Abstract
Nowadays, environmentally friendly processes are of great interest and are considerably needed due to the environmental pollution seems to be a problem worldwide. For this reason, in this study, silver nanoparticles were synthesized using environmentally-friendly methods and their effectiveness as wood preservatives was investigated. Scots pine (Pinus sylvestris L.) samples were impregnated with an autoxidized soybean oil polymer containing Ag nanoparticles (Agsbox). Samples characterised by Fourier transform infrared spectroscopy (FTIR) were tested against brown rot (Coniophora puteana) and wood-destroying insects (Hylotrupes bajulus). In addition, decay tests were applied to mini-block samples leached according to the EN 84 standard. Results demonstrated that Agsbox increased decay resistance in the unleached samples. However, low efficacy was exhibited against newborn H. bajulus larvae. As a results of FTIR measurement, impregnated with the nanocomposites showed significant changes at the 2910 cm−1 (C–H) and 1712 cm−1 (C=O) peaks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wood used in indoor and outdoor applications is exposed to the effects of temperature, humidity and biological agents. In wood with low natural durability, exposure to fungi and insects under appropriate humidity and temperature conditions leads to colour changes and structural damage of the wood (Meyer-Veltrup et al. 2017; Yang et al. 2017). The service life of wood can be extended by treating it with preservative that can be water-borne or solvent-based. However, nowadays, the first is the one most frequently used for construction applications. The most important water-based wood preservative known is copper chromium arsenic (CCA). Over the past 80 years, it has been used extensively worldwide. However, due to the toxic effect on the environment, its use is prohibited in some countries. Copper-based preservatives like alkaline copper quaternary (ACQ) and copper azole (CA) have been developed and utilized as replacements (Zhong et al. 2014) although these preservatives gradually leach out of the wood, causing significant losses in effectiveness (Liu et al. 2018).
Many biological approaches are used to obtain silver nanoparticles via ‘green’ methods including aqueous extracts of manna from the hedysarum plant and the soap-root (Acanthe phylum bracteatum) plant, culture supernatants of Aspergil lusterreus and leaf extracts of Euphorbia hirta L., Euphorbia milii and Foeniculum vulgare (Kuppusamy et al. 2016). In addition, basic amino acids such as l-lysine or l-arginine are also used to obtain silver nanoparticles (Bonde 2011; de Matos et al. 2011; Elumalai et al. 2010; Forough and Fahadi 2010; Li et al. 2011; Singh et al. 2010). In other studies, silver nanoparticles were synthesized using Polyalthia longifolia leaf extract (Kaviya et al. 2011) and Cleome viscosa plant extract (Lakshmanan et al. 2018). In addition, silver nanoparticles of 13 ± 3 nm were obtained using sulphate polysaccharides isolated from Porphyria vietnamensis (Venkatpurwar and Pokharkar 2011).
In general, nanocompounds penetrate into the wood cell walls more easily, while they are more difficult to leach out and have higher bio-durability. In recent years, several studies have been conducted on the antifungal activity of silver nanoparticle compounds (Can et al. 2018; Kim et al. 2009; Paril et al. 2017). However, the antifungal properties, leaching resistance and chemical characterisation of silver are less understood because most studies have been focused on viral pathogens and bacterial in animals (Klasen 2000; Silver 2003) and silver nanoparticle metals have been the subject of intense interest (Baker et al. 2005; Melaiye et al. 2005; Sondi and Salopek-Sondi 2004). Though the biocidal effects and mode of action of silver ions are known (Lakshmanan et al. 2018; Siddiqi et al. 2018), However, the antifungal activity and the mode of action of nano-Ag against fungi have for the most part not been determined and remain unknown. Although some studies have shown that silver nanoparticles are effective against decay fungi (Kim et al. 2009; Pulit et al. 2013), some studies have demonstrated that silver nanoparticles are less effective against decay fungi (Akhtari and Arefkhani 2013; Rezaei et al. 2011).
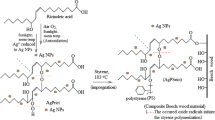
These days, metal nanoparticles are often obtained through green processes (Ahmed et al. 2016; Bonde 2011; de Matos et al. 2011; Elumalai et al. 2010; Forough and Fahadi 2010; Hazer and Akyol 2016; Li et al. 2011; Singh et al. 2010). Consequently, the present study examined the effect on wood of silver nanoparticles obtained naturally using soybean oil.
This study aimed to increase the leaching resistance of silver nanoparticles produced by the green chemistry approach. Furthermore, the biological durability and chemical properties of Scots pine (Pinus sylvestris L.) wood were investigated.
Experimental section
Materials
Scots pine (density 0.42 g cm−3) (Pinus sylvestris L.) specimens were selected from entirely sapwood boards, cut into sizes of 15 (Radial) × 25 (Tangential) × 50 (Longitudinal) mm3 for the decay and insect tests and 20 (R) × 20 (T) × 10 (L) mm3 for the leaching test. Two different sizes were used for the samples in the fungal tests. The first was the standard test size (15 × 25 × 50 mm3 along the grain), and the second (20 × 20 × 10 mm3) was that applied for the mini-block samples in the leaching test. The AgNO3 (purity ≥ 99.0%) was obtained from Sigma-Aldrich. Soybean oil used was supplied by Çotanak/Altas and was composed of 45.6% polyunsaturated fatty acids (linoleic acid and linolenic acid), 34 wt% monounsaturated fatty acids (oleic acid) and 15.9 wt% saturated fatty acids (stearic acid and palmitic acid).
Synthesis of silver nanoparticles (Agsbox)
The same procedure was applied as for the autoxidation, but without silver nanoparticles, on the fatty acids/oil mixed with AgNO3. 18 g of soybean oil was poured into a Petri dish (Φ = 14.5 cm, oil thickness: 1.0 mm), after which 0.50 g (2.94 mmol) of AgNO3 was added into this oil and occasionally mixed using a glass rod. The colourless oil layer exposed to the air turned a deep brown colour after 4 weeks due to the formation of silver nanoparticles. In order to obtain a clear nanocomposite liquid, the unreacted inorganic salts were filtered from the oxidized oil containing the silver nanoparticles (Can et al. 2018; Hazer and Kalaycı 2017).
Impregnation of wood
Two different solutions, chloroform (CL) and toluene (T), were prepared with the soybean oil–Ag nanocomposites for impregnation of the wood specimens. For this purpose (all w/w) 1.5% Agsbox was dissolved in chloroform (Agsbox-CL), and 1.5% Agsbox was dissolved in toluene (Agsbox-T). Samples to be treated with Agsbox were mixed in the chloroform and toluene using a magnetic stirrer for 2 h, followed by an additional 2 h of stirring after changing the chloroform and toluene. Following the stirring process, the samples were held in the lab for 2 h, and then dried in a vacuum oven at 40 ± 2 °C for 4 h subsequent to drying in an oven at 103 ± 2 °C. The control samples were also held in chloroform for 2 h for comparison with the treated samples. After drying at 103 ± 2 °C for 1 day, the samples were weighed and then exposed to the impregnation: vacuum (650 mmHg for 20 min) and atmospheric pressure (1 h) performed in a desiccator. The samples were then oven-dried for 1 day at 103 ± 2 °C and weighed again and the weight percent gain (WPG1) was calculated using Eq. (1).
where Mo represents oven-dry weights of the untreated samples and Mt oven dry weights of the treated samples.
Fourier transform infrared (FT-IR) spectroscopy analysis
The FT-IR spectra, with 32 scans for each sample, were collected via a Shimadzu IRAffinity-1 spectrometer equipped with an ATR pike MIRacle accessory (4 cm−1 resolution). The samples were measured from the earlywood section with an ATR apparatus and spectrum measurements were taken at eight different points from each sample surface (Ext.) and at a depth of 2 mm (Int.). The resulting single spectrum was obtained for each variation by calculating the average of the spectra in the programme of the device.
Colour measurement
Colour measurement was applied with a Konica Minolta spectrophotometer (Osaka, Japan) by measuring the L, a and b values on the samples. For each sample, four colour measurements were made and colour evaluation was carried out according to ISO 7724-2 standard (ISO 7724 1984). The changes in colour coordinates (∆L*, ∆a* and ∆b*) were determined by the difference between the initial and the final values. Hereafter, the total colour changes (ΔE *) were calculated according to the following equations:
Total colour change (∆E*) was calculated according to Eq. (2) below.
Leaching test
The leaching procedure was similar to the EN 84 (1997) with wood size modifications. Wood specimens with dimensions of 20 × 20 × 10 mm3 were placed into a 300-mL beaker filled with deionized water, and subjected to a vacuum (650 mmHg) to impregnate the blocks with the leaching solution. A set of six replicates was used for each treatment. At the end of each period, the leaching water was collected and its silver content was determined by ICP analysis. The silver content of the leached water was measured via inductively coupled plasma (ICP) analysis, using an ICP spectrometer (ICP-AES, Spectro Genesis), according to the AWPA standard (AWPA A7 1993). After leaching and conditioning, WPG2 was then calculated using Eq. (3).
where M1 is the oven dry weight of the leached wood blocks.
Decay test
The fungal test was conducted with the decay fungus Coniophora puteana (Schumacher ex Fries) Karsten strain BAM Ebw according to EN 113 (2006). In a glass container, where the fungal strain had been previously grown on a 4–2.5% malt-agar medium, one treated and one untreated sample were incubated at a constant condition of 20 ± 2 °C and 70 ± 5% R.H for 16 weeks. Six replicates were performed for each treatment.
Insect test
The insect test was performed based on the EN 47 (2005) standard to determine the effectiveness of the Agsbox against the larvae of Hylotrupes bajulus. Prior to the test, 24 sample with dimensions of 15 × 25 × 50 mm3 along the grain were conditioned at 20 ± 2 °C and 65 ± 5% RH. After exposure to the larvae, the test specimens were placed in jars and stored in a controlled chamber at 20 ± 2 °C and 70 ± 5% RH for 8 weeks. After the exposure, each sample was examined by X-ray analysis to check the status of the inserted larvae (dead, living, not recovered).
Statistical analysis
A descriptive analysis was developed (mean and standard deviation) for decay test. ANOVA was applied to verify the effect of treatment with the silver nanoparticles. Duncan’s test was set at 99% confidence level to determine the statistical difference between the means.
Results and discussion
As seen in previous publications, transmission electron microscopy (TEM) images of Agsbox showed a ‘bunch of grapes’ cluster of Ag nanoparticles approximately 5 nm in size (Hazer and Kalaycı 2017).
Fourier transform infrared spectroscopy (FTIR-ATR)
The assignments of characteristic absorption IR bands of the wood samples are given in Table 1 and the FTIR spectra of the treated and untreated samples are shown in Fig. 1.
The spectrum of pine wood (Fig. 1) shows the same basic structure as wood samples. 3300–4000 cm−1 peaks shows strong main OH stretching (1), 2800–3000 cm−1 shows C–H stretching in methyl and methylene groups (2–3). Two new characteristic peaks appeared in the samples treated with the Agsbox at 2910 cm−1 (2) and 2840 cm−1 (3). These peaks were more prominent for the surface areas of the samples. 2919 cm−1 and 2849 cm−1 peaks intensity increased after impregnation in the samples treated with the Agsbox. These peak values were higher with the Agsbox dissolved in chloroform than for that dissolved in toluene. Agsbox-CL-Ext. and Agsbox-CL-Int. gave similar peaks. This showed that the Agsbox-CL material exhibited behaviour similar to that of the wood surface even at depths of 2 mm. However, the same was not the case for Agsbox-T because lower peak values were obtained at a depth of 2 mm for the samples impregnated with Agsbox-T.
No distinctive peak was observed in the range between 2700 and 1800 cm−1. The region from 1800 to 700 cm−1 included the sharp and discrete functional groups characterised for wood components (cellulose, hemicellulose, and lignin) (Liu et al. 2018). The modification process made with Agsbox caused significant changes in this region. The band at 1712 cm−1 (4) indicating an unconjugated C=O stretch in the xylan. This peak increased sharply. Modification of acetyl and carbonyl groups is the main reason for the increase in peak values. Agsbox-Ext. showed a more salient band than the other groups at 1712 cm−1. The peak at 1224 cm−1 (8) showed the CO and OH groups in the hemicellulose, cellulose and lignin (Faix 1991). The CO and OH peaks of the silver-impregnated Agsbox-CL specimens increased compared to the control samples, whereas there were no significant changes for the Agsbox-T specimens.
The IR bands at 1016 cm−1 (11) on behalf of cellulose and hemicellulose changed after Agsbox impregnation. 1016 cm−1 peak was increased after impregnation with Agsbox-CL, while no change was seen in samples impregnated with Agsbox-T.
Weight percent gain (WPG %) and leachability of nanoparticles
The WPG values for the impregnated specimens are given in Table 2. In addition, Duncan’s homogeneity groups are indicated with letters (p < 0.05) for the WPG results. In the homogeneity groups, each column is evaluated within itself.
Higher WPG values were obtained in the samples impregnated with Agsbox-CL than in those impregnated with Agsbox-T in both the mini-block and the standard test samples. Both the nano-Ag and the soybean oil in the material used caused the high WPG values, which resulted from the anatomical structure and porosity of the Scots pine wood species. The high WPG values caused colour changes on the wood surface of the specimens, which attained a dark-brown colour when treated with the silver nanoparticles (Fig. 2 and Table 3).
Table 3 shows the colour changes (∆L*, ∆a*, ∆b*, ∆E*) of wood after treatment. Negative lightness stability (∆L*) indicate a tendency of wood surface to become darker. The original colour of wood samples was noticeably changed by Agsbox impregnation. Positive values show the tendency of a wood surface to become reddish for ∆a*, yellowish for ∆b* and negative values represent greenish for ∆a*, bluish for ∆b* (Sivrikaya and Can 2016). Agsbox treatment caused the surface to become reddish. Agsbox caused a yellowing of the surface, as evinced by increased ∆b* values. The maximum total colour change (∆E*) was obtained in the samples impregnated with Agsbox. Chloroform and toluene are capable of breaking down the polymeric components such as tannin, lignin, and cellulose present in tree bark, sawdust and pinecones. Fragmentation of tannin and lignin causes colour change on wood surfaces. The colourless oil layer exposed to the air turned a deep brown colour in 3–4 weeks due to the formation of the silver nanoparticles. An intensive bright-green colour was observed for the nanocomposite samples under UV illumination, while the samples appeared brown in daylight. In previous studies, it has been reported that silver had a dark brown appearance (Hazer and Kalaycı 2017; Paril et al. 2017; Lakshmanan et al. 2018).
Negative WPG values were obtained in the leached mini-block test samples. This indicated that silver and also some wood components had been leached from the wood (Fig. 3). According to the results, 0.0023% of the silver was leached out when chloroform was used and 0.0025% when toluene was used. After the leaching test, most of the − 0.46% and − 0.72% WPG2 values obtained were from other substances which were leached from the wood.
After the leaching test, the final amount of Ag released was 0.156 ppb for Agsbox-CL and 0.182 ppb for Agsbox-T, which represented approximately 1.8% and 2.2% of the silver content, respectively, that was in the unleached samples. This finding was satisfactory because silver compounds such as silver nitrate have a water-soluble structure.
The highest leaching rate was realized after the initial leaching period. The amount of leached substances gradually decreased after 48 h. A similar leaching progression was presented by Paril et al. (2017) for silver- and copper-based preservatives. The accessibility of the silver deposited at a higher concentration on the wood surface layer was considered as the main reason for this. The results indicated that the silver nanoparticles were more sensitive to leaching, but many factors, including size, type of stabilizers or species treated, could have influenced the final behaviour.
There is a global movement to ban silver nanoparticles in antimicrobials. Several health maintenance organizations have already banned the use silver nanoparticle antibiotics in many of the products they use. However, the leaching of the nano silver was very low due to our method to obtain nano silver. According to the leaching results, only 0.0023–0.0025% nano silver was leached. It is necessary to conduct further research on toxicity of low amounts of nano-silver (Faunce and Watal 2010; Pulit-Prociak and Banach 2016).
Decay test
Figure 4 shows the weight loss values in the samples after the fungal test. In the Duncan test results, the unleached samples were evaluated among themselves, and the leached samples were evaluated among themselves. When the unleached samples were evaluated among themselves, it was seen that all test samples were in the same homogeneity group. In the leached samples, all other variations except Agsbox-T were in the same homogeneity group.
According to Fig. 4, at the end of the 8 weeks decay test, unleached control specimens suffered mass loss at the rate of 37.39%. The decay test standard (EN 113 2006) accounted for the mass loss of 20% in the untreated samples. At the end of 8 weeks, the test was terminated because a weight loss of more than 20% had occurred. Compared to the high weight losses in the unleached control samples, the weight loss was improved in the modified samples at the rate of 50.54% for Agsbox-CL. The lowest weight loss obtained in the leached Agsbox-T samples was also seen in the unleached samples. The control leached specimens exhibited mass loss at the rate of 32.30%. Lower weight losses were obtained in the test samples compared to the controls. However, these weight loss values were above the value of 3% specified by the EN 113 standard.
The results indicated that the decay resistance of wood modified by Agsbox was improved against the brown-rot fungus (C. puteana) when compared to that of untreated Scots pine. Many studies have investigated the efficacy of silver material in the wood protection field. In these studies, it has been emphasized that nano silver material is effective against white-rot fungi, but has less effect on brown-rot fungi (Moya et al. 2014, 2017; Bak and Nemeth 2018).
Vegetable oils, due to their chemical formulations, have no fungicidal and/or insecticidal effects on the structure. For this reason, the introduction of a fungicide and insecticide into the wood structure would increase the effectiveness of the oil against fungi and insects. In our study, the fungal resistance in the wood samples was enhanced by the effect of the extensive use of the silver nanoparticles in the soybean oil. In the study by Tomak et al. (2011), by adding boron compounds to oils obtained from vegetable sources (hazelnut, sunflower, canola, soybean, etc.), weight loss in the fungal decay test was 7–13% after the impregnation process. These weight losses were reported to be due to the fact that the oils obtained from plant materials were not effective against fungi, and only prevented water from entering the cells so that the fungus could not get the moisture it required.
Insect test
Figure 5 shows the mortality rates after the insect test. The 4-week and 2-month results were compared and the Duncan test results were calculated from the final (2-month) results only.
The larvae mortality is presented in Fig. 5. According to the figure, 4 weeks after the first inclusion of the larvae into the treated wood, the chloroform formulations had the same probability of causing the death of H. bajulus larvae as the treatment with toluene formulations. In the first 4 weeks, the mortality rates with the silver-based variations were higher than that of the control. At the end of the 8-week test, high-level rates of larval death had occurred only in the samples impregnated with chloroform and toluene. Mortality rates obtained with Agsbox-CL and Agsbox-T were higher than in the control, while they were lower than the mortality rates when chloroform and toluene were used alone. The study was terminated after 8 weeks. For this reason, it can be said that mortality rates were low in the samples containing nano silver. In other words, Agsbox exhibited low activity against the H. bajulus beetle in the 8-week period.
It can be said that the soybean oil used in the production of Agsbox was a nutritive additive for the larvae. In order to survive, insects require proteins, carbohydrates, fat and vitamins, although there are variations in the specific requirements of different insect species (Nair 2007). The statistical analysis revealed that chloroform and toluene were in the same homogeneity class while there were significant differences from the control (p < 0.05).
Conclusion
This study was undertaken to characterize wood impregnated with Agsbox and to determine the performance of it against C. puteana fungus and H. bajulus larvae. Further, it was investigated the leaching of Ag nanoparticles from wood after the impregnation. By the FTIR spectra a significant increase in the asymmetric and the symmetric stretching vibrations for C–H after the Agsbox impregnation was observed.
Regarding the insect test, the soybean oil could have provided a nutritive effect and therefore, the Agsbox was not significantly effective against the H. bajulus larvae. In the decay tests, no significant differences was found on non leached samples treated with Agbox and two solvents, the only significant difference was found in the control as we can expected. By the decay results seemed that even the solvents alone can gives some effectiveness against fungi, probably due to extraction of some component into the wood. Further investigation on the effect of solvents such as toluene and chloroform on wood resistance against fungus C. puteana could be performed in the future.
References
Ahmed S, Ahmad M, Swami BL, Ikram S (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res 7:17–28
Akhtari M, Arefkhani M (2013) Study of microscopy properties of wood impregnated with nano-particles during exposed to white-rot fungus. Agric Sci Dev 2:116–119
AWPA A7 (1993) Standard for wet ashing procedures for preparing wood for chemical analysis. American Wood Preservers. Association Standard, Granbury
Bak M, Nemeth R (2018) Effect of different nanoparticle treatments on the decay resistance of wood. BioResources 13:7886–7899
Baker C, Pradhan A, Pakstis L, Pochan DJ, Shah SI (2005) Synthesis and antibacterial properties of silver nanoparticles. J Nanosci Nanotechnol 5:244–249
Bonde S (2011) A biogenic approach for green synthesis of silver nanoparticles using extract of Foeniculum vulgare and its activity against Staphylococcus aureus and Escherichia coli. Bioscience 3:59–63
Can A, Sivrikaya H (2017) Chemical characterization of fungal deterioration in Populus alba by FT-IR. J Bartin Fac Forest 19:139–147
Can A, Sivrikaya H, Hazer B (2018) Fungal inhibition and chemical characterization of wood treated with novel polystyrene-soybean oil copolymer containing silver nanoparticles. Int Biodeterior Biodegrad 133:210–215
de Matos RA, da Silva Cordeiro T, Samad RE Jr, Vieira ND, Courrol LC (2011) Green synthesis of stable silver nanoparticles using Euphorbia milii latex. Colloids Surf A: Physicochem Eng Asp 389:134–137
Elumalai EK, Prasad TNVKV, Venkata K, Nagajyothi PC, David E (2010) Green synthesis of silver nanoparticle using Euphorbia hirta L. and their antifungal activities. Arch Appl Sci Res 2:76–81
European Committee for Standardization EN 47 (2005) Wood preservatives. Determination of the toxic values against larvae of Hylotrupes bajulus (Linnaeus). (Laboratory method). European Committee for Standardization, Brussels
European Committee for Standardization EN 84 (1997) Wood preservatives. Accelerated ageing of treated wood prior to biological testing. Leaching procedure. European Committee for Standardization, Brussels
European Committee for Standardization EN 113 (2006) Wood preservatives. Test method for determining the protective effectiveness against wood destroying basidiomycetes—determination of the toxic values. European Committee for Standardization, Brussels
Faix O (1991) Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 45:21–28
Faunce T, Watal A (2010) Nanosilver and global public health: international regulatory issues. Nanomedicine 5:617–632
Forough M, Fahadi K (2010) Biological and green synthesis of silver nanoparticles. Turkish J Eng Environ Sci 34:281–287
Hazer B, Akyol E (2016) Efficiency of gold nano particles on the autoxidized soybean oil polymer: fractionation and structural analysis. J Am Oil Chem’ Soc 93:201–213
Hazer B, Kalaycı ÖA (2017) High fluorescence emission silver nano particles coated with poly (styrene-g-soybean oil) graft copolymers: antibacterial activity and polymerization kinetics. Mater Sci Eng: C 74:259–269
ISO 7724-2 (1984) Paints and varnishes. Colorimetry. Part 2: colour measurement. International Organization for Standardization, Geneva, p 6
Kaviya S, Santhanalakshmi J, Viswanathan B (2011) Green synthesis of silver nanoparticles using Polyalthia longifolia leaf extract along with D-sorbitol: study of antibacterial activity. J Nanotech J Nanotech 2019:1–5
Kim KJ, Sung WS, Suh BK, Moon SK, Choi JS, Kim JG, Lee DG (2009) Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals 22:235–242
Klasen HJ (2000) A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 26:131–138
Kuppusamy P, Yusoff MM, Maniam GP, Govindan N (2016) Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm J 24:473–484
Lakshmanan G, Sathiyaseelan A, Kalaichelvan PT, Murugesan K (2018) Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: assessment of their antibacterial and anticancer activity. Karbala Int J Modern Sci 4:61–68
Li G, He D, Qian Y, Guan B, Gao S, Cui Y, Wang L (2011) Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int J Mol Sci 13:466–476
Liu M, Zhong H, Ma E, Liu R (2018) Resistance to fungal decay of paraffin wax emulsion/copper azole compound system treated wood. Int Biodeterior Biodegrad 129:61–66
Melaiye A et al (2005) Silver(I)-imidazole cyclophane gem-diol complexes encapsulated by electrospun tecophilic nanofibers: formation of nanosilver particles and antimicrobial activity. J Am Chem Soc 127:2285–2291
Meyer-Veltrup L et al (2017) The combined effect of wetting ability and durability on outdoor performance of wood: development and verification of a new prediction approach. Wood Sci Technol 51:615–637
Moya R, Berrocal A, Rodriguez-Zuniga A, Veja-Baudrit J, Noguera SC (2014) Effect of silver nanoparticles on white-rot wood decay and some physical properties of three tropical wood species. Wood Fiber Sci 46:527–538
Moya R, Rodriguez-Zuniga A, Berrocal A, Vega-Baudrit J (2017) Effect of silver nanoparticles synthesized with NPsAg-ethylene glycol (C2H6O2) on brown decay and white decay fungi of nine tropical woods. J Nanosci Nanotech 17:5233–5240
Nair KS (2007) Tropical forest insect pests: ecology, impact, and management. Cambridge University Press, Cambridge
Pandey KK (2005) A note on the influence of extractives on the photo-discoloration and photo-degradation of wood. Polym Degrad Stab 87:375–379
Paril P, Baar J, Čermák P, Rademacher P, Prucek R, Sivera M, Panáček A (2017) Antifungal effects of copper and silver nanoparticles against white and brown-rot fungi. J Mater Sci 52:2720–2729
Pulit J, Banach M, Szczygłowska R, Bryk M (2013) Nanosilver against fungi. Silver nano-particles as an effective biocidal factor. Acta Biochim Pol 60:795–798
Pulit-Prociak J, Banach M (2016) Silver nanoparticles—a material of the future…? Open Chem 14:76–91
Rezaei VT, Usefi A, Soltani M (2011) Wood protection by nano silver against white rot. In: 5th symposium on advances in science & technology, Mashhad, pp 1–9
Siddiqi KS, Husen A, Rao RA (2018) A review on biosynthesis of silver nanoparticles and their biocidal properties. J Nanobiotech 16:14
Silver S (2003) Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev 27:341–353
Singh A, Jain D, Upadhyay MK, Khandelwal N, Verma HN (2010) Green synthesis of silver nanoparticles using Argemone mexicana leaf extract and evaluation of their antimicrobial activities. Dig J Nanomater Bios 5:483–489
Sivrikaya H, Can A (2016) Effect of weathering on wood treated with tall oil combined with some additives. Maderas Ciencia y Tech 18:723–732
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for gram-negative bacteria. J Colloid Interface Sci 275:177–182
Tomak ED, Viitanen H, Yildiz UC, Hughes M (2011) The combined effects of boron and oil heat treatment on the properties of beech and Scots pine wood. Part 2: water absorption, compression strength, color changes, and decay resistance. J Mater Sci 46:608–615
Venkatpurwar V, Pokharkar V (2011) Green synthesis of silver nanoparticles using marine polysaccharide: study of in vitro antibacterial activity. Mater Lett 65:999–1002
Yang Z, Jiang Z, Hse CY, Liu R (2017) Assessing the impact of wood decay fungi on the modulus of elasticity of slash pine (Pinus elliottii) by stress wave non-destructive testing. Int Biodeterior Biodegrad 117:123–127
Yilgor N, Dogu D, Moore R, Terzi E, Kartal SN (2013) Evaluation of fungal deterioration in Liquidambar orientalis Mill. heartwood by FT-IR and light microscopy. BioResources 8:2805–2826
Zhong H, Wang JM, Tang SH, Ma EN (2014) CA-B/new-type paraffin emulsion compound system: study on the mould preservation property of treated wood. Agric Sci Technol 15:2053–2056
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Can, A., Palanti, S., Sivrikaya, H. et al. Physical, biological and chemical characterisation of wood treated with silver nanoparticles. Cellulose 26, 5075–5084 (2019). https://doi.org/10.1007/s10570-019-02416-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02416-x