparticles (Sugano et al. 2016) are also expensive, and TEMPO-mediated oxidation system requires preparation step (Isogai et al. 2018) The oxidation of cellulose with the catalytic aqueous mixture of H5PV2Mo10O40 + H2SO4 with molecular oxygen (Lu et al. 2016) is a very slow process compared to our method.
Thermochemical conversion of cellulosic materials can also be achieved by microwave heating (Motasemi and Afzal 2013; Al Shra’Ah and Helleur 2014; Wang et al. 2008). In conventional thermal heating, the energy is transferred from the source to the center of material via radiation, convection, and conduction, while in the microwave or dielectric heating the electromagnetic energy is converted to thermal energy from inside the sample.
The microwave-assisted reactions can be completed more efficiently in comparison with other thermal methods due to efficient heat transfer profiles. For this reason, microwave heating appears as one of the promising techniques for carrying out the biomass pyrolysis process, accelerating chemical reactions and reducing the processing costs. The combination of microwave radiation and KClO3 is novel and was apparently not reported previously.
Thermogravimetric analysis (TGA) of thermal degradation of oxidized MCC
The TGA analysis in Fig. 3 shows the curves of the thermal decomposition of MCC and DMCC obtained after potassium chlorate treatment under microwave conditions. The thermal decomposition (Td) point of the DMCC was approximately 38 °C, which was lower than the original decomposition temperature of pristine cellulose (300 °C). From 30 to 300 °C, the mass of cellulose was almost unchanged. In contrast, the onset decomposition temperature for DMCC was 38 °C, and the weight loss continues until 90 °C. The difference between the two curves is clear indicating the absence of cellulose from DMCC. The difference between MCC and DMCC is also reflected in their stability. The highest boiling point of the degradation products (AA, FA and GA) is 118 °C which belongs to AA. The curves of DMCC show a continuous weight loss at temperatures below 200 ºC. At 450 °C, MCC and DMCC show residual weights of 0 and 23%. DMCC was collected from the reaction mixture after the completion of the oxidation process (Rosli et al. 2015). The 18% of DMCC after heating to 800 °C is perhaps carbon char that was left from the decomposition of the degradation products.
Scanning electron microscope (SEM)
SEM helps to reveal the morphological and topographical features of the precursors and the resulting carbon composites. The E-SEM images of cellulose are presented in Fig. 4a (before) and Fig. 4b (after) oxidation. Before oxidation and degradation, the cellulose particles resembled blocks and logs of wood. After degradation, the original crystallinity of the cellulose particles is lost. The morphology of the cellulose crystals changed after oxidation, which substantiates the TGA results on the successful degradation of the cellulose.
X-ray diffraction (XRD)
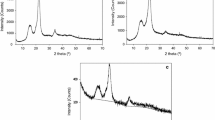
To further determine the change in crystallinity, the XRD patterns of MCC and DMCC are shown in Fig. 5. For MCC there are crystallographic diffraction peaks around 2θ = 34.45°, 22° and 15° (Pang et al. 2014), indicating the characteristic cellulose I crystal structure. After oxidation and degradation, the crystalline peaks near 22° and 35° disappeared, and the peak around 2θ = 15° widened and became less sharp. These results demonstrate that the crystallinity of cellulose was noticeably decreased. The peak at 30° belongs to the holder, as it can be seen in the curve of the empty holder that contains peaks around 2θ = 30° and 41°. This result is in accordance with the SEM results, confirming that the oxidation reaction decomposes the cellulose polymer and diminishes its crystallinity.
X-ray photoelectron spectroscopy (XPS)
XPS analysis provides information about the surface elemental composition and functional groups. The images in Fig. 6 obtained from XPS patterns of MCC and the DMCC present the difference in the functional groups that appear in the product. Both products consist mainly of carbon and oxygen. The cellulose polymer has two types of functional groups—the multiple hydroxyl groups per unit of the polymer had one primary and two secondary alcohols, and one ether group. Figure 6a shows the C1s spectra of MCC which is deconvoluted into two peaks located at 285.0 and 286.3 eV, representing C–C/C–H and C–O–C/C–OH groups. However, the C1s spectrum of DMCC reveals additional peaks at 288.0 and 289.3 eV, corresponding to C=O and COO− as well as a shoulder at 286.3 eV assigned to C–OH. These peaks belong to the organic acids after degradation (FA, AA and GA). Figure 6c and d compare the oxygen (O1s) spectra of MCC and DMCC and reveal the new bonds in the product. The peaks in the O1s spectrum of DMCC disclosed three main classes of oxygen bonds: (Table 3).
All the characterization experiments lead to the same conclusion—the DMCC product differs from the cellulose. In this work, we found that only potassium chlorate was able to oxidize the MCC. The other nine well-known oxidizing agents were not successful in this oxidation process neither by microwave nor hydrothermally. Even for the chlorate ions, negative results were obtained when the reaction was carried out hydrothermally at 80 °C for 8 h. No degradation of cellulose was detected for the other oxidizing agents. We are therefore left with two major questions: 1) why only ClO3− can oxidize the MCC under microwave (MW) radiation and 2) why these products are obtained only by MW irradiation?
First, we tried to correlate the oxidizing power to the standard oxidation potential (Table 4). The table indicates that the chlorate is not the strongest oxidizing agent. However, to this table, we must add the basic conditions under which the reaction is performed, namely pH ~ 11. Under these basic conditions, chlorate is the strongest oxidizing agent, while for example K2S2O8 decomposes fast under these conditions to HSO−4. Potassium permanganate is also a stronger oxidizing agent than ClO3− but this is only true for acidic medium; it is a weak oxidant in both neutral and alkaline medium (Peroxide et al. 2019). Potassium dichromate acts as an oxidizing agent only in an acidic medium. It does not act as oxidizing agent in basic medium, which involves a non-redox reaction whereby it forms chromate ion. In basic medium, Cr has a + 6 oxidation state in both dichromate and chromate forms and reacts according to the following reaction:
The main products in the one-step oxidation of cellulose by chlorate ions were FA, GA and AA.
The proposed mechanism is speculative. To comprehend the mechanism of the cellulose degradation, we examined whether the degradation mechanism involves first the decomposition of the cellulose to glucose. This was done by a control experiment in which identical conditions were used to oxidize the glucose as for the cellulose. The NMR results (Fig.S1) display a different spectrum compared with the peaks in Fig. 1b. The NMR peaks indicate that different products such as glucuronic acid and glucose were obtained in the oxidation of glucose. Moreover, unlike cellulose the glucose was not completely degraded. It is still speculated that glucose is an intermediate product, but perhaps it is formed in its excited state and therefore leading to different products. These results show that the degradation of cellulose does not proceed via glucose.
Returning to the second question regarding why MW radiation, MW is a well-known means for accelerating chemical reactions especially for reactants or catalysts with a dipole moment. The ability to accelerate a chemical reaction is attributed to heating effect in which the “real” temperature is higher than the measured temperature. This is frequently termed superheating (Tao et al. 2021) or the existence of hot spot (Liu et al. 2020). The other explanation is a drastically reduced activation energy allowing the reaction to progress along a new mechanistic route. This is particularly useful when the transition state has a dipole moment which strongly interacts with the MW radiation. These two factors play an important role in accelerating the reaction. The presence of many ClO3− and OH− ions plus their counter ions helps to absorb the radiation and leads to hot spots. In addition, the transition state of the reaction also has a dipole moment and speeds up this chemical reaction. In the first step, the base hydrolysis forms the glucose. In the next step, the glucose oxidizes by KClO3 and forms a different product with MW condition, shown in Scheme 2. The formation of the acids was confirmed by NMR techniques (Wang et al. 2018a).
Conclusion
In conclusion, we presented a fast and facile method to convert the highly micro-crystalline commercial cellulose into a precious and significant product like formic, acetic, or glycolic acid. The novelty of this strategy for decomposing cellulose comprises two concepts: (1) to use an inorganic oxidizing reagent to oxidize the cellulose and (2) expose the degradation of cellulose by microwave irradiation for a maximum of 5 min. Therefore, the practicability of using cellulose as a feedstock for extraction of important chemicals is demonstrated.
References
Aguilo A, Horlenko T (1980) Formic acid. Hydrocarb Process 59:120–130. https://doi.org/10.4324/9780429447341-40
Al Shra’Ah A, Helleur R (2014) Microwave pyrolysis of cellulose at low temperature. J Anal Appl Pyrolysis 105:91–99. https://doi.org/10.1016/j.jaap.2013.10.007
Davaritouchaee M, Hiscox WC, Martinez-Fernandez J et al (2019) Effect of reactive oxygen species on biomass structure in different oxidative processes. Ind Crops Prod 137:484–494. https://doi.org/10.1016/j.indcrop.2019.05.063
Delbecq F, Len C (2018) Recent advances in the microwave-assisted production of hydroxymethylfurfural by hydrolysis of cellulose derivatives—a review. Molecules. https://doi.org/10.3390/molecules23081973
Deng W, Zhang Q, Wang Y (2014) Catalytic transformations of cellulose and cellulose-derived carbohydrates into organic acids. Catal Today 234:31–41. https://doi.org/10.1016/j.cattod.2013.12.041
Gromov NV, Taran OP, Delidovich IV et al (2016) Hydrolytic oxidation of cellulose to formic acid in the presence of Mo-V-P heteropoly acid catalysts. Catal Today 278:74–81. https://doi.org/10.1016/j.cattod.2016.03.030
Heinze T, Koschella A (2005) Solvents applied in the field of cellulose chemistry: a mini review. Polímeros 15:84–90. https://doi.org/10.1590/s0104-14282005000200005
Herrick RA, Lipták BG (2003) Carbon dioxide. Instrum Eng Handb Process Meas Anal Fourth Ed. https://doi.org/10.1016/b0-12-227090-8/00095-6
Hussin MH, Husin NA, Bello I, et al (2018) Isolation of microcrystalline cellulose (MCC) from oil palm frond as potential natural filler for PVA-LiClO4 polymer electrolyte. Int J Electrochem Sci 13:3356–3371. https://doi.org/10.20964/2018.04.06
Isogai A, Atalla RH (1998) Dissolution of cellulose in aqueous NaOH solutions. Cellulose 5:309–319. https://doi.org/10.1023/A:1009272632367
Isogai A, Hänninen T, Fujisawa S, Saito T (2018) Review: Catalytic oxidation of cellulose with nitroxyl radicals under aqueous conditions. Prog Polym Sci 86:122–148. https://doi.org/10.1016/j.progpolymsci.2018.07.007
Li S, Deng W, Wang S et al (2018) Catalytic transformation of cellulose and its derivatives into functionalized organic acids. Chemsuschem 11:1995–2028. https://doi.org/10.1002/cssc.201800440
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69:627–642. https://doi.org/10.1007/s00253-005-0229-x
Liu Z, Meng H, Li C et al (2020) Degradation of biologically treated coking wastewater over CuOx/PAC, CuOx/GAC, and CuOx/ACF catalysts under microwave irradiation in the presence of H2O2. J Environ Eng 146:04020014. https://doi.org/10.1061/(asce)ee.1943-7870.0001653
Liu K, Du H, Zheng T et al (2021) Recent advances in cellulose and its derivatives for oilfield applications. Carbohydr Polym 259:117740. https://doi.org/10.1016/j.carbpol.2021.117740
Lu T, Niu M, Hou Y et al (2016) Catalytic oxidation of cellulose to formic acid in H5PV2Mo10O40 + H2SO4 aqueous solution with molecular oxygen. Green Chem 18:4725–4732. https://doi.org/10.1039/c6gc01271j
Lu T, Hou Y, Wu W et al (2018) Catalytic oxidation of cellulose to formic acid in V(V)-Fe(III)-H2SO4 aqueous solution with O2. Fuel Process Technol 173:197–204. https://doi.org/10.1016/j.fuproc.2018.02.001
Milanovic JZ, Milosevic M, Jankovic-Castvan I et al (2021) Capillary rise and sorption ability of hemp fibers oxidized by non-selective oxidative agents: hydrogen peroxide and potassium permanganate. J Nat Fibers 16:1–6. https://doi.org/10.1080/15440478.2020.1870609
Motasemi F, Afzal MT (2013) A review on the microwave-assisted pyrolysis technique. Renew Sustain Energy Rev 28:317–330. https://doi.org/10.1016/j.rser.2013.08.008
Newman RH, Davies LM, Harris PJ (1996) Solid-state 13C nuclear magnetic resonance characterization of cellulose in the cell walls of Arabidopsis thaliana leaves. Plant Physiol 111:475–485. https://doi.org/10.1104/pp.111.2.475
Okushita K, Komatsu T, Chikayama E et al (2012) Statistical approach for solid-state NMR spectra of cellulose derived from a series of variable parameters. Polym J 44:895–900. https://doi.org/10.1038/pj.2012.82
Pang Q, Wang L, Yang H et al (2014) Cellulose-derived carbon bearing–Cl and–SO 3 H groups as a highly selective catalyst for the hydrolysis of cellulose to glucose. RSC Adv 4:41212–41218
Peroxide H, Kelly MT, Blaise A, et al (2019) Permanganate food and nutritional analy—sis|wine physical and chemical treatment processes for leachate redox transformations spectrophotometry|inorganic compounds fundamentals: ligands, complexes, synthesis, purification, and structure secondar
Rosli N, Ambak K, Daniel BD et al (2015) Jurnal Teknologi 1:1–6
Sugano Y, Kumar N, Peurla M et al (2016) Specific electrocatalytic oxidation of cellulose at carbon electrodes modified by gold nanoparticles. ChemCatChem 8:2401–2405. https://doi.org/10.1002/cctc.201600190
Sundarraj AA, Ranganathan TV (2018) A review on cellulose and its utilization from agro-industrial waste. Drug Invent Today 10:89–94
Tao Y, Teng C, Musho TD et al (2021) Direct measurement of the selective microwave-induced heating of agglomerates of dipolar molecules: the origin of and parameters controlling a microwave specific superheating effect. J Phys Chem B 125:2146–2156. https://doi.org/10.1021/acs.jpcb.0c10291
Toshikj E, Tarbuk A, Grgić K et al (2019) Influence of different oxidizing systems on cellulose oxidation level: introduced groups versus degradation model. Cellulose 26:777–794. https://doi.org/10.1007/s10570-018-2133-4
Wang X, Chen H, Luo K et al (2008) The influence of microwave drying on biomass pyrolysis. Energy Fuels 22:67–74. https://doi.org/10.1021/ef700300m
Wang T, Park YB, Caporini MA et al (2013a) Sensitivity-enhanced solid-state NMR detection of expansin’s target in plant cell walls. Proc Natl Acad Sci 110:16444–16449. https://doi.org/10.1073/pnas.1316290110
Wang Z-L, Yan J-M, Ping Y et al (2013b) An efficient CoAuPd/C catalyst for hydrogen generation from formic acid at room temperature. Angew Chemie 125:4502–4505. https://doi.org/10.1002/ange.201301009
Wang G, Meng Y, Zhou J, Zhang L (2018a) Selective hydrothermal degradation of cellulose to formic acid in alkaline solutions. Cellulose 25:5659–5668. https://doi.org/10.1007/s10570-018-1979-9
Wang M, Ma J, Liu H et al (2018b) sustainable productions of organic acids and their derivatives from biomass via selective oxidative cleavage of C–C bond. ACS Catal 8:2129–2165. https://doi.org/10.1021/acscatal.7b03790
Zacharska M, Podyacheva OY, Kibis LS et al (2015) Ruthenium clusters on carbon nanofibers for formic acid decomposition: effect of doping the support with nitrogen. ChemCatChem 7:2910–2917. https://doi.org/10.1002/cctc.201500216
Zhang S, Metin Ö, Su D, Sun S (2013) Monodisperse AgPd alloy nanoparticles and their superior catalysis for the dehydrogenation of formic acid. Angew Chemie - Int Ed 52:3681–3684. https://doi.org/10.1002/anie.201300276
Zhou L, Li N, Shu J et al (2018) One-pot preparation of carboxylated cellulose nanocrystals and their liquid crystalline behaviors. ACS Sustain Chem Eng 6:12403–12410. https://doi.org/10.1021/acssuschemeng.8b02926
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that they are relevant to the content of this article.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jabareen, L., Maruthapandi, M., Saravanan, A. et al. Effective degradation of cellulose by Microwave irradiation in alkaline solution. Cellulose 28, 11275–11285 (2021). https://doi.org/10.1007/s10570-021-04274-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-04274-y