Abstract
In this study, the biodegradation of wood and chemical changes caused by the Hylotrupes bajulus beetle were revealed for the first time using FTIR spectroscopy. In the study, Scots pine (Pinus sylvestris), fir (Abies nordmanniana), and spruce (Picea orientalis) wood species were exposed to damage by H. bajulus larvae for four months. Morphological measurements and chemical analysis were carried out for the fine powdery dust (FPD) and frass (FR) produced by H. bajulus and compared to control samples. In addition, the FPD and FR of the larvae were examined by FTIR analysis and changes in the wood structure were determined. According to the morphological measurement data obtained from the study, the dimensions of the larval FR were 1 × 0.55 mm screened at 20-mesh, and 0.37 × 0.24 mm at 60-mesh. Holocellulose analysis showed that less holocellulose was detected in the FR and FPD than in the control wood. In the sugar analysis performed via HPLC, the glucose, xylose, galactose, mannose, and arabinose components decreased compared to the control wood, whereas the content of acid-insoluble lignin increased. The FTIR spectra of the wood species (especially in Scots pine) showed that the carbohydrate band intensity at 1735, 1370, 1321, and 897 cm−1 decreased and the carbohydrate bands at 1735, 1321, 1369, and 897 cm−1 were nearly absent in the FPD and FR after H. bajulus larva degradation. However, absorption band intensity at 1508, 1458, 1268, and 1031 cm−1 related to the lignin bands increased significantly. According to these results, H. bajulus larvae degraded cellulose and hemicellulose from the wood components, but they did not degrade lignin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hylotrupes bajulus (old house borer) is among the chief wood borers that damage wood. It generally feeds on softwoods including the sapwood of pine, fir, and spruce (Robinson and Cannon 1979; Akbulut et al. 2008). However, in some cases, H. bajulus larvae have even been reported to feed on heartwood when they reach the mature stage (Eaton and Hale 1993). The adults are 20–30 mm in length when fully grown and the larvae are 22–25 mm. The adults do not feed on wood. The main damage to wood is done by the larvae. They reduce the economic value of wood. The cream-colored larvae are similar to typical Cerambycidae larvae. It has been reported that their development can continue for 1–6 years and sometime up to 10 years (Grosser 1985). Females generally lay eggs in the cracks of coniferous trees because they are attracted by the alpha-pinene and beta-pinene in coniferous species. The reason they do not attack hardwoods is that toxic substances in these tree species prevent the development of the larvae (Cymorek 1981; Fettköther et al. 2000).
The structure of wood is composed of various amounts of hemicellulose, cellulose, and lignin and is formed by the arrangment of the cell walls and cells in a certain order. Some insect species living in wood can digest the hemicellulose, cellulose, and lignin that comprise the main components of wood. Different mechanisms have been proposed to explain the digestion of cellulose in insects. Some insect species have a symbiotic relationship with bacterial species in their hindguts enabling them to digest wood components, and other insect species can produce their own enzymes to digest wood (Chiappini et al. 2010). The digestion of cellulose in insects is based on the principle that it is accomplished by cellulase enzymes, which are also responsible for the digestion of cellulose in fungi (Martin et al. 1991).
Cellulose is composed of glucose sub-units that can be hydrolyzed via the effect of acids and enzymes and bonded via β bonds. Biological hydrolysis of cellulose is accompanied by the activity of cellulases, including endoglucanases and cellobiohydrolases or exocellulases and beta glucosidases. The hydrolysis of cellulose to glucose occurs as a result of hydrolysis of the cellulose β-1,4 bonds by cellulase enzymes. The complete hydrolysis of cellulose can have a triple synergistic effect, with glucose as the final product (Wilson and Irwin 1999; Laurent et al. 2000).
The source and degradation of enzymes in the intestines of wood-destroying insects is still controversial (Zverlov et al. 2003). According to Martin et al. (1991), the digestion of cellulose has been shown in 8 orders and 78 species belonging to 20 families, including Anobiidae (furniture beetle and death watch beetle), Cerambycidae (long-horned beetles), Buprestidae (metallic wood borers), Blattodea (termites), Siricidae (wood wasps), and Scarabaeidae (scarab beetles). The insects most effective at digesting cellulose are termites, at a 99% degradation rate. It was reported that xylophagus larvae such as Anobidae, Siricidae, and Cerambycidae have the somewhat lower digestion rate for cellulose of 12–68%.
Previous studies have proven that no other microorganisms are involved in the digestion of cellulose and hemicellulose for the H. bajulus beetle. Therefore, the cellulase enzyme in this insect is endogenous. Although 20% of cellulose and hemicellulose can be digested by H. bajulus cellulase enzyme, the other 80% is formed into pellets with feces (frass). The morphological properties of the frass play a determining role in the identification of these insects. Like other wood borers, the long life-cycle confirms their necessity to obtain energy from the cellulose and protein of the wood components in which they live (Chiappini et al. 2010). Studies in the literature have proven that protein additives to the H. bajulus diet accelerate the growth of their larvae (Schuch 1937). On the other hand, Höll et al. (2002) reported that in diet studies, starch had no effect on the development of H. bajulus.
Many studies have been conducted to explain the chemical changes and characterization of degradation in wood (Green and Highley 1997; Howell et al. 2009; Tomak et al. 2013). Since infrared spectroscopy is a rapid and non-destructive technique (Pandey 1999), it is often used in the determination of cellulose, hemicellulose, and lignin content as well as other substrates in wood and to detect changes in wood structure caused by fungi and bacteria (Pandey and Pitman 2003; Pandey and Nagveni 2007; Popescu et al. 2007). To date, although the changes in wood structure due to fungi have been investigated many times, unfortunately, the chemical characterization of the changescaused by major wood borers have not been revealed.
This study investigated the biodegradation and chemical changes occurring in wood caused by the major wood borer, H. bajulus. The frass (FR) and fine powdery dust (FPD) produced by the larvae from the digestion of Scots pine, fir, and spruce wood specieswere analyzed and their chemical characterization was carried out for the first time using Fourier transform infrared (FTIR) spectroscopy. Morphological examination and additional analyses were conducted for holocellulose, alpha-cellulose, and extractive substances in addition to consumption rate and HPLC analyses in order to determine the feeding properties of H. bajulus.
Materials and methods
Preparation of wood samples and exposure to larval destruction
The larvae cultures were obtained from the Division of Biodeterioration and Reference Organisms at the Federal Institute for Materials Research and Testing (BAM), Berlin, Germany. Scots pine (Pinus sylvestris) sapwood samples (obtained from Alaplı, Zonguldak Province, Turkey) were prepared in dimensions of 50 × 25 × 15 mm as specified in EN 46 (2016). A total of 75 prepared Scots pine samples were impregnated with peptone and yeast and newly hatched H. bajulus larvae were inserted into these wood samples. The samples were then kept at 26 ± 2 °C and 75 ± 5% relative humidity. The larvae thus grew more rapidly than they would in their natural life cycle. The larvae in this group were classified as the smaller larvae (< 0.05 g) and their frass was used only in the HPLC and FTIR analyses for Scots pine samples. The larvae reaching the specified size were transferred to the Scots pine (Pinus sylvestris) (SPW), fir (Abies nordmanniana) (FW), and spruce (Picea orientalis) (SW) wood species samples prepared in dimensions of 60 × 55 × 55 mm. However, the wood samples in this group were natural wood, i.e., not impregnated with peptone and yeast extract. The larvae in these samples continued to feed at 26 °C and 75% relative humidity. The larvae in this group were classified as the larger (> 0.05 g) larvae and their frass (FR) and fine powdery dust (FPD) were used for the analyses. The experimental design of the study and the analyses carried out are given in the Table 1.
Determination of consumption rate
Consumption rate tests were carried out using the larger larvae (> 0.05 g). The weights of the SPW, FW, and SW samples were measured on an assay balance. Three replicates were used for each wood species. One larva was placed in each wood sample and the wood blocks were kept at 75% relative humidity and 24 °C. At the end of 120 days, the larvae were removed from the wood blocks and the feces and dust were cleaned away. The wood blocks were weighed again on an assay balance and the daily consumption rates of the larvae were calculated according to Eq. (1).
where CR is the consumption rate, M1 is the pre test weight of the wood blocks, and M2 is the post test weight of the wood blocks.
Morphological properties
Samples of FR and FPD were collected from the larger (> 0.05 g) larvae fed only on SPW for four months (Fig. 1). After passing through 20-, 40-, and 60-mesh sieves, the dimensional classification of the larval FR was carried out. Fourteen frass pellets (FR) were selected randomly from each mesh type for the measurements. The FR dimensions (length and width) were measured under a stereo microscope.
Chemical analysis
Determination of holocellulose content
Determination of holocellulose was carried out according to the chlorite method of Wise and John (1952). The method was applied to the SPW flour (control samples) and the FR, and FPD produced by the larger (> 0.05 g) larvae. Samples (5 g) of oven-dried 40-mesh control flour, FR, and FPD were placed in a 250-mL flask with 160 mL of water, 1.5 g of NaClO2, and 0.5 mL of glacial acetic acid and kept at 78 °C for 1 h. The flask was shaken and stirred during the reaction. After one h, 1.5 g of NaClO2 and 0.5 mL of glacial acetic acid were added to the mixture and heating was continued for 1 h. This process was repeated four times. When chlorination was completed, the mixture was filtered through a glass crucible (por 2). The residue was washed repeatedly with acetone and then with cold distilled water and dried at 105 ± 3 °C. The amount of holocellulose was then determined.
Determination of alpha-cellulose content
The alpha-cellulose ratio was determined according to TAPPI T 203cm-09 (2009) standard using 17.5% NaOH on the holocellulose samples. About 2 g of oven-dried holocellulose was placed in a beaker and 10 mL of 17.5% NaOH solution was added. This was mixed with 5 mL of 17.5% NaOH solution twice at 5-min intervals and then kept in a water bath at 20 °C for 30 min. Afterwards, 33 mL of distilled water was added to the mixture and kept at 20 °C for 60 min and then filtered through a por 2 crucible. The residue in the crucible was washed, first with 100 mL of 8.3% NaOH solution, followed by 15 mL of 10% acetic acid and 250 mL of distilled water and then weighed after drying at 105 ± 3 °C. Finally, the % α-cellulose content was determined relative to the oven- dry holocellulose.
Determination of extractives
The SPW was ground using a hammer mill and sieved to 40-mesh size for the control. Samples of about 5 g each of SPW flour (control), FPD, and FR were subjected to extraction with a toluene: acetone: ethanol mixture [4/1/1, (v/v)] for 6 h using a soxhlet extractor. Three replicates were used for each substrate type. The extracted samples were filtered through a glass crucible (por 2) using a vacuum, and then oven-dried at 103 ± 2 °C for 12 h. They were then transferred to a desiccator containing phosphorus pentoxide and kept for for 15 min, after which the extractive amount was calculated.
HPLC analysis
Within the scope of the study, 0.3 g control samples (moisture determined) of SPW, FW, SW, FPD, and FR were weighed. Three replicates were used for each wood species control, FR, and FPD sample in the study (a total of 36 replicates). Samples were subjected to hydrolysis according to the method determined by NREL Laboratory Analytical Procedures (LAP) of the National Renewable Energy Laboratory (Sluiter et al. 2004). Accordingly, 0.3 g-samples of wood flour (control), FPD, and FR and 3 mL of 72% H2SO4 were kept in a hot water bath at 30 °C for 1 h and mixed with a glass stirring rod every 10 min. The mixture was then placed in a flask and 84 mL of distilled water was added. It was immediately sterilized by autoclaving for 60 min. For neutralization, 20 mL of sterilized sample and 1 g of CaCO3 were mixed and centrifuged. The mixture was then transferred in a vial to the HPLC device. The HPLC analyses were performed using the Agilent 1200 refractive index detector (RID) at 20 µL injection volume with an ultra-pure water mobile phase and the SHODEX SP 0810 column at a flow rate of 0.6 mL/min at 80 °C. The sugar proportions in the samples were calculated in micro-liters as a percentage of the original sample.
FTIR analysis
The samples (control, FPD, and FR for SPW, FW and SW) were dried in an oven at 60 °C for 48 h and used for the FTIR analyses. The analyses were performed at the Duzce University Scientific and Technological Research Application and Research Center (Duzce, Turkey) using an IR Prestige-21 instrument (Shimadzu Benelux B.V., Hertogenbosch, Netherlands) fitted with an ATR attachment. Analyses were carried out at 4 cm−1 for 32 scans and the spectra were recorded in the wavenumber range of 600–1800 cm−1 (fingerprint region).
Statistical analyses
Statistical analyses of the data were performed using SPSS 19 software (IBM Corp., Armonk, NY, USA). The averages were evaluated using a one-way analysis of variance (ANOVA). A mean separation test (i.e., Duncan’s multiple range test) was applied at the α = 0.05 level to determine the significant differences among the paired comparisons.
Results
Morphological properties
Evaluation of the dimensional measurements of the FR produced by H. bajulus larvae revealed that the FR size decreased from the 20-mesh to the 80-mesh (Fig. 2). According to the FR measurements, the length and width of the FR in the 20-mesh were on average 1 mm and 0.55 mm, whereas the 40 mesh were 0.61 mm and 0.30 mm. When all dimensional measurements were considered, the FR length varied between 1 mm and 0.36 mm and the width between 0.55 mm and 0.24 mm. When the color properties were examined, a light color was observed on the FPD and a darker color on the FR pellets. The larvae pellets (FR) were clearly cylindrical or sausage-like in shape (Fig. 1).
Chemical analyses
The extractive substance analysis of the SPW flour (control), FPD, and FR, showed a higher proportionate amount of extractive substance in the FR and FPD than in the control. An average of 1.29% extractive material was determined in the control and 2.64% and 3.38% in the FPD and FR, respectively. No statistically significant difference was found between the amount of extractive substance in the FPD and FR (Table 2).
Among the wood species, the highest daily consumption rate by the H. bajulus (average 0.05 g) larvae was determined in the SPW as an average of 43.6 mg/day, whereas the lowest was found in the FW, with an average of 11.8 mg/day. Daily consumption amount differences among the wood species were found to be statistically significant (p ≤ 0.05) (Table 2).
The findings of the SPW control, FPD, and FR samples were examined for holocellulose and it was found to be higher in the control wood compared to the FPD and FR (Fig. 3). As a result, 76.8% holocellulose was detected in the control, and 67.2% and 68.4% in the FPD and FR, respectively. Differences between the control and FR were also statistically significant. The alpha-cellulose ratios are shown in Fig. 3. According to the data, no difference was found between the control and FR samples in terms of average alpha-cellulose ratios. When the residual acid-insoluble lignin ratios were examined (Table 2), differences were found between the control samples and the FPD and FR, with a higher rate of lignin in the FR compared to the control.
The HPLC analysis findings for the FPD, FR, and control wood flour were revealed after degradation in different woods by H. bajulus (Table 3) and the HPLC chromatograms were then generated. Chromatograms are given for the FW control sample, FPD, and FR in Figs. 4, 5, and 6. The findings in Table 3 showed that H. bajulus larvae had consumed glucose, xylose, galactose, mannose, and arabinose sugar components, which are the basic units of cellulose and hemicellulose. In general, there were statistically significant differences in the wood species between the glucose sugar levels of the control, FR, and FPD. For example, a glucose level of 21.3% was detected in the SPW control samples, but this ratio decreased to 18.9% and 17.6% in the FPD and FR samples, respectively (larvae weight < 0.05 g). A similar situation was also observed in the FW and SW samples.
FTIR analyses
The evaluation of the peaks in the FTIR spectra is given in the Table 4. This evaluation was based on the data obtained from the literature by examining studies on cellulose, hemicellulose, and lignin degradation in wood (Pandey and Pitman 2003; Oevering et al. 2003; Mohebby 2005; Popescu et al. 2007; Mico et al. 2011; Elmas and Yilgor 2020).
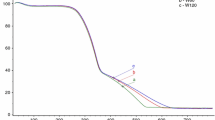
The FTIR spectra of the control wood samples are given in Fig. 7, which shows important peaks occurring at 897, 1031, 1055, 1268, 1365, 1423, 1467, 1508, and 1735 cm−1 wavelengths in the region defined as the “fingerprint region” (1800–600 cm−1). The peaks that occurred in this region were attributed to the lignin and polysaccharides in the wood.
Different lignin peaks are shown at 1596 and 1508 cm−1 wavelengths, whereas peaks at 1467, 1320, 1268, and 1120 cm−1 indicate lignin and polysaccharides and the 1735, 1365, and 897 cm−1 wavelengths belong only to polysaccharides. The polysaccharide peak at the wavelength of 1735 cm−1 belongs to hemicellulose, and the peak at the 897 cm−1 wavelength belongs to cellulose (see also Table 4). Unconjugated C = O stretching was represented in the xylan at a wavelength of 1735 cm−1 and acetyl and carboxyl vibrations in the xylan were represented at the 1735 and 1235 wavelengths in the hemicellulose. The peaks in the absorption band at 1375–1365 cm−1 were assigned to the C–H deformation in the cellulose and hemicellulose, whereas 897 cm−1 was only assigned to the C–H deformation in cellulose. Aromatic skeletal vibrations and C = O stretching in the lignin were found at 1596 and 1508 cm−1 wavelengths. The peak at 1268 cm−1 can be attributed to guaiacyl ring breathing, the C–O stretching in lignin, and the C–O linkage in the guaiacyl aromatic methoxyl groups. Although no major differences were observed among the FTIR spectra of the control woods, the main difference occurred as the higher peak intensity in cellulose and hemicellulose in the SPW sample at 897 and 1735 cm−1 wavelengths.
Examination of the SPW FTIR spectra (Figs. 8 and 9) showed that the intensities of the carbohydrate bands at 1735, 1370, 1321, and 897 cm−1 decreased. The carbohydrate bands at 1735, 1321, 1369, and 897 cm−1 (Fig. 9) were nearly absent in the FPD and FR after H. bajulus larva degradation. However, intensity of the absorption bands at 1508, 1458, 1268, and 1031 cm−1 related with the lignin bands increased significantly and a new peak appeared in the FPD and FR at the 1421 cm−1 wavenumber (Fig. 9).
In terms of the wood species, the highest peak change occurred in the SPW impregnated with peptone and yeast. Yeast and peptone impregnation is a feeding method specified in EN 46-1 (2016) to accelerate the development of H. bajulus larvae. The SPW impregnated with nutrients appeared to be more digested and structurally changed by the larvae. Examination of the FTIR spectra for the larger larvae (> 0.05 g) (Fig. 8) showed that they had caused minimal changes in the peaks belonging to cellulose and hemicellulose (1735, 897 cm−1) compared to the smaller larvae (< 0.05 g). However, they caused increases in the heights of the lignin peaks (1508, 1268, and 1263 cm−1). As shown by the FTIR spectra, H. bajulus larvae were not able to digest the lignin component, although they digested the cellulose and hemicellulose from the polysaccharides. For this reason, the findings obtained from the determination carried out of the holocellulose in SPW (Fig. 3) and the findings obtained from the FTIR spectra confirmed each other.
In the case of the FW and SW (Figs. 10 and 11), the FR and FPD peaks were similar to each other, unlike with the control wood. When the peaks of the control wood, FPD, and FR were examined, increases were observed in the peaks of lignin in the absorption bands at 1508, 1268, 1220 cm−1.
When the spruce wood was examined, a significant increase was observed in the height of the bands of the lignin component at 1596, 1508, 1423, 1268, and 1220 cm−1 wavelengths for the FR and FPD compared to the control woods. Minimal changes were detected in the wavelengths of the peaks of cellulose and hemicellulose.
Discussion
Regarding the morphological properties of the H. bajulus FR, Bobadilla et al. (2015) found similar findings in their study. They reported that H. bajulus FR was homogeneous and clearly cylindrical with flattened or very slightly rounded ends and ranging from light to darker in color. H. bajulus digest cellulose and hemicellulose while leaving the lignin. Thus, darker colors may be expected as they occur in wood decayed by brown rot fungi.
The main reason for the proportionately higher amount of extractive substance in the FR was the consumption of cellulose and hemicelluloses by the H. bajulus larvae. Similar results were also found in the study by Sen et al. (2017), in which the extractive substance ratio was found to be 2.95% in the SPW control samples, whereas this rate increased to 4.86% in the FR of Spondylis buprestoides (Coleoptera: Cerambycidae) larvae.
The resistance of wood against insects depends on the species. Previous studies have found the SPW species to be more susceptible to H. bajulus than SW and FW (Graf et al. 1989). One study reported the H. bajulus larvae daily consumption amount of SPW to be 11.2 mg/day during a 124-day feeding period (Nerg et al. 2004). Compared to Nerg et al. (2004), the daily consumption amount of SPW by H. bajulus was found to be higher in the present study. This is probably due to different larvae dimensions/weights used in the studies.
When the lignin present in the wood structure is removed, the remaining cellulose and hemicellulose components are called holocellulose (Rabemanolontsoa and Saka 2012). In the literature, similar results have been found for S. buprestoides (Coleoptera: Cerambycidae) larvae, showing more lignin detected in frass than in control wood. Sen et al. (2017) revealed that S. buprestoides larvae degraded holocelluloses rather than lignin and extractives. Because the H. bajulus beetle is a cellulose- and hemicellulose-degrading beetle, the lignin content was found to be higher in the frass.
The digestion of the cellulose and hemicellulose components by coleopteran beetles can be accomplished by using three different types of enzymes that are also found in fungi. The insects synthesize these enzymes in their guts. The cellulase enzymes produced by the insects include three major classes of hydrolytic enzymes: endoglucanases (Cx-cellulases), cellobiohydrolases (C1-cellulases), and β-glucosidases (Chararas and Chipoulet 1983). It is believed that digestion of natural cellulose is initiated by the endoglucanases enzymes attacking the isolated amorphous regions of the cellulose matrix. Endoglucanases enzymes create nicks in the linear cellulose chains. The cellobiohydrolases attack these nick sites on cellulose chains caused by the endoglucanase and liberate the cellobiose. The endoglucanases and cellobiohydrolases then convert the original cellulose to cellobiose units and a mixture of soluble linear oligosaccharides. Finally, the cellobiose is hydrolyzed to glucose and other oligosaccharides by the cellobiase. Digestion of cellulose does not occur if one of these three enzymes is missing (Martin 1983).
The reduction in sugar content in the FPD indicated that the digestion of cellulose had occurred in the foregut of the H. bajulus larvae and that the enzymes were active in this section. Cazemier et al. (1997) demonstrated beta -glucosidase and carboxy methylcellulasis activity in the H. bajulus gut, with very high enzyme activity observed in the foregut.
The decrease or disappearance of the peaks at 1735 cm−1 for the FR and FPD was due to the decrease in the carbonyl (unconjugated C = O) absorption band intensity in the hemicellulose xylan structure after larval attack. The deformation of the C–H groups in the cellulose structure at the 897 cm−1 wavelength from the effect of the cellulase enzyme secreted by the larvae caused the peak height in this band to decrease or disappear for the FR and FPD compared to the control. However, the height of the C–O stretch peak in the lignin guaiacyl ring at 1268 cm−1 increased due to the xylan C–O stretching vibration in the frass. Because of the C–H deformation in the carbohydrates at 1421 and 1458 cm−1, the lignin was not deformed in the larval FR; hence, a new peak was formed at 1421 cm−1 and an increase was observed in the lignin peak height at 1458 cm−1. Our results are in agreement with Mico et al. (2011), who demonstrated the existence of cellulolysis in the saproxylic scarab larvae, Cetonia aurataeformis (Coleoptera: Scarabaeoidea: Cetoniidae).
Wood is a complex material consisting of cellulose, hemicelluloses, and, lignin. Coniferous trees (softwoods) contain 33–42% cellulose, 22–40% hemicellulose, 27–32% lignin, and 2–3.5% extractives, whereas the content of deciduous trees (hardwoods) includes 38–51% cellulose, 17–38% hemicellulose, 21–31% lignin, and 3% extractives (Tarasov et al. 2018). H. bajulus larvae prefer softwoods (Yalcin et al. 2018) and can digest the cellulose and hemicellulose contents of wood and structurally alter it by using the cellulase enzymes secreted from their midgut epithelium. The changes in cellulose and hemicellulose for the functional groups in the FTIR spectra resulted from the activity of the enzymes secreted from the insect intestines after larval attack. Cellulose and hemicelluloses were degraded into smaller glucose molecules that could be used by the insect (Chararas and Chipoulet 1983). However, the lignin could not be digested because H. bajulus produces no ligninolytic enzymes such as laccase, lignin peroxidase, or manganese peroxidase. The enzyme systems of the insects play an important role in making this distinction. A key-lock match is required between enzyme and substrate in order for enzymes to digest the cellulose in wood (Yalçın et al. 2019). The presence of the cellulase enzyme that degrades cellulose in the H. bajulus larval intestine was recorded in Busconi et al. (2014). Ligninolytic enzymes have not been reported in H. bajulus to date in any literature studies.
The increase in lignin bands is due to the proportional increase in the substrate resulting from the degradation of hemicellulose and cellulose (Pandey and Pitman 2003, Can and Sivrikaya 2020). Similar studies have previously been carried out to explain the wood digestion mechanism of insects. Mico et al. (2011) studied the digestion mechanisms of Cetonia aurataeformis (Coleoptera: Scarabaeoidea: Cetoniidae) on litter and Betula alba and Quercus pyrenaica woody structures. They found that Cetonia aurataeformis larvae changed the relative intensity of the polysaccharide and lignin peaks. They reported that the insect species used in the study could digest both lignin and polysaccharides. However, they demonstrated that the polysaccharides were more decomposed than the lignin because of the lower-strength hemicelluloses found in polysaccharides. In the current study, the hemicellulose peaks (1735 cm−1 wavelength) for the peptone-impregnated SPW samples disappeared in both the FPD and FR.
A similar study was also conducted by Oevering et al. (2003) to reveal the relationship of the wood degradation mechanism and digestion of insects. In the study, larvae and adults of the Pselactus spadix (Coleoptera: Curculionidae: Cossoninae) beetle were used, and results showed that the larvae degraded more cellulose and hemicellulose than the adults. As in the current study, although there was a decrease in the FTIR spectra of the hemicelluloses (1738 cm−1) and cellulose (1375 and 898 cm−1) wavelengths for the FR, the relative intensity and peak heights of the 1230 cm−1 and 1268 cm−1 lignin wavelengths increased.
The smaller (< 0.05 g) larvae were fed with peptone-impregnated wood, which may have caused more cellulose and hemicellulose degradation in the FTIR spectra for the larvae in this group. Protein-containing peptone has also been shown to affect the growth of H. bajulus larvae (Schuch 1937).
Conclusion
The biodegradation and chemical changes occurring in wood caused by the H. bajulus beetle was revealed for the first time by FTIR spectroscopy. Moreover, FTIR was found to be an appropriate technique for determining the structural changes caused by H. bajulus in the wood materials. The highest consumption rate among the wood species was obtained in SPW. As a result of the chemical analysis, holocellulose ratios and sugar components were found to be lower in the FR, whereas lignin and extractive substance ratios were higher. The chemical analysis and FTIR results showed that H. bajulus larvae had changed the structure of the FPD samples as well. The absorption values and relative intensity of hemicellulose and cellulose (1735, 1370, 1321, and 897 cm−1) decreased, although the relative intensity and peak heights of lignin (1508, 1458, 1268, and 1031 cm−1) increased. The smaller (< 0.05 g) H. bajulus larvae caused greater structural changes in the wood compared to the larger larvae. The results showed that the H. bajulus larvae digested the carbohydrates, while leaving the lignin undigested.
References
Abidi N, Cabrales L, Haigler CH (2014) Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr Polym 100:9–16
Akbulut S, Keten A, Yüksel B (2008) Wood destroying insects in Düzce province. Turk J Zool 32(3):343–350
Bobadilla I, Arriaga F, Luengo E, Martínez R (2015) Dimensional and morphological analysis of the detritus from six European wood boring insects. Maderas-Cienc Tecnol 17(4):893–904
Busconi M, Berzolla A, Chiappini E (2014) Preliminary data on cellulase encoding genes in the xylophagous beetle, Hylotrupes bajulus (Linnaeus). Int Biodeter Biodegr 86:92–95
Can A, Sivrikaya H (2020) Evaluation of marine wood boring organism’s attack on wood materials in the black sea coastal region. BioResources 15(2):4271–4281
Cazemier AE, den Camp HJMO, Hackstein JHP, Vogels GD (1997) Fibre digestion in arthropods. Comp Biochem Phys A 118:101–109
Chararas C, Chipoulet JM (1983) Studies on the digestion of cellulose by the Larvae of the Eucalyptus Borer, Phoracantha semipunctata (Coleoptera: Cerambycidae). Aust J Biol Sci 36(3):223. https://doi.org/10.1071/bi9830223
Chiappini E, Molinari P, Busconi M, Callegari M, Fogher C, Bani P (2010) Hylotrupes bajulus (L.) (Col., Cerambycidae): nutrition and attacked material. In: Proceedings of the 10th international working conference on stored product protection, vol 27, pp 97–103. https://doi.org/10.5073/jka.2010.425.241
Cymorek S (1981) The house longhorn beetle Hylotrupes bajulus (L.),(Col., Cerambycidae), in hardwood: experiments with hardwood species, testing of the effect of lignin components and Gingko biloba, observations on Hesperophanes. Mitt Dtsch Ges Allg Angew Entomol 3(1/3):90–96
Eaton RA, Hale MDC (1993) Wood decay, pest and protection. Chapman and Hall, London
Elmas GM, Yılgor N (2020) Chemical and Thermal Characterizations of Pinus sylvestris and Pinus pinaster. BioResources 15(2):3604–3620
EN 46-1 (2016) Wood preservatives—Determination of the preventive action against recently hatched larvae of Hylotrupes bajulus (Linnaeus)—Part 1: Application by surface treatment (laboratory method)
Fettköther R, Reddy G, Noldt U, Dettner K (2000) Effect of host and larval frass volatiles on behavioural response of the old house borer, Hylotrupes bajulus (L.) (Coleoptera: Cerambycidae), in a wind tunnel bioassay. Chemoecology 10:1–10
Graf E, Manser P, Schmitter M (1989) Einfluß der Vitalität von Fichten [Picea abies (L.) Karst.] und Tannen (Abies alba) auf die Resistenz des Bauholzes gegen Eilarven des Hausbockes (Hylotrupes bajulus L.). Material und Organismen 24:93–105
Green F, Highley TL (1997) Mechanism of brown-rot decay: paradigm or paradox. Int Biodeter Biodegr 39(2–3):113–124. https://doi.org/10.1016/s0964-8305(96)00063-7
Grosser D (1985) Pflanzliche und tierische Bau- und Werkholzscha¨dlinge. DRW, Leinfelden-Echterdingen, p 159
Höll W, Frommberger M, Strassl C (2002) Soluble carbohydrates in the nutrition of house long horn beetle larvae, Hylotrupes bajulus (L.) (Col., Cerambycidae): from living sapwood to faeces. J Appl Entomol 126:463–469
Howell C, Steenkjær Hastrup AC, Goodell B, Jellison J (2009) Temporal changes in wood crystalline cellulose during degradation by brown rot fungi. Int Biodeter Biodegr 63(4):414–419. https://doi.org/10.1016/j.ibiod.2008.11.009
Laurent P, Buchon L, Guespin-Michel JF, Orange N (2000) Production of pectate lyases and cellulases by Chryseomonas luteola strain MFCL0 depends on the growth temperature and the nature of the culture medium: evidence for two critical temperatures. Appl Environ Microbiol 66(4):1538–1543
Martin MM (1983) Cellulose digestion in insects. Comp Biochem Physiol Part A Mol Integr Physiol 75(3):313–324. https://doi.org/10.1016/0300-9629(83)90088-9
Martin MM, Jones CG, Bernays EA (1991) The evolution of cellulose digestion in insects. Philos Trans R Soc B 333(1267):281–288. https://doi.org/10.1098/rstb.1991.0078
Mico E, Juárez M, Sánchez A, Galante E (2011) Action of the saproxylic scarab larva Cetonia aurataeformis (Coleoptera: Scarabaeoidea: Cetoniidae) on woody substrates. J Nat Hist 45(41–42):2527–2542
Mohebby B (2005) Attenuated total reflection infrared spectroscopy of white-rot decayed beech wood. Int Biodeter Biodegr 55(4):247–251. https://doi.org/10.1016/j.ibiod.2005.01.003
Nerg AM, Heijari J, Noldt U, Viitanen H, Vuorinen M, Kainulainen P, Holopainen JK (2004) Significance of wood terpenoids in the resistance of scots pine provenances against the old house borer, Hylotrupes bajulus, and Brown-Rot Fungus, Coniophora puteana. J Chem Ecol 30(1):125–141
Oevering P, Pitman AJ, Pandey KK (2003) Wood digestion in Pselactus spadix Herbst—a Weevil attacking marine timber structures. Biofouling 19:249–254. https://doi.org/10.1080/0892701021000051635
Pandey KK (1999) A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J Appl Polym Sci 71(12):1969–1975
Pandey KK, Nagveni HC (2007) Rapid characterisation of brown and white rot-degraded chirpine and rubberwood by FTIR spectroscopy. Holz Roh Werkst 65:477–481
Pandey K, Pitman A (2003) FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int Biodeter Biodegr 52(3):151–160. https://doi.org/10.1016/s0964-8305(03)00052-0
Popescu CM, Popescu MC, Singurel G, Vasile C, Argyropoulos DS, Willfor S (2007) Spectral characterization of eucalyptus wood. Appl Spectros 61:1168–1177
Rabemanolontsoa H, Saka S (2012) Holocellulose determination in biomass. In: Yao T (ed) Zero-Carbon Energy Kyoto 2011. Green Energy and Technology. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54067-0_14
Robinson WH, Cannon KF (1979) The life history and habits of the old house borer Hylotrupes bajulus and its distribution in Pennsylvania USA. Melsheimer Entomol Ser 27:30–34
Schuch K (1937) Experimentelle Untersuchungen über den Nahrungswert von Kiefern-und Fichtenholz für dielarve des Hausbockkäfers (Hylotrupes bajulus L.). Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz 4:572–585
Sen S, Yalçın M, Tasçioglu C, Özbayram AK (2017) Larvicidal activities of some bark and wood extracts against wood-damaging insects. Maderas-Cienc Tecnol 19(3):273–284
Shi J, Xing D, Lia J (2012) FTIR studies of the changes in wood chemistry from wood forming tissue under inclined treatment. Energy Procedia 16:758–762
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2004) NREL biomass program: Determination of structural carbohydrates and lignin in biomass, Biomass Analysis Technology Team, Laboratory Analytical Procedure 2004, Department of Energy, USA
TAPPI T203 cm-09 (2009) Alpha-, beta- and gamma-cellulose in pulp. TAPPI Press, Atlanta
Tarasov D, Leitch M, Fatehi P (2018) Lignin–carbohydrate complexes: properties, applications, analyses, and methods of extraction: a review. Biotechnol Biofuels 11:269. https://doi.org/10.1186/s13068-018-1262-1
Tomak ED, Topaloglu E, Gumuskaya E, Yildiz UC, Ay N (2013) An FT-IR study of the changes in chemical composition of bamboo degraded by brown-rot fungi. Int Biodeter Biodegr 85:131–138. https://doi.org/10.1016/j.ibiod.2013.05.029
Wilson DB, Irwin DC (1999) Genetics and properties of cellulases. Adv Biochem Eng/Biotechnol: Recent Prog Bioconver 65:1–21
Wise LE, John EC (1952) Wood chemistry, vol 2. Reinhold Publishing Co., New York
Yalcin M, Taşçıoğlu C, Plarre R, Akçay Ç, Busweiler S (2018) Investigation of natural durability of some native and exotic wood species against Hylotrupes bajulus (Cerambycidae) and Anobium punctatum (Anobiidae). Kastamonu Univ J For Facul 18(1):83–91
Yalçın M, Doğan HH, Akçay Ç (2019) Identification of wood-decay fungi and assessment of damage in log depots of Western Black Sea Region (Turkey). For pathol 49(2):e12499
Zverlov VV, Höll W, Schwarz WH (2003) Enzymes for digestion of cellulose and other polysaccharides in the gut of longhorn beetle larvae, Rhagium inquisitor L. (Col., Cerambycidae). Int Biodeter Biodegr 51(3):175–179
Acknowledgments
This study was not supported by any project or fund agency in the public, commercial, or non- profit sectors. The author would like to thank Recai Aslan and Suayip Okumus for their kind support during the HPLC and FTIR analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akcay, C., Yalcin, M. Morphological and chemical analysis of Hylotrupes bajulus (old house borer) larvae-damaged wood and its FTIR characterization. Cellulose 28, 1295–1310 (2021). https://doi.org/10.1007/s10570-020-03633-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03633-5