Abstract
Surface hydrophobic modification for oil–water separation based on membrane filtration is essential but still challenging in dealing with various industrial and environmental problems like sewage treatment and oil spill. Chemical graft technology can arm membranes with more efficient durability for applications due to the stronger chemical interaction between modified layers and surfaces compared with physical coating technology. As cellulose-based material, cotton filter fabric is a flexible and degradable material with abundant hydroxyl groups, providing great possibility for graft reactions. Stearyol chloride has both hydrophobic alkyl chain and active acyl group, making it easily to be grafted onto cotton filter fabrics. Therefore, we propose to use stearoyl chloride as hydrophobic layer and one-step modify cotton filter fabric after activation. The obtained hydrophobic modified cotton filter fabric exhibits high contact angles (CAmax = 147o, CAavg = 141.8o), which only decrease slightly after 5 h droplet holding time (CAmax = 141o, CAavg = 126.3o). Besides, experiments also confirm that this modified membrane displays excellent anti-fouling property against different drinks as well as anti-washing property for 10 cycles. Much more surprised is that the obtained membrane displays outstanding anti-abrasion performance that the CA values can remain above 131o after 390 cycles abrasion with hektogram counterweight. As for filtration application, the modified membrane also shows hydrophobic durability after three times gravity-driven oil–water separation (CAmax = 137o, CAavg = 132.6o). This hydrophobic modified cotton filter fabric may have potential application in future oilwater separation and the stearoyl chloride graft technology is an both effective and efficient way in surface hydrophobic modification.
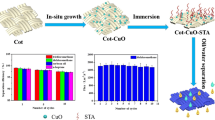
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oil–water separation is an inevitable process in many industries to protect environment and ecology. Sewage treatment, oil spill and other industrial fields need oil–water separation. Due to cost-efficiency and facile operation, membrane filtration method has caught wide attention of both academia and industry. One of the oil–water separation way is to fabricate hydrophobic membranes that only oil could permeate but water would be hold. There are various eco-friendly hydrophobic materials, such as natural waxes (Toruna et al. 2019; Guan et al. 2018), fatty acids (Heale et al. 2018; Dong et al. 2019), proteins (Liu et al. 2019; Shome et al. 2019), cellulose derives (Huang et al. 2018; Guo et al. 2016), biomass and agricultural waste (Anitha et al. 2018; Shishodia et al. 2019). Within these, cellulose based fabrics is a flexible, degradable and cost-less material suitable for further development into useful hydrophobic membranes.
As shown in Scheme 1, physical coating is one of the hydrophobic modification methods of cellulose-based materials. Nanoparticles with low surface energy such SiO2 (Wang et al. 2019), TiO2 (Ren et al. 2020) and ZnO (Thi et al. 2017) exhibit hydrophobic properties. For example, the hexadecyl polysiloxane modified SiO2 could act as a kind of superhydrophobic coating on glass slides, which exhibits a contact angle (CA) of 163.9o (Zhao et al. 2019). AlxNix(Bi2O3)z coating synthesized by electrophoretic deposition and perfluorodecyltriethoxysilane (FAS-17) modification showed high CA of 169o (Guo et al. 2017). Epoxy resins @ stearic acid-Mg(OH)2 coating (Si et al. 2016), benzoic acid incorporated Ag thin film coating (Sarkar et al. 2010) and other coatings (Biswas et al. 2021; Bayer 2020; Beshkar et al. 2020a, b; Joshi et al. 2020; Yu et al. 2019) also show excellent hydrophobicity.
However, physical bonding between coating and substrates is usually too weak to bear many injuries in applications. Thus, researchers put forward to replace this weak function by stronger chemical bonding. According to the difference in releasing groups, there are three major kinds of chemical grafted methods (Scheme 1). The first kind is non releasing group, mainly including radical addition and ring-opening reaction. For example, -SH can convert to -S· under UV radiation and then add with C = C group in organofunctional polysiloxanes, but in this situation, -OH on cellulose has to be changed into -SH in pretreatment process (Sun et al. 2016). Our group also used to modify cotton filter fabrics via activators regenerated by electron transfer atom transfer radical polymerization (ARGET-ATRP) (Li et al. 2019; Luo et al. 2020). Epoxy unit can directly reacts with the -OH on cellulose, but it will produce -OH after ring-opening reaction, which is not favor the hydrophobicity (Shang et al. 2010; Muresan et al. 2013; Ma et al. 2018). The second kind is H2O releasing. For example, carboxylic group can react with hydroxyl on cellulose fiber and release H2O (Huang et al. 2010; Bretel et al. 2018). The third kind is t-BuOH releasing. Using tert-butyl acetoacetate as co-reagent and releasing t-BuOH can make the low active acetoxyacetic group react with -OH. As the acetoxyacetic group is not a good hydrophobic layer, another compound such as octadecyl amine is needed to be further grafted onto cellulose via serial process (Rong et al. 2019). But it will create hydrophobic -NH group on the graft compound, which has passive impact on hydrophobicity. The forth kind, HCl releasing, is a novel kind we put forward in this work intrigued by the efficient reaction between -OH and -COCl (Wolfe 1997; Sano et al. 1999). High reactive efficiency can not only graft hydrophobic compounds onto cellulose surface easier and more uniform, but also reduce energy wastage.
Herein, we propose to use stearoyl chloride as hydrophobic layers via one-step graft on cotton filter fabrics after activation. Instead of multiple process or high reaction temperature, this hydrophobic modification can occur at room temperature, and the alkyl chains can be grafted onto cellulose via one activation process and one efficient reaction, which is both energy cost-less and time cost-less. Apart from fundamental properties such as anti-fouling, washing and abrasion performance characterization, we also evaluate the potential filtration application in oil–water separation of the obtained hydrophobic modified cotton filter fabric.
The mechanism scheme is shown as Fig. 1. In this experiment, N,N,N’,N’-tetramethyl ethylene diamine (TEMED) is acted as base to firstly react with stearoyl chloride and produce nitrenium ion precursor. Subsequently, hydroxyl on the glucose monomer reacts with nitrenium ion precursor via nucleophilic addition reaction. Finally, alkyl chains with 18 carbon atoms are grafted onto cotton filter fabrics. From the result, it is stearoyl chloride reacts with -OH and produces HCl, which will be neutralized by TEMED. After activation, the whole process occurs via only one step that put all reactants together and the reaction will finish at room temperature overnight. Details can be found in the supporting information. Of course, hydroxyl on the secondary carbon atom is more easier to react with stearoyl chloride than those on the third carbon atom due to less steric hindrance. But we calculate the stearoyl chloride dosage according to the ideal mole ratio in order to graft alkyl chain as more as possible. Table S1 shows different mole ratios and concentration of reactants and the resulted CA values are compared in Fig. S1. As can be seen, stearoyl chloride graft achieves nice hydrophobic modification compared with the blank cotton filter fabric because the blank sample shows hydrophilicity after few minutes droplet holding time but the modified samples can keep hydrophobicity for hours. Among the modified samples, it is found that too low concentration of stearoyl chloride and TEMED (sample 1) presents unexpected hydrophobic durability that the CA value drops to 120o after 3 h droplet holding time, although the initial CA can be larger than 140o. This suggests a low graft effect of stearoyl chloride on cotton filter fabric. On the other hand, too high concentration of hydrophobic layer and base (sample 3) is also not suitable for graft effect that the initial CA is only around 130o though it does not slide down too much after 5 h droplet holding time. When under a suitable concentration, the modified cotton filter fabric presents expected hydrophobic durability with high CA values before (CAmax = 147o, CAavg = 141.8o) and after 5 h droplet holding time (CAmax = 141o, CAavg = 126.3o). Besides, we tried different solvent at the optimal condition to confirm the adaptability of this graft method (Table S2). As can be seen from Fig. S2, the sample modified in DMF shows comparable hydrophobic durability (CAinitial = 139o, CA5h = 128o in average) with that in CH2Cl2. But the sample modified in THF seems much worse than in the above two solvent, showing unexpected hydrophobicity (CAinitial = 123o) and durability (CA3h = 102o). That means THF is not a suitable solvent for this system.
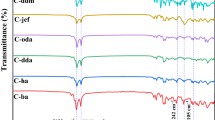
Figure 2a is the Fourier transform infrared spectroscopy (FTIR) of cotton filter fabrics before and after hydrophobic modification. There exist two characteristic peaks for alkyl chains after stearoyl chloride graft. Compared with the blank fabric, CH asymmetrical vibration at 2916 cm− 1 and CH symmetrical vibration at 2848 cm− 1 can be seen clearly from the grafted fabric, indicating a well grafted result. Thermal gravimetric analyzer (TG) under N2 was also involved to investigate the graft effect. There are two steps in the grafted fabric curve but only one in the blank fabric curve (Fig. 2b). The first step around 350 oC represents the decomposition of glucose, which points out the higher decomposition temperature of the cotton filter fabric after grafted, suggesting that alkyl graft can improve the thermal stability of fabrics. At around 380 oC, glucose is completely decomposed. The second step only existing in the grafted fabric curve at about 530 oC is the alkyl chain decomposed process, which is another evidence for the successful graft of alkyl chains. Apart from structure characterizations and thermal analyses, morphological measurements were also conducted to confirm the graft effect via this method. As can be seen from the scanning electronic microscopy (SEM) images (Fig. S3), the surface morphology of cotton filter fabrics in low magnification is similar before (Fig. S3a) and after (Fig. S3b) graft reaction. But in high magnification, the roughness of the grafted fiber surface (Fig. S3d) is obviously larger than that of the blank fiber (Fig. S3c). This also indicates the well graft effect via the stearoyl chloride graft technology, which is corresponding with the FTIR and TG results. Energy dispersive spectrometer (EDS) was performed to analyse the element distribution of the cotton filter fabrics. Fig. S4 displays the EDS spectra of the cotton filter fabrics before and after hydrophobic modification. After the modification, the carbon atom content was improved from 62.6 At% to 66.4 At%, suggesting the existence of alykl chains on the grafted fabric. Insets are the corresponding mapping, showing the uniform distribution of carbon and oxygen element.
To evaluate this stearoyl chloride graft technology more objectively, we compared the CA values both among our previous methods and the research of other groups. Figure 3a displays the hydrophobic durability among the three period studies of our group. The initial CA values can all be above 140o, but their CA values show more and more obvious distinguish as the droplet holding time increases. As for our first work, hydrophobic modification via ARGET-ATRP mechanism, the CA drops quickly below 120o within 1 h due to low alkyl chain density. In fact, the -OH on glucose monomer is active to be reacted after special treatment, but they are always tied with each other due to hydrogen bond interaction in macromolecule (Lee et al. 2020a, b). Relative researches has pointed out that CS2 can dissolve cellulose and make it decrystalization (Arce et al. 2020; Lee et al. 2020a, b), which may help to break up the hydrogen bond between hydroxyls on glucose monomers, making them exposed to reaction system and be more active to be reacted with hydrophobic layers. Therefore, in our second work, the hydrophobic durability was improved largely that the CA can remain above 120o after 2.5 h droplet holding time. However, it is hard to control active sites through radical polymerization, resulting in less uniform grafted alkyl chains. Besides, ARGET-ATRP method is too complex and time costly. To further improve hydrophobic durability and simplify the modification process, we utilize an efficient reaction between acyl chloride and hydroxyl. Unlike radical reaction, this reaction relies on the excessive TEMED and the activated -OH. Thus, the active sites distribution can be more uniform. As can be seen from Fig. 3a, the modified sample via RCOCl graft in this work exhibits the improved hydrophobic durability, whose CA can remain above 130o after 3 h and 120o after 5 h droplet holding time. The corresponding CA images are shown in Fig. S5. RCOCl is also comparable with other graft compounds. Many hydrophobic modified fabrics display high initial CA values above 140o, such as ODTES (Shang et al. 2010), GS (Muresan et al. 2013), CPDMS (Ma et al. 2018) and FAS (Yu et al. 2019) modified samples (Fig. 3b). As can be seen, all the materials shown in Fig. 3b including our sample display similar hydrophobicity. However, these reported materials have various unexpected aspects. Such as FAS, it is a fluorine-contained material, which is environmental unfriendly. Others like ODTES and GS, the graft compounds have to be synthesized firstly before graft reaction, which is not as convenient as the directed used RCOCl. Moreover, CPDMS and ODA methods involved high temperature reaction above 100oC, which is energy costly compared with RCOCl method in this work. Without the above mentioned drawbacks, the modified sample via RCOCl method can be facile synthesized and maintain comparable hydrophobic properties with other reported materials at the same time. Although it is not superior to other graft compounds, RCOCl can reach their levels. Besides, the cellulose-based substrate of the sample is both eco-friendly and costless.
In addition, relevant properties such as anti-fouling, washing and abrasion performance were also conducted in this work. As for anti-fouling property, we conducted three experiments. Fig. S6 compares the nano-TiO2 suspension droplets sliding down on the hydrophobic modified cotton filter fabric and the raw sample at a tilted angle of 20o. The raw sample absorbed the droplet as soon as it touched the surface. Instead, droplet slid down quickly on the modified sample surface and we have to slow down at × 0.2 speed, or we can not snapshot the sliding process pictures. Commercial aqueous drinks were also used to test the anti-fouling property of the modified sample. As shown in Fig. S7, tea, cola, vinegar and juice droplets can be held stably on the modified sample, while they are collapsed or adsorbed on the unmodified surface. These four drink droplets also slide down quick on the modified sample surface (Fig. S8). The above phenomena consistently confirm the good anti-fouling property of the hydrophobic modified cotton filter fabric. Washing and abrasion test were conducted to evaluated the mechanical durability of the hydrophobic modified cotton filter fabric. As can be seen from Fig. S9a, the CA values of the modified sample can remain at around 140o after washing for ten times, indicating a nice anti-washing performance. Fig. S9b displays the abrasion test for the modified sample. In the situation of 200 g counterweight put on the slide, the CA values can remain at around 140o after 180 cycles. When the counterweight was changed to 500 g, the CA values drops slightly after abrasion and finally maintains at around 132o after 210 cycles. In another word, the modified sample still exhibits well hydrophobicity after 390 cycles abrasion, displaying an outstanding anti-abrasion performance. These two performances both confirm a well mechanical property and durability of the modified sample, which is suitable for practical applications.
a Contact angles of the RCOCl-grafted cotton filter fabric after each oil-water separation. b Separation efficiency and oil flux of the RCOCl-grafted cotton filter fabric of each cycle. Insets are optical photos of the liquid before and after filtration. The Blue phase is CuSO4-dyed aqueous phase and the transparent phase is CHCl3 oil phase. c The first filtration process of oil–water separation
After fundamental characterizations, we performed a membrane filtration application research on the hydrophobic modified cotton filter fabric. Oil–water separation was conducted on an extraction filter with effective area of 3.14 cm2. For each turn, oil phase was 5 mL CHCl3 and aqueous phase was 5 mL CuSO4-dyed water. The above mixture was poured into the as-mentioned extraction filter and filtered drop by drop by gravity. The gravity-driven oil flux can be calculated by the formula shown in the supporting information. Figure 4a is the average CA values after each filtration, from which can be seen that there is only few angles declined compared with the initial one. After three times filtration, the average CA value can still be above 130o, suggesting a fine recyclability of the RCOCl-grafted membrane. Figure 4b displays the separation efficiency and oil flux of each cycle. Separation efficiency can remain at 99 % after each cycle. However, the oil flux decreases after every cycle from the beginning of 522. 08 L m− 2 h− 1 to 282.33 L m− 2 h− 1 then further decreases to 117.69 L m− 2 h− 1. This can be explained by the hydrophobic effect that the oil droplets will adhere during each separation process, making it fewer and fewer holes for oil pass through. Insets are the optical photo of the liquid before and after filtration, showing nearly 5 mL aqueous phase recovery. Figure 4c is the screenshots of the first oil–water separation video recording. We started to record as we poured the mixture into the extraction filter. The first oil drop filtered was observed at 13 s and the oil phase finished filtration at 2 min 03 s. And the aqueous phase could be hold for at least one and a half minutes by the obtained hydrophobic modified cotton filter fabric. The second and third filtration processes are also provided in Fig. S10 and S11. Similar with the first process, the aqueous phase holding times are also about one and a half minutes, indicating a maintaining aqueous phase holding property after three times separation.
In conclusion, we reported a facile hydrophobic modification method of CS2 activation and RCOCl graft on cotton filter fabric. Through a serial optimizing experiments, alkyl chains were successfully grafted on the glucose monomers, which is confirmed by FTIR and TG. The obtained fabric exhibits both high hydrophobicity (CA = 141.8o) and hydrophobic durability (CA5h = 126.3o), which is comparable with those of other reported grafted fabrics. Besides, the modified sample also exhibits excellent anti-fouling property against various aqueous drinks as well as anti-washing property with almost no CA decrease after 10 cycles washing. Much more surprised is that this RCOCl-grafted fabric displays outstanding anti-abrasion performance that it can bear 180 cycles abrasion with 200 g counterweight and 210 cycles under 500 g counterweight totally. The average CA value can remain above 131o finally. For membrane filtration application, the RCOCl-grafted fabric shows expective oil–water separation with 99 % separation efficiency and 522. 08 L m− 2 h− 1 gravity-driven oil flux. This cost-effective hydrophobic modified cotton filter fabric has potential application in future oil–water separation. And this time cost-less and energy cost-less chemical graft technology may rich the surface hydrophobic modification routes of cellulose-based substrates.
References
Anitha C, Mayavan S (2018) Salvinia inspired fluroine free superhydrophobic coatings. Appl Surf Sci 449:250–260
Arce C, Llano T, Garcı´a P, Coz A (2020) Technical and environmental improvement of the bleaching sequence of dissolving pulp for fibre production. Cellulose 27(7):4079–4090
Bayer IS (2020) Superhydrophobic Coatings from Ecofriendly Materials and Processes. Adv Mater Interfaces 7(13):2000095
Beshkar F, Salavati-Niasari M, Amiri O (2020) Superhydrophobic-superoleophilic copper-graphite/styrene-butadiene-styrene based cotton filter for efficient separation of oil derivatives from aqueous mixtures. Cellulose 27:4691–4705
Beshkar F, Salavati-Niasari M, Amiri O (2020) A reliable hydrophobic/superoleophilic fabric filter for oil-water separation. Cellulose 27:9559–9575
Biswas A, Jana NR (2021) Cotton Modified with Silica Nanoparticles. ACS Appl Nano Mater 4(1):877–885
Bretel G, Rull-Barrull J, Nongbe MC, Terrier J-P, Grognec EL, Felpin F-X (2018) Hydrophobic covalent patterns on cellulose paper through PhotothiolX ligations. ACS Omega 3(8):9155–9159
Dong X, Gao S, Huang J, Li S, Zhu T, Cheng Y, Lai Y (2019) A self-roughened and biodegradable superhydrophobic coating with UV shielding. J Mater Chem A 7(5):2122–2128
Guan Y, Yu C, Zhu J, Yang R, Li X, Wei D, Xu X (2018) Design and fabrication of vapor-induced superhydrophobic surfaces obtained from polyethylene wax and silica nanoparticles in hierarchical structures. RSC Adv 8(44):25150–25158
Guo J, Fang W, Welle A, Feng W, Filpponen I, Rojas OJ, Levkin PA (2016) Superhydrophobic and Slippery Lubricant-Infused Flexible Transparent Nanocellulose Films by Photoinduced Thiolene Functionalization. ACS Appl Mater Interfaces 8(49):34115–34122
Guo X, Li X, Lai C, Jiang X, Li X, Shu Y (2017) Facile approach to the green synthesis of novel ternary composites with excellent superhydrophobic and thermal stability property. Chem Eng J 309:240–248
Heale FL, Page K, Wixey JS, Taylor P, Parkin IP, Carmalt CJ (2018) Inexpensive and non-toxic water repellent coatings comprising SiO2 nanoparticles and long chain fatty acids. RSC Adv 8(48):27064–27072
Huang J, Wang S, Lyu S, Fu F (2018) Preparation of a robust cellulose nanocrystal superhydrophobic coating for self-cleaning and oil-water separation only by spraying. Ind Crops Prod 122:438–447
Huang W, Song Y, Xing Y, Dai J (2010) Durable hydrophobic cellulose fabric prepared with Polycarboxylic acid catalyzed Silica Sol. Ind Eng Chem Res 49(19):9135–9142
Joshi S, Kathuria H, Verma S, Valiyaveettil S (2020) Functional catechol–metal polymers via interfacial polymerization for applications in water purification. ACS Appl Mater Interfaces 12(16):19044–19053
Lee JH, Park SH, Kim SH (2020) Surface alkylation of cellulose nanocrystals to enhance their compatibility with polylactide. Polymers 12(1):178
Lee J, Jeon H, Park H, Oh S, Chung I (2020) Studies on the melt viscosity and physico-chemical properties of cellulose acetate propionate composites with lactic acid blends. Mol Cryst Liq Cryst 707(1):8–20
Li Z, He Z, Chen X, Tang Y, You S, Chen Y, Jin T (2019) Preparation of hydrophobically modified cotton filter fabric with high hydrophobic stability using ARGET-ATRP mechanism. RSC Adv 9(43):24659–24669
Liu H, Xie WY, Song F, Wang XL, Wang YZ (2019) Constructing hierarchically hydrophilic/superhydrophobic ZIF-8 pattern on soy protein towards a biomimetic efficient water harvesting material. Chem Eng J 369:1040–1048
Luo Y, Deng S, Li Z, Cao L, He Y, Chen Y, Jin T (2020) Effect of CS 2 /NaOH activation on the hydrophobic durability of cotton filter fabric modified via ARGET-ATRP. Eur Polym J 141:110087
Ma Y, Zhu D, Si Y, Sun G (2018) Fabricating durable. J Appl Polym Sci 135(25):46396
Muresan EI, Balan G, Popescu V (2013) Durable hydrophobic treatment of cotton fabrics with glycidyl stearate. Ind Eng Chem Res 52(18):6270–6276
Ren J, Tao F, Liu L, Wang X, Cui Y (2020) A novel TiO 2 @stearic acid/chitosan coating with reversible wettability for controllable oil/water and emulsions separation. Carbohydr Polym 232:115807
Rong L, Liu H, Wang B, Mao Z, Xu H, Zhang L, Zhong Y, Feng X, Sui X (2019) Durable antibacterial and hydrophobic cotton fabrics utilizing enamine bonds. Carbohyd Polym 211:173–180
Sano T, Ohashi K, Oriyama T (1999) Remarkably fast Acylation of Alcohols with Benzoyl Chloride promoted by TMEDA. Synthesis 7:1141–1144
Sarkar DK, Saleema N (2010) One-step fabrication process of superhydrophobic green coatings. Surf Coat Technol 204(15):2483–2486
Shang S, Li Z, Xing Y, Xin JH, Tao X (2010) Preparation of durable hydrophobic cellulose fabric from water glass and mixed organosilanes. Appl Surf Sci 257(5):1495–1499
Shishodia A, Arora HS, Babu A, Mandal P, Grewal HS (2019) Multidimensional durability of superhydrophobic self-cleaning surface derived from rice-husk ash. Prog Org Coat 136:105221
Shome A, Rather AM, Manna U (2019) Chemically reactive protein nanoparticles for synthesis of a durable and deformable superhydrophobic material. Nanoscale Adv 1(5):1746–1753
Si Y, Guo Z, Liu W (2016) A Robust Epoxy Resins @ Stearic Acid-Mg(OH)2 micronanosheet superhydrophobic omnipotent protective coating for real-life applications. ACS Appl Mater Interfaces 8(25):16511–16520
Sun D, Wang W, Yu D (2016) Preparation of fluorine-free water repellent finishing via thiol-ene click reaction on cotton fabrics. Mater Lett 185:514–518
Thi VHT, Lee B-K, Ngo C-V (2017) Durable superhydrophobic cotton filter prepared at low temperature for highly efficient hexane and water separation. J Taiwan Inst Chem E 71:527–536
Toruna I, Ruzi M, Er F, Onses MS (2019) Superhydrophobic coatings made from biocompatible polydimethylsiloxane and natural wax. Progress Organ Coat 136:105279
Wang F, Pi J, Li J-Y, Song F, Feng R, Wang X-L, Wang Y-Z (2019) Highly-efficient separation of oil and water enabled by a silica nanoparticle coating with pH-triggered tunable surface wettability. J Colloid Interface Sci 557:65–75
Wolfe MS (1997) N-Benzoyl-4-(dimethylamino)pyridinium Chloride. Synthetic Commun 27(17):2975–2984
Yu M, Wang Q, Yang W, Xu Y, Zhang M, Deng Q, Liu G (2019) Facile fabrication of magnetic. Polymers 11(3):442
Zhao X, Li Y, Li B, Hu T, Yang Y, Li L, Zhang J (2019) Environmentally benign and durable superhydrophobic coatings based on SiO2 nanoparticles and silanes. J Colloid Inter Sci 542:8–14
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human or animal rights
The work described in this article did not involve human participants and or animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, Y., Luo, Y., Li, Z. et al. Facile stearoyl chloride grafted cotton filter fabric and its application in oil-water separation. Cellulose 28, 8221–8228 (2021). https://doi.org/10.1007/s10570-021-04046-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-04046-8