Abstract
Thermochemical conversion process is an important and potential route to transform biomass feedstocks into powers, fuels, and a variety of chemical platforms. The chapter describes general characteristics of thermochemical processes of biomass including combustion, pyrolysis, liquefaction, and gasification, with the focus on the thermal decomposition of individual biomass components such as cellulose and hemicellulose. Thermochemical processes occur at a wide range of temperatures and pressures with or without catalysts, in which cellulose and hemicellulose undergo serial primary and secondary reactions to form a variety of product types and yields. Primary reactions of cellulose and hemicellulose are associated with the dehydration and depolymerization process to smaller fragments, monosaccharides units, and volatiles which further decompose to low molecular weight compounds at severe conditions of temperature, times, pressures, and catalysts. Thermochemical decomposition of cellulose and hemicellulose typically produces various fuel sources including bio-char, bio-oil, bio-crude, and syngas, along with diverse substances such as anhydrosugars (levoglucosan, mannosan, galactosan), furans (furfural, 5-hydroxymethylfurfural), organic acids (acetic acid, formic acid, levulinic acid), ketones, and aldehydes. Cellulose and hemicellulose are the most abundant constituents in lignocellulosic biomass. Understanding the mechanism of thermochemical conversion of cellulose and hemicellulose leads to the choice of suitable biomass feedstocks and the transformation process for targeted production.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
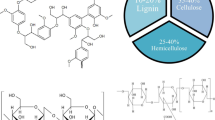
Thermochemical conversion processing is the use of heat to decompose and transform biomass feedstocks into power, fuels, and chemical products. Thermochemical processing occurs at high temperatures from several hundred to 1000 °C or even higher. Therefore, thermochemical processes take place in a short time, in seconds or minutes, compared to hours or days for biochemical processing. Thermochemical processes can be classified into combustion, pyrolysis, gasification, and liquefaction. Biomass combustion has long been used to supply heat and power in the industry. Combustion is the complete oxidation of all organic matters in biomass using sufficient oxygen, while gasification of biomass is performed by partial oxidation of solid biomass feedstocks to produce a mixture of gases at high temperatures using a controlled amount of oxygen. Pyrolysis is the thermal decomposition of biomass into bio-char, bio-oil, and syngas in the absence of oxygen. Liquefaction produces liquid fuels and various chemical platforms from biomass that occurs at mild temperatures in the presence of pressurized water or solvent (Buendia-Kandia et al. 2020; Robert and Kaige 2017). Each process has its own characteristics and uses different reaction conditions, such as temperature, heating rate, residence time, pressure, purge gas flow rate, and catalyst. The yield and composition of the product depend not only on the operating parameters but also on the physicochemical properties of biomass feedstock. Lignocellulosic biomass is mainly composed of cellulose, hemicelluloses, and lignin. Cellulose is rigid and dense because of its highly ordered structure. Hemicelluloses are heterogeneous polymers of hexose and pentose sugars and are less stable than cellulose. Lignin is much more difficult to completely decompose as compared to cellulose and hemicellulose due to its complex aromatic structure. These components that vary from one biomass to another are able to make interactions with each other during thermochemical processes, thus resulting in diverse products with different yields and qualities (Patel et al. 2020). For a better understanding of the mechanism of thermochemical biomass conversion, the processing of the individual components such as cellulose and hemicellulose have been widely performed and investigated. Understanding the decomposition mechanism of each biomass component can provide important information for the effective transformation of biomass into target products, such as energy, power, or chemical products. In this chapter, we discuss the characteristics and behaviors of thermochemical conversion processes of cellulose and hemicellulose including combustion, pyrolysis, liquefaction, and gasification, and their main products (Fig. 6.1).
6.2 Cellulose
Cellulose is the most abundant organic polymer on earth, which is an important structural component of the plant cell wall (Börjesson and Westman 2015). Cellulose is a linear homo-polysaccharide composed of D-glucose monomers linked together by β-1,4 glycosidic bonds. The degree of polymerization of cellulose depends on its source and ranges from several hundred to over ten thousand. Structurally, cellulose is made up of highly ordered crystalline regions and amorphous parts (Amenaghawon et al. 2021; Collard and Blin 2014; Hendriks and Zeeman 2009). These crystalline regions give mechanical stability, hydrophobicity, and chemical recalcitrance to cellulose microfibrils. These microfibrils are arranged and bound to biomass matrices such as hemicellulose and lignin to form bundles or macrofibrils. In addition, multiple hydroxyl groups in the cellulose can form intermolecular hydrogen bonds among different cellulose chains or intramolecular hydrogen bonds within the polymer itself. The high crystallinity and high degree of hydrogen bonds in the cellulose microfibrils give cellulose fibers strength and stiffness (Börjesson and Westman 2015; Pinkert et al. 2009).
6.3 Hemicellulose
Hemicellulose is a heterogeneous and complex polymer of different sub-constituents such as pentoses (xylose and arabinose), hexoses (glucose, mannose, galactose), hexuronic acids (4-O-methyl-D-glucuronic acid, D-glucuronic acid, and D-galacturonic acid), acetyl groups, and small amounts of L-rhamnose and L-fucose (Patel et al. 2020; Zhou et al. 2018). Hemicellulose accounts for 15–30% of lignocellulosic biomass compositions (Wang et al. 2021). Compared to cellulose, hemicellulose has a lower molecular mass and degree of polymerization. Unlike cellulose, hemicellulose is made up of amorphous regions and branched, making it less stable and more prone to decomposition than cellulose (Amenaghawon et al. 2021; Laureano-Perez et al. 2005). Hemicellulose is cross-linked with cellulose and lignin to strengthen the plant cell wall structure. The types and compositions of hemicellulose vary according to their source. Xylan is the predominant hemicellulose in hardwood and herbaceous biomass, while mannan is found mainly in softwood (Carrier et al. 2011; Zhou et al. 2016). Xylan usually consists of a backbone of β -(1–4)-linked xylose monomers which might attach side chains containing 4-O-methyl-glucuronic acid, glucuronic acid, arabinose, galactose, and glucose (Ebringerovμ et al. 2005). Mannan is composed of a backbone of β-(1–4)-linked mannose units or β-(1–4)-linked mannose and glucose (glucomannan) with/without galactose-containing side chains (Amenaghawon et al. 2021; Zhou et al. 2016) (Table 6.1).
6.4 Combustion
Combustion is the most feasible and conventional way to utilize biomass as a renewable energy source by cleaving the chemical bonds of fuel and generating a series of reactions in the presence of air or oxygen under the heat. To evaluate combustion performance, it is important to recognize the chemical composition and physical characteristics of biomass. Thermogravimetric analysis (TGA) is typically used to investigate thermal decomposition characteristics of solid materials based on weight change at the determined heating rate as a function of time or temperature. The structure and chemical composition of lignocellulosic fibers (cellulose, hemicellulose, and lignin) greatly influence their thermal degradation, and the differential thermal analysis is an effective method to investigate the thermal behavior of the biomass (Chen et al. 2017; Jiang et al. 2017; Sefain et al. 1985).
6.4.1 Combustion of Cellulose
TGA analysis showed that the combustion of pure cellulose showed only one peak, which could be explained by the occurrence of only the combustion of the volatile fraction of cellulose (Boukaous et al. 2018). Combustion kinetics can be gained by using a multi-Gaussian-distributed activation energy model and density-functional theory (Wang et al. 2021; Yu et al. 2021). For combustion of cellulose, reactive force field molecular dynamics (ReaxFF-MD) simulations were applied to study the thermal decomposition of amorphous and partially crystalline cellulose (i.e., microfibrils). By following the complete transformation of cellulose into low molecular weight products, scientists found the decomposition begins with glycosidic bond cleavage. Particularly, the crystallinity has no appreciable effects on the mechanism or kinetics of chain scission (which is generated by glycosidic bond cleavage occurs during cellulose decomposition), the evolution of the molecular weight distribution, or the low molecular weight products (Paajanen et al. 2021).
Stochastic reactive molecular dynamics (RMD) simulations were used to identify and analyze the primary thermal decomposition reactions of an isolated cellulose molecule at a range of temperatures from 1400 to 2200 K (1127–1927 °C), and the results suggest that the decomposition occurs primarily through random cleavage of the (1–4)-glycosidic bonds by an activation energy of (171 ± 2) kJ.mol-1(Paajanen and Vaari 2017). When cellulose is combusted at temperatures above 300 °C, levoglucosan (1,6-anhydro-β-D-glucopyranose) is the most abundant monosaccharide anhydride that was released. Levoglucosan is considered as a molecular marker for the combustion of cellulose (Kuo et al. 2008; Li et al. 2019; Ruan et al. 2020; Segato et al. 2021).
Cellulose combustion typically releases carbon monoxide and carbon dioxide. The mechanism of carbon monoxide release in cellulose combustion was investigated by using molecular dynamics (MD) simulations together with ReaxxFF to analyze reactions of cellobiose oxidation at different temperatures. Basically, this approach uses molecular dynamics simulations equipped with a reactive force field to study the formation of cellobiose from the cellulose oxidation process. The production of carbon monoxide is highly dependent on the abundance of formyl and carboxyl groups, which are formed through cellobiose decomposition. Elevated temperatures cause more carbon monoxide to be released. Subsequently, the formed carbon monoxide is oxidized into carbon dioxide, where reaction steps for the formation and decomposition of the carboxyl group are involved. The simulation results help to identify critical reaction steps and lead to the development of a method to reduce the concentration of free radicals, which then allows the formation of carbon monoxide to be reduced (Barzegar et al. 2020; Hao et al. 2020; Luo et al. 2018).
6.4.2 Recent Applications of Cellulose Combustion
A new synthetic method known as combustion synthesis (CS) has emerged and gained considerable research attention due to it being fast and economic and involving simple synthesis steps. Using a thin cellulose paper, the technique called “Cellulose Assisted Combustion Synthesis” (CACS) or “Impregnated Layer Combustion Synthesis” (ILCS) has been applied for the synthesis of electrode materials, metal oxides, ceramics, catalysts, plus other products (Kumar 2019). Cellulose-based materials with their excellent film-forming properties, mechanical properties, and flame-retardancy, have found application in the battery industry. In this process, cellulose was prepared in a membrane, with excellent infiltration of the electrolyte and flame-retardant performance, namely DOPO–Cinnamoyl Cellulose (DCC), then assembled into a lithium-ion battery. The corresponding battery characterization was then tested for its cycle and rate stability. The test results showed excellent characteristics and enhanced battery performance making the cellulose-based composite materials a very promising separator for high power applications, which broadens their uses in the field of energy storage devices (Liu et al. 2021).
Cellulose combustion can also be applied in making Ni–MgO catalysts with cellulose paper being impregnated with Mg(NO3)2, Ni(NO3)2, glycine solutions, and their different combinations. It was established that the combustion mechanism changes as a function of the impregnated media composition, and after combusting, the resulting materials had a highly porous, sponge-like microstructure (Danghyan et al. 2020). Using a single-step nitrate-cellulose combustion synthesis, a novel method was proposed to produce MnO/carbon composites, in which the MnO nanoparticles were embedded into a porous carbon matrix, that resulted in a MnO/carbon composite with enhanced cycling performance and capacity retention, as it had potential to be an anode alternative for high-performance lithium-ion battery (Zhu et al. 2016).
To have a deep understanding of the mechanism, scientists developed a novel model for the combustion of reactive solutions impregnated into a cellulose carrier, which were shown to be effective in the synthesis of metallic oxides with a nanoscale microstructure, that made the cellulose-assisted combustion materials suitable for catalyst applications. Basically, the model involved three reactions: (1) combustion of the carrier matrix, (2) an endothermic reaction related to the decomposition or gasification of the synthesis reaction precursors, and (3) the exothermic oxide synthesis reaction. The results indicated that manipulation of the cellulose burning reaction was the most favorable to increase the reaction yield of the composite materials (Lennon et al. 2011).
6.4.3 Combustion of Hemicellulose
Hemicellulose combustion occurs via a two-step process: (1) Reduced degree of polymerization in the first step; and (2) Decomposed into volatiles and char in the second step. The ignition temperature is the temperature at which the combustion reaction begins, while the final temperature indicates the end of the combustion reaction, which is essential to ensure the perfect design of the combustor and avoid unburned solid fuel at the outlet of the reactor. In the first step, hemicellulose requires the lowest ignition temperature and also gains the lowest final temperature. However, in the second step, the ignition and final temperature of hemicellulose are higher than those required for the other components and just only lower than that of lignin. TGA characteristic curves showed the presence of three peaks, two of them were completely overlapped and could be explained by the heterogeneity of hemicellulose, which is mainly constituted of xylose along with small parts of glucuronic acid and other sugars (Boukaous et al. 2018). Through the process, mannosan and galactosan are considered as molecular markers for the combustion of hemicellulose (Kuo et al. 2008; Li et al. 2019; Ruan et al. 2020; Segato et al. 2021).
6.5 Pyrolysis
Pyrolysis is the thermochemical conversion process of biomass feedstock in the absence of oxygen to break down the large complex polymers present in the biomass into smaller fragments and molecules. The pyrolysis process is initiated by the evaporation of water, followed by primary decomposition and secondary reactions. Primary decomposition consists of three pathways including char formation, depolymerization, and fragmentation (Collard and Blin 2014; Lu et al. 2011; Van de Velden et al. 2010; Wooten et al. 2003). The charring process involves the formation of aromatic polycyclic structures of chars in which benzene rings are formed, combined, and rearranged (Cho et al. 2010; McGrath et al. 2003). Depolymerization breaks down the linkages in the biomass polymers, resulting in a decrease in the degree of polymerization of the chains and releasing volatile compounds (Azeez et al. 2011; Madhua et al. 2020). Fragmentation occurs in the linkage of the polymers even within the monomer units, leading to the formation of small condensable organic compounds and incondensable gases (Collard and Blin 2014; Kostetskyy and Broadbelt 2020). Secondary reactions include cracking or recombination (Chen et al. 2019a). Cracking reactions involve the breaking of chemical bonds within the unstable and volatile compounds, which results in the formation of lower molecular weight molecules (Collard and Blin 2014; Neves et al. 2011). In contrast, released volatile compounds can be recombined together to yield higher molecular weight molecules (Orcid et al. 2017; Wei et al. 2006). The products of pyrolysis are divided into three groups including carbon-rich solids (char), liquid products of condensable vapors (tars and oils), and non-condensable species (Abhijeet et al. 2020; Patel et al. 2020; Robert and Kaige 2017). The yield of each fraction depends on the heating rate and residence time. The slow heating rates and long residence times favor the production of solid char, while high heating rates and short residence times facilitate the production of vapor products. It is considered as slow pyrolysis when the heating rate is below 10 °C/s, fast pyrolysis when it is higher than 100 °C/s, and flash pyrolysis when the process is performed at higher than 500 °C/s. Biomass pyrolysis has been used for centuries and continually improved since it provides many benefits and serves as a sustainable means of producing biofuels, biochemicals, and other commodities (Amenaghawon et al. 2021; Robert and Kaige 2017).
6.5.1 Slow Pyrolysis
Slow pyrolysis is the oldest form of biomass pyrolysis that usually occurs over a long period of time using slow heating rates to produce charcoal or bio-char as the main product. Lower heating rates and long residence times facilitate the re-polymerization reactions of the biomass constituents, which may generate a polycyclic carbon structure and maximize the yield of solid bio-char. Most of the slow pyrolysis literature focused primarily on the production of carbon-rich solids, thus the process is called carbonization (Gabhane et al. 2020). Carbonization is carried out over a wide temperature range from 300 to 900 °C.
Temperature plays the most important role in the production of bio-char by slow pyrolysis. The processing temperature determines the structural and physicochemical properties of bio-char such as surface area, pore structure, surface functional groups and elemental composition (Tag et al. 2016). It was found that high pyrolysis temperature resulted in increased specific surface area, pore-volume, and carbon content of bio-char but reduced bio-char yield, nitrogen, oxygen, and hydrogen content (Chatterjee et al. 2020; Dhar et al. 2020; Ferraro et al. 2021; Moradi-Choghamarani et al. 2019; Salam et al. 2020). Bio-char with a high specific surface area, large pore size, and elevated carbon content have potential applications in pollution remediation, soil fertility improvement, and carbon sequestration (Agnieszka et al. 2020; Tag et al. 2016).
Torrefaction is another type of slow pyrolysis which is performed at mild temperatures between 200 and 300 °C in the absence of oxygen to produce torrefied biomass (Boateng, 2020). During the torrefaction process, water and volatile compounds in the biomass feedstock are released resulting in the formation of a dark solid material (torrefied biomass) that exhibits higher energy density and greater homogeneity compared to the original feedstock (Amenaghawon et al. 2021). Currently, torrefaction has been investigated intensively and is gaining a high capability to be implemented at an industrial production scale.
6.5.1.1 Torrefaction of Cellulose
Previous studies mainly investigated the effects of torrefaction pretreatment on the chemical structure and pyrolysis behaviors of whole biomass, while a few studies focused on the structural transformation of cellulose. The reaction temperature significantly affects the distribution of the products from cellulose pyrolysis. The torrefaction of cellulose slowly occurred at 200 °C to produce solid char as the main product, along with a small amount of bio-oil, but no gaseous products were generated at below 250 °C. As the temperature was increased, the yield of bio-char decreased while bio-oil production was elevated (Zhang et al. 2021). The formation of an intermediate product called “active cellulose” or anhydrocellulose was reported during the processing below 300 °C (Paajanen and Vaari 2017). Active cellulose is obtained from partial depolymerization of the cellulose, whereas anhydrocellulose is formed after the dehydration reactions. For application, cellulose can be directly used for the production of hydrocarbon-rich bio-oil using integrated microwave torrefaction and ZSM-5 catalyst, but the results are not equal in comparison to those from biomass (Bu et al. 2021; Shen and Gu 2009; Zhou et al. 2020). Significant influences of metal salts, such as chlorides (CaCl2, ZnCl2, MgCl2, and Al2O3), hydroxides (Ca(OH)2 and Mg(OH)2), and acetates (Ca(CH3COO)2) on the torrefaction of cellulose, indicated that the conversion mechanism of torrefaction process needed to be intently considered before scaling up to the industrial application (Atienza-Martínez et al. 2017; Tancredi et al. 2017; Zhao et al. 2020; Zhou et al. 2020).
6.5.1.2 Torrefaction of Hemicellulose
According to the TGA profile, the thermal decomposition of hemicellulose occurs at a temperature range of 200–350 °C (Collard and Blin 2014; Zhou et al. 2016). The lower thermal stability of hemicellulose compared to cellulose is attributed to its amorphous structure and its lower degree of polymerization. Therefore, hemicellulose requires lower temperature and activation energy for thermal decomposition (Chen et al. 2019b; Negi et al. 2020; Zhou et al. 2016). The structural changes in hemicellulose after torrefaction, even disappeared at higher torrefaction temperatures (250–300 °C), probably is caused by the removal of hydroxyls in hemicellulose resulting in the generation of carboxyl and conjugated ketone, based on two-dimensional perturbation correlation analysis using Fourier transform infrared spectroscopy (Chen et al. 2015; Wang et al. 2016a). After torrefaction, oxygen content decreases significantly, leading to the increase of a high heating value. The results obtained from analyzing two-dimensional perturbation correlation based on diffuse reflectance infrared Fourier transform spectroscopy showed that the dehydration of hydroxyls and the dissociation of branches were the main reactions at low torrefaction temperature, but when the temperature was increased, the depolymerization of hemicellulose and the fragmentation of monosaccharide residues occurred. Via the activation energy model, the results showed that hemicellulose torrefaction process enhanced the activation energy but decreased the yields of torrefied products (Cahyanti et al. 2021; Wang et al. 2016b). The torrefaction process strongly affects the degradation of hemicellulose, that leads to the increase of carbon content, decreased H/C and O/C ratios, increased mass energy density, higher heating value, better grindability, higher hydrophobicity, and resistance to biodegradation (Niu et al. 2019; Zheng et al. 2021).
6.5.2 Fast Pyrolysis
In contrast to slow pyrolysis, fast pyrolysis involves the use of a high heating rate, short residence time (<2 s), and rapid vapor cooling to obtain bio-oil, mixed gases, and solid char (Pawar et al. 2020). During the fast pyrolysis process, rapid decomposition of the biomass occurs with the formation of vapors and aerosols, which are condensed after quenching to recover a darkish brown liquid known as bio-oil. Slow pyrolysis gives high solid yields with low liquid yields, while fast pyrolysis gives high liquid yields with low solid yields (Amenaghawon et al. 2021). Fast pyrolysis is the most popular technique for the production of liquid fuels and various commodity chemicals.
Recently, bio-oil produced through the fast pyrolysis process has attracted considerable attention since it provides potential uses as renewable biofuels source, biofuel additives, and as a precursor for the production of specialty chemicals (Patel et al. 2020). The quality and yield of bio-oil produced by fast pyrolysis are affected by not only processing conditions but also the chemical composition of the biomass feedstock. Many studies have shown that the optimum temperatures for obtaining high liquid yields were between 450 and 550 °C, and bio-oil yields varied according to the type of biomass used. It was reported that biomass feedstocks with moisture content less than 10% and particle size of 2–3 mm were the primary requirements for achieving a high heating rate and heat transfer during fast pyrolysis (Pandey et al. 2015).
Moreover, the pyrolysis reactor must be designed and controlled to heat up the biomass rapidly and cool the vapor phase to make it condense to form the bio-oil products in seconds (Robert and Kaige 2017). Several configurations of reactors are used for pyrolysis including bubbling fluidized bed, circulating fluidized bed, conical spouted bed, ablative reactor, rotating cone, vacuum pyrolysis reactor, entrained flow reactor, wire mesh reactor, and auger (twin screw) reactor. The fluidized bed is the most widely used pyrolysis reactor because it is simple to design, construct, and operate. Moreover, it is proven to exhibit a high heat transfer rate, good temperature control, temperature uniformity, and large heat storage capacity (Boateng 2020; Pandey et al. 2015). A circulating fluidized bed reactor is similar to a bubbling fluidized bed except for the velocity of the gas used to fluidize the bed. In the circulating fluidized beds, the gas velocity is set high enough to transport char and heat carrier particles (e.g., sands) to the second combustor. The sand stream is reheated through the char combustion process and then recirculated to the fluidized bed to heat up the biomass feedstock. Circulating fluidized bed reactors have been used for obtaining a high yield of bio-oil from sugarcane bagasse (78%) (Treedet and Suntivarakorn 2018), sawdust, giant Miscanthus, and empty fruit bunch (60%) at pilot scale (Park et al. 2019). Another technology is the rotating cone reactor in which biomass feedstock and hot sand are fed near the bottom of a cone at the same time. The centrifugal forces generated by the rotation of the cone enable the particles to move upwards without the need for large volumes of carrier gas. Hot sand is then recycled back to the rotating cone reactor. The Biomass Technology Group (BTG) has commercialized this reactor that converts biomass feedstock into bio-oil as the main product in just 2 s. It has been reported that a conical spouted bed reactor is suitable for the fast pyrolysis to obtain high bio-oil yields from rice husk (70%) (Alvarez et al. 2014) and eucalyptus waste (75.4%) (Maider Amutio et al. 2015). The outstanding features of the spouted bed reactor include high heating and mass transfer rates, short residence time, and continuous char removal, which accelerate de-volatilization reactions and minimize the cracking of these components (Alvarez et al. 2014; Amutio et al. 2012).
6.5.3 Flash Pyrolysis
Efforts have been carried out to increase the heating rate and minimize the residence time of the vapor phase to improve the production yield of bio-oil from biomass pyrolysis. High bio-oil yields of 75–80% can be achieved by flash pyrolysis occurring at high temperatures (>800 °C) using very high heating rates (>1000 °C/s) and very short residence times (<0.5 s) (Pawar et al. 2020). Feedstock particle size less than 0.2 mm is a primary requirement to achieve such a high heating rate and heat transfer rate (Amenaghawon et al. 2021; Balat et al. 2009; Dada et al. 2021). Rotating cone and conical spouted bed reactors are considered good configurations for the flash pyrolysis process due to their high heat transfer rates and short residence time (Amutio et al. 2012; Papari and Hawboldt 2015).
6.5.4 Pyrolysis of Cellulose
TGA is the most common analytical method used to study the mechanism of polymer degradation. TGA of cellulose showed that the cellulose is thermally degraded at a temperature range of 300–400 °C with the highest decomposition rate between 330 and 370 °C (Collard and Blin 2014; Zhou et al. 2016). The reaction temperature significantly affects the distribution of the products from cellulose pyrolysis. Bio-oil yield increased with increasing temperatures and was maximized at 450–500 °C. Further increase in the temperature resulted in a gradual decrease in the yield of tar and bio-oil. In contrast, the conversion of cellulose into gaseous products was dominant at above 600 °C and the yield of syngas including CO, CO2, CH4, and H2 significantly increased with increasing temperatures (Zhang et al. 2021).
Pyrolysis of cellulose produces several products classified into three groups: anhydrosugars, low molecular weight (LMW) compounds, and furans. Anhydrosugars are formed by transglycosylation reactions (Junior et al. 2020). Levoglucosan (LG) is the most abundant anhydrosugar (up to 80% in relative peak area) from cellulose pyrolysis (Collard and Blin 2014; Junior et al. 2020; Yang et al. 2020a). The yield of levoglucosan is affected by processing temperatures, residence times, and the degree of polymerization of the cellulose chains (Wang et al. 2020a; Yang et al. 2020a). The subsequent decomposition of LG produces LMW compounds, such as 1-pentene-3,4-dione, acetaldehyde, 2,3-dihydroxypropanal, and propanedialdehyde from C −O bond breaking reactions (Zhang et al. 2012). The levoglucosan vaporized above 500 °C contributes mainly to gaseous and liquid streams rather than solid char formation (Banyasz et al. 2001; Basu 2013). Other anhydrosugars were found in much smaller proportions than LG, such as levoglucosenone, 1,4:3,6-dianhydro-α-D-glucopyranose, 2,3-anhydro-D-mannosan, and 1,6-anhydro-β-D-glucofuranose. LMW compounds from cellulose pyrolysis include glycoaldehyde, pyruvaldehyde, hydroxyacetone, and glyceraldehyde (Patwardhan et al. 2011; Yang et al. 2020a). Organic acids such as formic acid, acetic acid, and propionic acid also were detected among the LMW pyrolytic products (Patwardhan et al. 2011; Wamg et al. 2012, 2020b). Furans are formed by open-ring reactions and dehydration from the glucopyranose structure (Junior et al. 2020). The yields of furans (5-hydroxymethyl furfural, furfural, 2-furan methanol, 3-furan methanol, and 5-methyl furfural) varied with operating conditions. It is noted that the furan ring is very stable, but its side groups (methyl or oxygenated groups) are less stable and are readily cleaved or rearranged with increasing temperatures. Decomposition of furans led to an increase in the formation of low molecular weight compounds and gaseous species. 5-Hydroxymethyl furfural (5-HMF) and furfural, the attractive platform chemicals, were found to be the most abundant furans (Patwardhan et al. 2011; Yang et al. 2020a). In a previous study, the furans content reached the highest value, about 35% (in relative peak area), and changed little with increasing temperature. It is proven that the thermal degradation of pentose sugars (xylose and arabinose) tends to yield furfural, whereas the processing of hexose sugars (glucose, mannose, and galactose) tends to produce 5-HMF (Zhou et al. 2016). Further decomposition of 5-HMF via fast pyrolysis at 600 °C was performed and resulted in the release of 72.8% furfural. This evidence demonstrated the presence of furfural as a predominant secondary product of cellulose pyrolysis reported in the literature (Collard and Blin 2014; Wang et al. 2012).
6.5.5 Pyrolysis of Hemicellulose
According to the TGA profiles, the entire thermal degradation of hemicellulose could take place in three steps. The first step includes the dehydration and cleavage of the side chains of hemicellulose with slight mass loss. The dissociation of side chains in xylan occurs readily due to its relatively low energy of activation (Dai et al. 2021). Acetic acid was reported as the major product in xylan pyrolysis which was formed by the cleavage of acetyl groups attached to the backbone of xylan (Zhou et al. 2016). The main pyrolysis, which occurs in the second step, is responsible for the most mass loss through a sequence of reactions such as dehydration, decarboxylation, and decarbonylation. Finally, in the third step, further decomposition of hemicellulose occurs to release volatiles from the residue resulting in a slight mass loss (Collard and Blin 2014; Peng and Wu 2010).
Xylan is the most abundant hemicellulose in nature. Xylan is typically used as a model compound for understanding the mechanism of hemicellulose pyrolysis. Pyrolysis of xylan typically yields 20–30% char, 10–20% non-condensable gas species, and 40–60% bio-oil (Zhou et al. 2018). The non-condensable gaseous products include H2, CO, CO2, and light hydrocarbons such as CH4, C2H6, and C3H8. Bio-oil mainly includes acids (acetic acid and formic acid), furans (furfural), anhydrosugars (anhydroxylose and dianhydroxylose), aldehydes, ketones, and minor aromatic compounds (Zhou et al. 2016, 2018).
Hemicellulose is composed of heterogeneous and diverse monomers. Pyrolysis of hemicellulose generates more complicated and various product distributions compared to cellulose pyrolysis. Hemicellulose pyrolytic products are mainly categorized into two groups including light oxygenated compounds and furans. Light oxygenated compounds mainly include glycoaldehyde, acetaldehyde, 1-hydroxy-2-propanone, 4-hydroxy-5,6-di-hydro-(2 H)-pyran-2-one, and 1-hydroxy-2-butanone, among which glycoaldehyde is detected as the most abundant compound (Werner et al. 2014). Furfural is widely reported as a major furan in hemicellulose pyrolysis (Zhou et al. 2018). Furfural is derived from thermal degradation of pentose units or by secondary decomposition of 5-hydroxymethyl furfural (5-HMF) (Usino et al. 2020). Hemicellulose rich in acetyl groups attached to its backbone yields more acetic acid as a major product (Wang et al. 2015). Furans and acetic acid contents decrease with increasing temperatures (Wang et al. 2015).
6.6 Liquefaction
Liquefaction (hydrothermal liquefaction) is a process responsible for the conversion of lignocellulosic biomass into bio-liquid and/or crude oil-like products at temperatures of 280–370 °C and pressures of 10–25 MPa in the absence of oxygen (Khatiwada et al. 2021; Yang et al. 2020a). This technology has much attraction to produce renewable fuels due to its low operating temperatures and fast reaction rates, and the use of wet feedstocks without the need for an energy-intensive drying process (Amarasekara and Reyes 2020; Khatiwada et al. 2021; Song et al. 2020). There are two types of liquefaction, which include aqueous liquefaction (using water) and non-aqueous liquefaction (using organic solvents such as methanol, ethanol, isopropanol, phenol, and others). The aqueous liquefaction is typically carried out in subcritical water and requires catalysts such as Brønsted or Lewis acids and metal oxides such as MnO, CaO, CeZrOx, Raney Ni-NaOH, Na2CO3, Fe-zeolite, Na2CO3-Fe. Aqueous liquefaction generally gives higher quality and quantity of bio-oil compared to non-aqueous liquefaction (Amarasekara and Reyes 2020; Feng et al. 2018; Song et al. 2020; Yang et al. 2020a).
6.6.1 Liquefaction of Cellulose
Under thermochemical liquefaction, cellulose is mainly hydrolyzed into monosaccharides and further to acids, aldehydes, ketones, and other products. Cellulose is decomposed into soluble sugars (cellobiose, lactose, and glucose) as primary products in subcritical water at 200–300 °C. Soluble sugars subsequently undergo secondary reactions of isomerization, dehydration, and retro-aldol condensation to form D-erythrose, glycolaldehyde, and furfural, which are further degraded to smaller species. A significant decrease in soluble products was observed with increasing reaction times because the sugars were readily degraded to carboxylic acids, ketone, and aldehydes (Gagic et al. 2018; Wang et al. 2020a). These products then can be upgraded to liquid fuels, platform biochemicals, and commodity chemicals such as ethanol, liquid alkanes, 5-hydroxymethylfurfural (5-HMF), furfural, and acetic acid. The results obtained in several studies showed that cellulose could be liquefied with high efficiency (Li et al. 2020; Peng et al. 2018; Wang et al. 2020b; Xu et al. 2019).
Catalyst is one of the key factors that affect the quantity and quality of liquefaction products. In addition to acidic, alkali, and metal catalysts, glycol organosolv and supercritical ethanol (with 2,2,6,6-Tetramethylpiperidinooxy-TEMPO) processes have recently received much attention, due to their environmental benefits, low viscosity, and high solubility (Jasiukaitytė-Grojzdek et al. 2021; Liu et al. 2020; Sun et al. 2020a). Hydrothermal liquefaction with metallic-Fe catalyst is still the most effective process for producing water-soluble fraction, thereby enhancing the hydrocarbon yield (Hirano et al. 2020).
Cellulose liquefaction shows great promise for using cellulose as a supporting substrate or template material in photothermal plastic due to its renewability, degradability, abundant availability, and low cost. In fact, cellulose composite materials have been successfully developed and applied in various fields such as antibacterial compounds, UV shielding, catalysis, flame retardant, fluorescence, metal ion adsorption, and supercapacitor (Sun et al. 2021b; Zimmermann et al. 2021). In liquefaction technology, solvent is one of the key factors. Water, low-carbon alcohols, supercritical fluids, and hydrocarbon-based solvents such as n-alkanes are often employed in cellulose liquefaction (Li et al. 2021).
The increasing trends of using biomass as an alternative to fossil fuels lead to the formation of the biorefinery concept, which now utilizes a range of biomass and known conversion technologies to produce green chemicals and polymers without impacting food and feed security. Among them, biopolyols and polyurethane obtained by direct conversion of separated cellulose or lignocellulosic biomass through liquefaction technology were listed among the “Top 10” platform chemicals in a report prepared by the Pacific Northwest National Laboratory (PNNL) and the National Renewable Energy Laboratory (NREL) (Ge et al. 2018). Interestingly, polyurethane foams were produced via cellulose liquefaction in the presence of crude glycerol, which indicated that cellulose could be a good alternative material to produce polyurethane (Ge et al. 2018; Kosmela et al. 2018).
6.6.2 Liquefaction of Hemicellulose
Due to its heterogeneous and complex structure, the product profile of hydrothermal liquefaction of hemicellulose is complicated and includes a variety of chemicals such as oligosaccharides, monosaccharides, furfural, glyceraldehyde, acetic acid, lactic acid, plus others (Ghimire et al. 2021; Song et al. 2020; Yang et al. 2020a). Hemicellulose decomposition in subcritical water at below 220 °C yielded monosaccharide sugars, mainly xylose and 4-O-methylglucuronic acid, which were further degraded into small molecule products such as furfural, formic acid, and acetic acid at temperatures higher than 220 °C. Xylan usually is used as a model compound in many studies. Xylan liquefaction resulted in different product distributions through hydrolysis and oxidation reactions depending on operating conditions. Hydrolysis occurs via the depolymerization of xylan to soluble sugars, while oxidation promotes the formation of organic acids. The released organic acids subsequently catalyze the degradation of the hydrolyzed products into small molecules. Catalysts are typically used in liquefaction. The presence of a high concentration of oxidizing agent (H2O2) and/or pressurization facilitates strong oxidative reaction and/or acidic hydrolysis, yielding xylo-oligosaccharides, xylose, arabinose, glucose, acetic acid, and their decomposition compounds, which include furans and organic acids (Phaiboonsilpa et al. 2020; Zhou et al. 2016). In the presence of ethylene glycol, xylan was decomposed and transferred to the liquid phase, with the average molecular weight of xylan significantly decreasing after liquefaction. The products from the liquefaction of xylan in ethylene glycol are ethylene glycol derivatives, alcohols, aldehydes, ketones, some acids, and their esters (Wang et al. 2016a).
6.7 Gasification
Gasification is a thermochemical conversion process that involves complex reactions, pressure changes, and heat and mass transfer. In general, gasification is used to convert solid fuels (coal or biomass) into value-added products or to release heat for heating and power generation at high temperatures without combustion. Gasification requires oxygen, air, and steam to convert carbonaceous materials into gaseous fuels. Fixed bed and fluidized bed gasifiers are common technologies. Basically, gasification consists of four steps: (1) Drying or dehydration, in which evaporation of moisture occurs under 150 °C; (2) Pyrolysis, in which devolatilization occurs at a temperature range of 150–700 °C; (3) Combustion, in which fuel constituents oxidize and exothermic reactions occur in the temperature range of 700–1500 °C; and (4) Reduction, in which endothermic reactions occur in the temperature range of 800–1100 °C (Ong et al. 2019; Soomro et al. 2018; Yang et al. 2020a; Yu et al. 2018).
6.7.1 Gasification of Cellulose
Cellulose consists of several hundred to many thousands of β-glycosidic bonds linking D-glucose units, which are not stable and tend to cleave at high temperatures. It was observed that cellulose began to decompose at 180 °C and the reaction rate was much faster with increasing temperature and residence time, leading to sugar degradation to carboxylic acids, ketone, and aldehydes. Glucose was completely decomposed in 60 s at 300 °C, but only in 0.5 s at 460 °C (Yang et al. 2020a). Degradation of levoglucosan, the major volatile intermediate being produced from cellulose gasification, was completed at a temperature range of 550–700 °C within only 0.11–0.45 s (Fukutome et al. 2017).
Normally, gasification requires a catalyst to increase efficiency while reducing temperature and time. Among these, alkali and alkaline earth metals and Ni-based catalysts are commonly used in the gasification process (Ong et al. 2019). Cellulose gasification produces the largest amount of H2 in the absence of steam and catalyst, but adding the Ni-based catalyst significantly increases the gas yield, particularly for H2 production (Hassan et al. 2020). Moreover, nickel-based catalysts were used and gained the highest H2 yield from gasification, especially in the presence of SiO2 (Sun et al. 2020b; Taylor et al. 2020). To enhance H2 production, scientists have proposed using pure cellulose instead of lignocellulosic biomass and also suggested that a combination of gasification of cellulose and dark fermentation may give a higher hydrogen yield (Hassan et al. 2020). Ca-Fe oxygen carrier is a potential material for efficient lignocellulose conversion and hydrogen-enriched syngas production, acting as catalysts to promote cellulose decomposition (Tang et al. 2021). CaO sorption enhanced the gasification efficiency of biomass for hydrogen-rich syngas production, demonstrating its potential as an inexpensive catalyst for the gasification process (Mbeugang et al. 2021). In addition, the highest carbon monoxide (CO) concentration was found in cellulose gasification compared to those originated from hemicellulose and lignin, which may be due to the abundance of C–O compounds in cellulose (Hassan et al. 2020).
Reliance on catalysts is one of the bottlenecks of gasification, so scientists have proposed other options, in which physical catalysts (Ce/Fe, Ni, etc.) were not used but replaced by a plasma-catalyst system to produce a cleaner syngas. The results provided an alternative and cleaner way for gasification, although it still needs to be evaluated further (Craven et al. 2020; Zou et al. 2018). Recently, scientists proposed a very low temperature gasification system for cellulose (around 50 °C) by using glow-discharge plasma. Due to the low temperature, pyrolysis did not occur, but a very long retention time (90 h) was required. Interestingly, no tar formation was observed, indicating that all of the cellulose was decomposed into gaseous products. This achievement demonstrated that clean and complete gasification of cellulose and/or hemicellulose could be achieved with plasma technology (Minami et al. 2018).
6.7.2 Gasification of Hemicellulose
Xylose and 4-O-methylglucuronic acid were mainly detected in the degradation of hemicellulose at a temperature below 220 °C, but low molecule weight products such as furfural, formic acid, and acetic acid were observed at higher temperatures (Berthet et al. 2016; Hassan et al. 2020). Xylose was completely decomposed in less than 5 s at a temperature of 300–450 °C and 25 MPa. The decomposition behavior was similar to that of glucose as they have a homoeologous molecule structure, except that xylose has one less CH-OH group than glucose. The formation of furfural via dehydration of xylose was considered to be analogous to that of dehydration of glucose (Fukutome et al. 2015; Hassan et al. 2020; Tang et al. 2021).
Hydrothermal gasification in either subcritical or supercritical water is an attractive approach to produce hydrogen from cellulose and hemicellulose, in which hydrogen yields from hemicellulose normally were higher than those from cellulose (García-Jarana et al. 2020; Okolie et al. 2020). Moreover, the use of hemicellulose isolated from biomass not only helps to understand and analyze the catalytic gasification characteristics of natural biomass material with high hemicellulose content, but also gives a considerable method to produce H2 from only hemicellulose, with the highest amount of H2 produced but a lower amount of gasification solid residues than the whole biomass used (Gökkaya et al. 2020).
6.8 Conclusion
For efficient use of biomass, technologies that employ thermochemical conversions, including combustion, pyrolysis, liquefaction, and gasification, have demonstrated high efficiencies and improved environmental performance, leading to their recognition worldwide. Process mechanisms and characteristics of each technology applying to cellulose and hemicellulose, which are two main components of biomass, have been investigated and the reported results have been helpful in identifying potential applications and useful products. Before they can be implemented in industrial applications, the related technologies need more research and development efforts followed by a demonstration at semi-commercial scales. Among the thermochemical conversion processes, combustion offers potential direct applications, especially in the innovation of new materials, whereas liquefaction may become a new route for the production of liquid fuels. Pyrolysis, particularly torrefaction, has been applied widely for heat and electricity generation, but the technology required sophisticated designs for specific equipment in industrial scales, while gasification often consumes extensive energy to reach the required temperatures and pressures. Advances in biomass processing such as pretreatments and fractionation as well as in system and engineering process design may enhance the effectiveness of biomass thermochemical conversion into fuels and value-added products with better performance and cost competitiveness.
References
Abhijeet P, Swagathnath G, Rangabhashiyam S, Asok Rajkumar M, Balasubramanian P (2020) Prediction of pyrolytic product composition and yield for various grass biomass feedstocks. Biomass Convers Biorefinery 10(3):663–674
Agnieszka T, Zofia S, Patrycja B (2020) Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev Environ Sci Biotechnol 19:24
Alvarez J, Lopez G, Amutio M, Bilbao J, Olazar M (2014) Bio-oil production from rice husk fast pyrolysis in a conical spouted bed reactor. Fuel 128:8
Amarasekara AS, Reyes CDG (2020) Pd/C catalyzed room-temperature, atmospheric pressure hydrogenation of furanic bio-oils from acidic ionic liquid catalyzed liquefaction of biomass in acetone. Fuel Process Technol 200:106320
Amenaghawon AN, Anyalewechi CL, Okieimen CO, Kusuma HS (2021) Biomass pyrolysis technologies for value-added products: a state-of-the-art review. Environ Dev Sustain
Amutio M, Lopez G, Alvarez J, Olazar M, Bilbao J (2015) Fast pyrolysis of eucalyptus waste in a conical spouted bed reactor. Biores Technol 194:8
Amutio M, Lopez G, Artetxe M, Elordi G, Olazar M, Bilbao J (2012) Influence of temperature on biomass pyrolysis in a conical spouted bed reactor. Resour Conserv Recycl 59:9
Atienza-Martínez M, Rubio I, Fonts I, Ceamanos J, Gea G (2017) Effect of torrefaction on the catalytic post-treatment of sewage sludge pyrolysis vapors using γ-Al2O3. Chem Eng J 308:264–274
Azeez A, Meier D, Odermatt J (2011) Temperature dependence of fast pyrolysis volatile products from European and African biomasses. J Anal Appl Pyrolysis 90:12
Balat M, Balat M, Kirtay E, Balat H (2009) Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: pyrolysis systems. Energy Convers Manag 50:11
Banyasz J, Li S, Lyons-Hart J, Shafer K (2001) Cellulose pyrolysis: the kinetics of hydroxyacetaldehyde evolution. J Anal Appl Pyrol 57:223–248
Barzegar R, Yozgatligil A, Olgun H, Atimtay AT (2020) TGA and kinetic study of different torrefaction conditions of wood biomass under air and oxy-fuel combustion atmospheres. J Energy Inst 93(3):889–898
Basu P (2013) Biomass gasification, pyrolysis and torrefaction: practical design and theory, 2nd edn. Elsevier
Berthet M-A, Commandré J-M, Rouau X, Gontard N, Angellier-Coussy HJM (2016) Torrefaction treatment of lignocellulosic fibres for improving fibre/matrix adhesion in a biocomposite. Mater Design 92:223–232
Boateng AA (2020) Pyrolysis of biomass for fuels and chemicals. Elsevier
Börjesson M, Westman G (2015) Crystalline nanocellulose—preparation, modification, and properties. In: Cellulose: fundamental aspects and current trends. InTechOpen, pp 35
Boukaous N, Abdelouahed L, Chikhi M, Meniai A-H, Mohabeer C, Bechara TJE (2018) Combustion of flax shives, beech wood, pure woody pseudo-components and their chars: a thermal and kinetic study 11(8):2146
Bu Q, Cao M, Wang M, Zhang X, Mao H (2021) The effect of torrefaction and ZSM-5 catalyst for hydrocarbon rich bio-oil production from co-pyrolysis of cellulose and low density polyethylene via microwave-assisted heating. Sci Total Environ 754:142174
Buendia-Kandia F, Brosse N, Petitjean D, Mauviel G, Rondags E, Guedon E, Dufour A (2020) Hydrothermal conversion of wood, organosolv, and chlorite pulps. Biomass Convers Biorefinery 10(1):1–13
Cahyanti MN, Doddapaneni TRKC, Madissoo M, Pärn L, Virro I, Kikas T (2021) Torrefaction of agricultural and wood waste: comparative analysis of selected fuel characteristics. Energies 14(10):2774
Carrier M, Loppinet-Serani A, Denux D, Lasnier JM, Ham-Pichavant F, Cansell F, Aymonier C (2011) Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenerg 35(1):10
Chatterjee R, Sajjadi B, CheN W-Y, Mattern LD, Hammer N, Raman V, Dorris A (2020) Effect of pyrolysis temperature on physicochemical properties and acoustic-based amination of biochar for efficient CO2 adsorption. Front Energy Res 8(85):18
Chen C, Zhao L, Wang J, Lin S (2017) Reactive molecular dynamics simulations of biomass pyrolysis and combustion under various oxidative and humidity environments. Ind Eng Chem Res 56(43):12276–12288
Chen D, Zheng Z, Fu K, Zeng Z, Wang J, Lu M (2015) Torrefaction of biomass stalk and its effect on the yield and quality of pyrolysis products. Fuel 159:27–32
Chen W-H, Wang C-W, Ong HC, Show PL, Hsieh T-H (2019a) Torrefaction, pyrolysis and two-stage thermodegradation of hemicellulose, cellulose and lignin. Fuel 258:116168
Chen Y, Fang Y, Yang H, Xin S, Zhang X, Wang X, Chen H (2019b) Effect of volatiles interaction during pyrolysis of cellulose, hemicellulose, and lignin at different temperatures. Fuel 248:7
Cho J, Davis JM, Huber GW (2010) The Intrinsic Kinetics and Heats of Reactions for Cellulose Pyrolysis and Char Formation. Chemsuschem 3:4
Collard F-X, Blin J (2014) A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew Sustain Energy Rev 38:15
Craven M, Wang Y, Yang H, Wu C, Tu X (2020) Integrated gasification and non-thermal plasma-catalysis system for cleaner syngas production from cellulose. IOP SciNotes 1(2):024001
Dada TK, Sheehan M, Murugavelh S, Antunes E (2021) A review on catalytic pyrolysis for high-quality bio-oil production from biomass. Biomass Convers Biorefinery
Dai G, Wang G, Wang K, Zhou Z, Wang S (2021) Mechanism study of hemicellulose pyrolysis by combining in-situ DRIFT, TGA-PIMS and theoretical calculation. Proc Combust Inst 38(3):9
Danghyan V, Orlova T, Roslyakov S, Wolf E, Mukasyan A (2020) Cellulose assisted combustion synthesis of high surface area Ni-MgO catalysts: mechanistic studies. Combust Flame 221:462–475
Dhar SA, Sakib TU, Hilary LN (2020) Effects of pyrolysis temperature on production and physicochemical characterization of biochar derived from coconut fiber biomass through slow pyrolysis process. Biomass Convers Biorefinery
Ebringerovμ A, Hromμdkovμ Z, Heinze T, Valantinavičius M (2005) Polysaccharides I, vol 186. Springer, Berlin, pp 67
Feng S, Wei R, Leitch M, Xu CC (2018) Comparative study on lignocellulose liquefaction in water, ethanol, and water/ethanol mixture: roles of ethanol and water. Energy 155:234–241
Ferraro G, Pecori G, Rosi L, Bettucci L, Fratini E, Casini D, Rizzo AM, Chiaramonti D (2021). Biochar from lab-scale pyrolysis: influence of feedstock and operational temperature. Biomass Convers Biorefinery
Fukutome A, Kawamoto H, Saka S (2015) Processes forming gas, tar, and coke in cellulose gasification from gas-phase reactions of levoglucosan as intermediate. ChemSusChem 8(13):2240–2249
Fukutome A, Kawamoto H, Saka S (2017) Kinetics and molecular mechanisms for the gas-phase degradation of levoglucosan as a cellulose gasification intermediate.J Anal Appl Pyrolysis 124:666–676
Gabhane JW, Bhange VP, Patil PD, Bankar ST, Kumar S (2020) Recent trends in biochar production methods and its application as a soil health conditioner: a review. SN Appl Sci 2(7):1307
Gagic T, Perva-Uzunalic A, Knez Z, Skerget M (2018) Hydrothermal degradation of cellulose at temperature from 200 to 300 °C. Ind Eng Chem Res 57:9
García-Jarana MB, Portela JR, Sánchez-Oneto J, Martinez de la Ossa EJ, Al-Duri B (2020) Analysis of the supercritical water gasification of cellulose in a continuous system using short residence times. Appl Sci 10(15):5185
Ge X, Chang C, Zhang L, Cui S, Luo X, Hu S, Qin Y, Li Y (2018) Conversion of lignocellulosic biomass into platform chemicals for biobased polyurethane application. In: Advances in bioenergy, vol 3. Elsevier, pp 161–213
Ghimire N, Bakke R, Bergland WH (2021) Liquefaction of lignocellulosic biomass for methane production: a review. Bioresour Technol 125068
Gökkaya DS, Sert M, Sağlam M, Yüksel M, Ballice L (2020) Hydrothermal gasification of the isolated hemicellulose and sawdust of the white poplar (Populus alba L.). J Supercrit Fluids 162:104846
Hao H, Chow CL, Lau D (2020) Carbon monoxide release mechanism in cellulose combustion using reactive forcefield. Fuel 269:117422
Hassan N, Jalil A, Vo D, Nabgan W (2020) An overview on the efficiency of biohydrogen production from cellulose. Boimass Convers Biorefinery 1–23
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellu- losic biomass. Bioresour Technol 100(1):9
Hirano Y, Miyata Y, Taniguchi M, Funakoshi N, Yamazaki Y, Ogino C, Kita Y (2020) Fe-assisted hydrothermal liquefaction of cellulose: effects of hydrogenation catalyst addition on properties of water-soluble fraction. J Anal Appl Pyrolysis 145:104719
Jasiukaitytė-Grojzdek E, Vicente FA, Grilc M, Likozar B (2021) Ambient-pressured acid-catalysed ethylene glycol organosolv process: liquefaction structure-activity relationships from model cellulose-lignin mixtures to lignocellulosic wood biomass. Polymers 13(12):1988
Jiang X, Chen D, Ma Z, Yan J (2017) Models for the combustion of single solid fuel particles in fluidized beds: a review. Renew Sustain Engery Rev 68:410–431
Junior II, Do Nascimento MA, de Souza ROMA, Dufourd A, Wojcieszaka R (2020) Levoglucosan: a promising platform molecule? Green Chem 22:22
Khatiwada JR, Shrestha S, Sharma HK, Qin W (2021) Bioconversion of hemicelluloses into hydrogen. In: Sustainable bioconversion of waste to value added products. Springer, pp 267–280
Kosmela P, Hejna A, Formela K, Haponiuk J, Piszczyk Ł (2018) The study on application of biopolyols obtained by cellulose biomass liquefaction performed with crude glycerol for the synthesis of rigid polyurethane foams. J Polym Environ 26(6):2546–2554
Kostetskyy P, Broadbelt LJ (2020) Progress in modeling of biomass fast pyrolysis: a review. Energy Fuels 34(12):22
Kumar A (2019) Current trends in cellulose assisted combustion synthesis of catalytically active nanoparticles. Ind Eng Chem Res 58(19):7681–7689
Kuo L-J, Herbert BE, Louchouarn P (2008) Can levoglucosan be used to characterize and quantify char/charcoal black carbon in environmental media? Organic Geochem 39(10):1466–1478
Laureano-Perez L, Teymouri F, Alizadeh H, Dale BE (2005) Understanding factors that limit enzymatic hydrolysis of biomass. Appl Biochem Biotechnol 124(1–3):19
Lennon E, Tanzy M, Volpert V, Mukasyan A, Bayliss AJ (2011) Combustion of reactive solutions impregnated into a cellulose carrier: modeling of two combustion fronts. Chem Eng 174(1):333–340
Li J, Qin G, Tang X, Xiang C (2021) Fe/HZSM-5 catalyzed liquefaction of cellulose assisted by glycerol. Catal Commun 151:106268
Li Q, Hu X, Liu D, Song L, Yan Z, Li M, Liu Q (2020) Comprehensive evaluation of hydro-liquefaction characteristics of lignocellulosic subcomponents. J Energy Inst 93(4):1705–1712
Li Q, Wang N, Barbante C, Kang S, Callegaro A, Battistel D, Argiriadis E, Wan X, Yao P, Pu T (2019) Biomass burning source identification through molecular markers in cryoconites over the Tibetan Plateau. Environ Pollut 244:209–217
Liu J, Qin D, Chen K, Wang B, Bian H, Shao Z (2021) The preparation of intrinsic dopo-cinnamic flame-retardant cellulose and its application for lithium-ion battery separator. Mater Res Express 8(7):076404
Liu Y, Wu S, Zhang H, Xiao R (2020) Preparation of carbonyl precursors for long-chain oxygenated fuels from cellulose ethanolysis catalyzed by metal oxides. Fuel Process Technol 206:106468
Lu Q, Yang X, Dong C (2011) Influence of pyrolysis temperature and time on the cellulose fast pyrolysis products: analytical Py-GC/MS study. J Anal Appl Pyrolysis 92:9
Luo J, Li Q, Meng A, Long Y, Zhang Y (2018) Combustion characteristics of typical model components in solid waste on a macro-TGA. J Therm Anal Calorim132(1):553–562
Madhua P, Dhanalakshmia CS, Mathew M (2020) Multi-criteria decision-making in the selection of a suitable biomass material for maximum bio-oil yield during pyrolysis. Fuel 277:12
Mbeugang CFM, Li B, Lin D, Xie X, Wang S, Wang S, Zhang S, Huang Y, Liu D, Wang Q (2021) Hydrogen rich syngas production from sorption enhanced gasification of cellulose in the presence of calcium oxide. Energy 228:120659
McGrath T, Chan W, Hajaligol M (2003) Low temperature mechanism for the formation of polycyclic aromatic hydrocarbons from the pyrolysis of cellulose. J Anal Appl Pyrolysis 66:20
Minami E, Fujimoto S, Saka SJ (2018) Complete gasification of cellulose in glow-discharge plasma. J Wood Sci 64(6):854–860
Moradi-Choghamarani F, Moosavi AA, Baghernejad M (2019) Determining organo-chemical composition of sugarcane bagasse-derived biochar as a function of pyrolysis temperature using proximate and Fourier transform infrared analyses. J Therm Anal Calorim 138
Negi S, Jaswal G, Dass K, Mazumder K, Elumalai S, Roy JK (2020) Torrefaction: a sustainable method for transforming of agri-wastes to high energy density solids (biocoal). Rev Environ Sci Biotechnol 19(2)
Neves D, Thunman H, Matos A (2011) Characterization and prediction of biomass pyrolysis products. Progr Energy Combust Sci 37:20
Niu Y, Lv Y, Lei Y, Liu S, Liang Y, Wang D (2019) Biomass torrefaction: properties, applications, challenges, and economy. Renew Sustain Energy Rev 115:109395
Okolie JA, Nanda S, Dalai AK, Kozinski JA. J. I. J. o. H. E. (2020). Optimization and modeling of process parameters during hydrothermal gasification of biomass model compounds to generate hydrogen-rich gas products. Int J Hydrog Energy 45(36):18275–18288
Ong HC, Chen W-H, Farooq A, Gan YY, Lee KT, Ashokkumar V (2019) Catalytic thermochemical conversion of biomass for biofuel production: a comprehensive review. Renew Sustain Energy Rev 113:109266
Orcid ER, Debiagi PEA, Frassoldati A (2017) Mathematical modeling of fast biomass pyrolysis and bio-oil formation. Note II: secondary gas-phase reactions and bio-oil formation. ACS Sustain Chem Eng 5(4):15
Paajanen A, Rinta-Paavola A, Vaari J (2021) High-temperature decomposition of amorphous and crystalline cellulose: reactive molecular simulations. Cellulose 1–19
Paajanen A, Vaari J (2017) High-temperature decomposition of the cellulose molecule: a stochastic molecular dynamics study. Cellulse 24(7):2713–2725
Pandey A, Stöcker M, Bhaskar T, Sukumaran RK (2015) Recent advances in thermochemical conversion of biomass. Elsevier
Papari S, Hawboldt K (2015) A review on the pyrolysis of woody biomass to bio-oil: Focus on kinetic models. Renew Sustain Energy Rev 52:16
Park JY, Kima J-K, Oh C-H, Park J-W, Kwon EE (2019) Production of bio-oil from fast pyrolysis of biomass using a pilot-scale circulating fluidized bed reactor and its characterization. J Environ Manag 234:7
Patel A, Agrawal B, Rawal BR (2020) Pyrolysis of biomass for efficient extraction of biofuel. Energy Sources Part A Recover Util Environ Effects 42:13
Patwardhan PR, Dalluge DL, Shanks BH, Brown RC (2011) Distinguishing primary and secondary reactions of cellulose pyrolysis. Bioresour Technol 102(8):5
Pawar A, Panwar NL, Salvi BL (2020) Comprehensive review on pyrolytic oil production, upgrading and its utilization. J Mater Cycles Waste Manag 22:11
Peng J, Liu X, Bao Z (2018) Experimental study on the liquefaction of cellulose in supercritical ethanol. Paper presented at the IOP conference series: materials science and engineering
Peng Y, Wu S (2010) The structural and thermal characteristics of wheat straw hemicellulose. J Anal Appl Pyrolysis 88(2):6
Phaiboonsilpa N, Champreda V, Laosiripojana N (2020) Comparative study on liquefaction behaviors of xylan hemicellulose as treated by different hydrothermal methods. Energy Rep 6:714–718
Pinkert A, Marsh KN, Pang S, Staiger MP (2009) Ionic liquids and their interaction with cellulose. Chem Rev 109:17
Robert CB, Kaige W (2017) Fast pyrolysis of biomass advances in science and technology
Ruan Y, Mohtadi M, Dupont LM, Hebbeln D, van der Kaars S, Hopmans E, Schouten S, Hyer EJ, Schefuß E (2020) Interaction of fire, vegetation, and climate in tropical ecosystems: a multiproxy study over the past 22,000 years. Global Biogeochem Cycles 34(11):e2020GB006677
Salam A, Bashir S, Khan I, Hu H (2020) Biochar production and characterization as a measure for effective rapeseed residue and rice straw management: an integrated spectroscopic examination. Biomass Convers Biorefinery
Sefain MZ, El‐Kalyoubi SF, Shukry N (1985) Thermal behavior of holo‐and hemicellulose obtained from rice straw and bagasse 23(5):1569–1577
Segato D, Villoslada Hidalgo MDC, Edwards R, Barbaro E, Vallelonga P, Kjær HA, Simonsen M, Vinther B, Maffezzoli N, Zangrando R, Turetta C (2021) Five thousand years of fire history in the high North Atlantic region: natural variability and ancient human forcing. Clim Past 17(4):1533–1545
Shen D, Gu S (2009) The mechanism for thermal decomposition of cellulose and its main products. Biores Technol 100:6496–6504
Song C, Zhang C, Zhang S, Lin H, Kim Y, Ramakrishnan M, Du Y, Zhang Y, Zheng H, Barcelo D (2020). Thermochemical liquefaction of agricultural and forestry wastes into biofuels and chemicals from circular economy perspectives. Sci Total Environ 141972
Soomro A, Chen S, Ma S, Xu C, Sun Z, Xiang W (2018) Elucidation of syngas composition from catalytic steam gasification of lignin, cellulose, actual and simulated biomasses. Biomass Bioenergy 115:210–222
Sun J, Xie X-A, Fan D, Wang X, Liao W (2020a) Effect of TEMPO and characterization of bio-oil from cellulose liquefaction in supercritical ethanol. Renew Energy 145:1949–1956
Sun J, Xu L, Dong G-H, Nanda S, Li H, Fang Z, Kozinski JA, Dalai AK (2020b) Subcritical water gasification of lignocellulosic wastes for hydrogen production with Co modified Ni/Al2O3 catalysts. J Supercrit Fluids 162:104863
Sun L, Li L, An X, Qian X (2021) Mechanically strong, liquid-resistant photothermal bioplastic constructed from cellulose and metal-organic framework for light-driven mechanical motion. Molecules 26(15):4449
Tag A-T, Duman G, Ucar S, Yanik J (2016a) Effects of feedstock type and pyrolysis temperature on potential applications of biochar. J Anal Appl Pyrolysis 120:7
Tancredi N, Gabús M, Yoshida MI, Suárez AC (2017) Thermal studies of wood impregnated with ZnCl 2. Eur J Wood Wood Prod 75(4):633–638
Tang G, Gu J, Huang Z, Yuan H, Chen Y (2021) Cellulose gasification with Ca-Fe oxygen carrier in chemical-looping process. Energy
Taylor MJ, Michopoulos AK, Zabaniotou AA, Skoulou V (2020) Probing synergies between lignin-rich and cellulose compounds for gasification. Energies 13(10):2590
Treedet W, Suntivarakorn R (2018) Design and operation of a low cost bio-oil fast pyrolysis from sugarcane bagasse on circulating fluidized bed reactor in a pilot plant. Fuel Process Technol 179:25
Usino D, Supriyanto Ylitervo P, Pettersson A, Richards T (2020) Influence of temperature and time on initial pyrolysis of cellulose and xylan. J Anal Appl Pyrol 147:104782
Van de Velden M, Baeyens J, Brems A (2010) Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction. Renew Energy 35:11
Wang C, Zhang H, Guo H, Shi S, Xiong L, Chen X (2016a) Part A: Recovery, Utilization,, & Effects, E. (2016a). Real-time monitoring of hemicellulose liquefaction in ethylene glycol. Energy Sources, Part A Recover Util Environ Effects 38(7):2517–2523
Wang S, Dai G, Ru B, Zhao Y, Wang X, Zhou J, Luo Z, Cen K (2016b). Effects of torrefaction on hemicellulose structural characteristics and pyrolysis behaviors. Bioresour Technol 218:1106–1114
Wang C, Yang S, Xu DJ, Li Y, Li J, Zhang Y (2020a). Hydrothermal liquefaction and gasification of biomass and model compounds: a review. Green Chem 22, 24, 22, 24.
Wang Q, Song H, Pan S, Dong N, Wang X, Sun S (2020b) Initial pyrolysis mechanism and product formation of cellulose: An Experimental and Density functional theory(DFT) study. Sci Rep 10:18
Wang S, Ru B, Lin H, Sun W (2015) Pyrolysis behaviors of four O-acetyl-preserved hemicelluloses isolated from hardwoods and softwoods. Fuel 150:9
Wang S, Guo X, Liang T, Zhou Y, Luo Z (2012) Mechanism research on cellulose pyrolysis by Py-GC/MS and subsequent density functional theory studies. Bioresour Technol 104(2012):722–728, 104, 7
Wang S, Zou C, Yang H, Lou C, Cheng S, Peng C, Wang C, Zou H (2021) Effects of cellulose, hemicellulose, and lignin on the combustion behaviours of biomass under various oxygen concentrations. Bioresour Technol 320:124375
Wei L, Xu S, Zhang L (2006) Characteristics of fast pyrolysis of biomass in a free fall reactor. Fuel Process Technol 9
Werner K, Pommer L, Broström M (2014) Thermal decomposition of hemicelluloses. J Anal Appl Pyrolysis 110:8
Wooten JB, Seeman JI, Hajaligol MR (2003) Observation and Characterization of cellulose pyrolysis intermediates by 13C CPMAS NMr. A new mechanistic model. Energy Fuels 2003(18):15
Xu Y-H, Li M-F (2021) Hydrothermal liquefaction of lignocellulose for value-added products: Mechanism, parameter and production application. Bioresour Technol 342:126035
Xu Z-X, Cheng J-H, He Z-X, Wang Q, Shao Y-W, Hu X (2019) Hydrothermal liquefaction of cellulose in ammonia/water. Boiresour Technol 278:311–317
Yang C, Wang S, Yang J, Xu D, Li Y, Li J, Zhang Y (2020a) Hydrothermal liquefaction and gasification of biomass and model compounds: a review. Green Chem 22:24
Yang X, Fu Z, Han D, Zhao Y, Li R, Wu Y (2020b) Unveiling the pyrolysis mechanisms of cellulose: Experimental and theoretical studies. Renew Energy 147:11
Yu C, Ren S, Wang G, Xu J, Teng H, Li T, Huang C, Wang C (2021) Kinetic analysis and modeling of maize straw hydrochar combustion using multi-Gaussian-distributed activation energy model. Int J Miner Metall Mater
Yu H, Wu Z, Chen G (2018) Catalytic gasification characteristics of cellulose, hemicellulose and lignin. Renew Energy 121:559–567
Zhang C, Li C, Zhang Z, Zhang L, Li Q, Fan H, Zhang S, Liu Q, Qiao Y, Tian Y, Wang Y, Hu X (2021) Pyrolysis of cellulose: Evolution of functionalities and structure of bio-char versus temperature. Renew Sustain Energy Rev 135(2021):110416, 135, 20
Zhang X, Yang W, Blasiak W (2012) Thermal decomposition mechanism of levoglucosan during cellulose pyrolysis. J Anal Appl Pyrolysis 96:10
Zhao L, Zhou H, Xie Z, Li J, Yin Y (2020) Effects of potassium on solid products of peanut shell torrefaction. Energy Sources Part A Recovery Util Environ Effects 42(10):1235–1246
Zheng A, Li L, Zhao Z, Tian Y, Li H (2021). Effect of torrefaction pretreatment on chemical structure and pyrolysis behaviors of Cellulose. Paper presented at the IOP conference series: earth and environmental science
Zhou H, Zhao L, Fu X, Zhu Y, Pan Y (2020) Effects of typical alkaline earth metal salts on cellulose torrefaction: solid products and thermal gravimetric analysis. Bioresources 15(1):1678–1691
Zhou X, Li W, Mabon R, Broadbelt LJ (2016) A critical review on hemicellulose pyrolysis. Energy Technol 4:29
Zhou X, Li W, Mabonb R, Broadbelt LJ (2018) A mechanistic model of fast pyrolysis of hemicellulose. Energy Environ. Sci 11:1240, 11, 21
Zhu C, Han C-G, Saito G, Akiyama T (2016) Facile synthesis of MnO/carbon composites by a single-step nitrate-cellulose combustion synthesis for Li ion battery anode. J Alloys Compd 689:931–937
Zimmermann A, Visscher C, Kaltschmitt M (2021) Plant-based fructans for increased animal welfare: provision processes and remaining challenges. Biomass Convers Biorefinery 1–19
Zou J, Oladipo J, Fu S, Al-Rahbi A, Yang H, Wu C, Cai N, Williams P, Chen H (2018). Hydrogen production from cellulose catalytic gasification on CeO2/Fe2O3 catalyst. Energy Convers Manag 171:241–248
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nguyen, A.Q., Trinh, L.T.P. (2022). Thermochemical Conversion of Cellulose and Hemicellulose. In: Nghiem, N.P., Kim, T.H., Yoo, C.G. (eds) Biomass Utilization: Conversion Strategies. Springer, Cham. https://doi.org/10.1007/978-3-031-05835-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-05835-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-05834-9
Online ISBN: 978-3-031-05835-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)