Abstract
This study evaluated the effect of hydraulic retention time (HRT) on the simultaneous hydrogen and ethanol production in two anaerobic fluidized bed reactors (55 °C) from mono-fermentation of cellulosic hydrolysate (AFBR-C) and the fermentation of glucose and xylose as co-substrates (AFBR-GX). In AFBR-C, the HRT was decreased from 24 to 8 h, while in AFBR-GX, the HRT was decreased from 16 to 0.5 h. The carbohydrate concentration was maintained at 4 g/L (AFBR-GX) and 2 g/L (AFBR-C). In AFBR-C, the main results observed by decreasing the HRT from 24 to 8 h were the increase in H2 yield (0.6–1.1 mol H2/mol hexose) and ethanol concentration (0.02–0.48 g/L). However, the H2 yield in AFBR-GX decreased from 0.4 to 0.1 mol H2/mol hexose by decreasing the HRT from 16 to 0.5 h. Additionally, the shortest HRTs applied to the AFBR-C (8 h) and AFBR-GX (0.5 h) resulted in the maximum hydrogen production rates of 115.7 and 279.9 mL H2/h L, the maximum energy yields of 7.4 and 47.7 kJ/h L, and EtOH molar fractions of 58.9 and 50.2%, respectively.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The agricultural sector is one of the main bases of the Brazilian economy. According to data from the Brazilian Institute of Geography and Statistics, Brazil produced more than 1064 Mt of agricultural products in 2018 (IBGE 2018). The main products of the country are sugarcane (64% of the total), soy (11%), corn (7%) and cassava (2%) (IBGE 2018). However, intense agricultural production is associated with the generation of large amounts of lignocellulosic residues. It is estimated that in Brazil, 200 Mt of agricultural residues are generated per year (Araújo et al. 2018).
Lignocellulose is the most abundant biomass on the planet (Liu et al. 2017), and it is composed mostly of cellulose (30–56%), hemicellulose (10–27%) and lignin (3–30%) (Saripan and Reungsang 2014). When cellulose and hemicellulose are hydrolyzed, they release mainly glucose and xylose, which account for 34–46% and 5–25%, respectively, of the lignocellulosic biomass (Nissilä et al. 2012). The ability to utilize glucose and xylose from lignocellulosic materials in the fermentative process is the key to the feasible production of value-added compounds. However, xylose conversion to more valuable compounds is inefficient when performed by most microorganisms. Thus, it is necessary to investigate microbial processes that can naturally ferment both xylose and glucose (Hniman et al. 2011; Zhao et al. 2018). Studies in the literature have reported the use of glucose and xylose as co-substrates for the production of lactic acid (HLa) (Novy et al. 2018), fumaric acid (HFu) (Liu et al. 2017), ethanol (EtOH) (Nielsen et al. 2017) and hydrogen (H2) (Zhao et al. 2018).
Continuous hydrogen production is one of the most efficient ways to recover valuable products and generate bioenergy from lignocellulosic residues or their derivatives (glucose and xylose) (Hniman et al. 2011). Currently, research on the use of glucose and xylose as co-substrates for hydrogen production is limited to mostly batch reactors (Ren et al. 2008) and a few studies in continuous reactors (Zhao et al. 2013; Sittijunda et al. 2013; Zhao et al. 2018), observing the simultaneous production of hydrogen and EtOH (Ren et al. 2008; Zhao et al. 2013; Sittijunda et al. 2013). Although the EtOH production reduces the available substrates for H2 fermentation (Wu et al. 2007), the simultaneous production of hydrogen and ethanol is promising because of the total energy gain (Wu et al. 2007; Barros and Silva 2012). Still, the ethanol-type fermentation is known for the restriction of other unwanted metabolic pathways, resulting in a higher hydrogen production rate (HPR) (Ren et al. 2007; Han et al. 2017).
Thus, there is a need for the development of high rate anaerobic reactors that can efficiently produce H2 and EtOH as second generation biofuels from lignocellulosic biomass. The anaerobic fluidized bed reactor (AFBR) has better mass transfer efficiency than other high rate reactors, leading to higher HPR (Wu et al. 2007). This reactor has been previously used for continuous H2 production from glucose (Wu et al. 2007; Shida et al. 2012; Barros and Silva 2012) and xylose (Dessì et al. 2018). However, to the authors’ knowledge, there are no studies in the literature comparing the single fermentation of cellulosic hydrolysate and combination of glucose and xylose as co-substrates for the fermentative H2 and EtOH production in thermophilic AFBRs.
In this regard, the objective of this study was to evaluate the effect of hydraulic retention time (HRT) on the simultaneous production of hydrogen and EtOH in thermophilic (55 ± 1 °C) AFBRs from the mono-fermentation of cellulosic hydrolysate (AFBR-C, total sugar concentration of 2 g/L) and from the fermentation of the glucose and xylose mixture (AFBR-GX, both at a concentration of 2.0 g/L). The HRT was decreased from 16 to 0.5 h in AFBR-GX and from 24 to 8 h in AFBR-C. Additionally, the profiles of glucose and xylose consumption in the AFBR-GX and glucose consumption in the AFBR-C were evaluated with energy production analysis.
Materials and methods
Anaerobic fluidized bed reactor (AFBR)
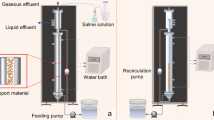
The reactors had a total volume of 1520 mL and were maintained at 55 °C with the aid of thermostatic baths that circulated water in the reactors’ external jackets. Expanded clay particles (with diameters between 2.80 and 3.35 mm) were used as the support material for biomass adhesion (Fig. 1 ). The flow rate of the recycling pump used in the reactors was adjusted in order to maintain the superficial velocity at 1.3 times the minimum fluidization velocity of the support material (1.24 cm/s).
Substrates and nutrient source
The AFBR-GX influent contained xylose and glucose, both at a concentration of 2.0 g/L. The AFBR-C influent contained hydrolysate obtained after the acid hydrolysis of microcrystalline cellulose (Avicel®). The hydrolysis methodology was based on the work of Nissilä et al. (2012). In the first step, the material was hydrolyzed with H2SO4 15% (v/v) in an autoclave for 30 min at 121 °C. In the second step, the hydrolysate was subjected to CaO liming to stabilize the pH in the range between 4.5 and 5.0. The material was then filtered and diluted to a concentration of 2.0 g glucose/L. During all the operational conditions of both reactors, the influents were supplemented with nutrient solution according to Barros and Silva (2012).
Experimental setup
The inoculum used to start the AFBR was from a thermophilic (55 °C) upflow anaerobic sludge blanket reactor (UASB) used to treat sugarcane vinasse generated at Usina São Martinho (Pradópolis, SP, Brazil). The inoculum was subjected to thermal treatment as proposed by Kim et al. (2006) in order to eliminate methanogenic archaea.
The reactors were initially operated in batch mode to allow the inoculum to adapt to the substrate and adhere to the support material. After 80% of the carbohydrate content was consumed, both reactors were operated in continuous mode at the HRT values of 24 h (AFBR-C) and 16 h (AFBR-GX). After reaching a steady state hydrogen production (determined by observing an HPR with a variation of less than 10% over 10 days), the operational conditions were changed according to the experimental scheme. In the AFBR-GX continuous mode, the HRT applied varied from 16 h to 12 h, 8 h, 4 h, 2 h, 1 h and 0.5 h. In AFBR-C, the HRT decreased from 24 h to 20 h to 16 h to 12 h to 8 h. Both reactors were operated for a total of 230 days.
Analytical methods
The analyses of chemical oxygen demand (COD), pH, sulfate, total suspended solids (TSS) and volatile suspended solids (VSS) were performed according to the Standard Methods for Examination of Water and Wastewater (APHA 2005). The biogas composition was analyzed by gas chromatography according to Santos et al. (2014). The volumetric biogas production was measured by the liquid displacement method (Walker et al. 2009). The concentration of total sugars was quantified in the influent and effluent of the reactors by the method proposed by Dubois et al. (1956). High-performance liquid chromatography (HPLC, Shimadzu) was used for the determination of the organic acids and alcohols produced by the fermentation process in the AFBR-C and AFBR-GX (Santos et al. 2014). The quantification of furfural, 5-hydroxymethylfurfural, was performed via HPLC according to the method described in the study of Gouveia et al. (2009).
Results and discussion
Substrate conversion
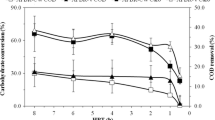
Figure 2 presents the profiles of glucose and xylose conversion in the AFBR-GX. In this reactor, glucose conversion remained stable between 96.5 ± 0.6% and 99.0 ± 0.1% at HRTs from 16 to 4 h. The glucose conversion decreased to 70.5 ± 2.9%, 55.2 ± 12.6% and 51.2 ± 6.0% by decreasing the HRT to 2, 1 and 0.5 h, respectively. The xylose conversion decreased from 56.7 ± 15.9% to 26.5 ± 5.7% by decreasing the HRT from 16 to 8 h and maintained constant values between 7.3 ± 2.0% and 12.8 ± 3.7% at HRTs from 4 to 0.5 h. In the AFBR-C, the glucose conversions remained stable between 92.4 ± 1.7% and 96.7 ± 1.2% with the decrease in the HRT from 24 to 8 h (data not shown).
A result similar to AFBR-C was observed by Pattra et al. (2008) in batch experiments for H2 production using sulfuric acid for the hydrolysis of sugarcane bagasse in an autoclave. The authors reported carbohydrate conversions above 83% using a glucose concentration of 1.97 g COD/L. Additionally Cao et al. (2009), using sulfuric acid for the hydrolysis of corn straw in an autoclave, observed that glucose was completely consumed (1.86 g/L) and the xylose consumption was 83% in batch experiments for H2 production.
Lower xylose conversions in comparison to glucose conversions can be explained by analyzing the metabolic pathways associated with these substrates. Initially, xylose is converted to xylulose, which in turn is phosphorylated to form xylulose-5-phosphate and then enters the glycolysis pathway as glyceraldehyde-3-phosphate, whereas glucose only needs to be phosphorylated to glyceraldehyde-3-phosphate (Wu et al. 2014). Thus, the hypothesis is that the mixture of these two sugars may be more advantageous than single carbohydrate fermentation because it prevents carbon source depletion and can ensure the stability of hydrogen production.
Haroun et al. (2016), using separate carbon sources, glucose (10 g/L) and xylose (10 g/L), for H2 production in a continuous stirred tank reactor (CSTR) (37 °C) at an HRT of 8 h, observed a carbohydrate consumption higher than 97% for both substrates. Qiu et al. (2016) used xylose for thermophilic (55 °C) H2 production in batch experiments and observed xylose conversions above 96% for all concentrations (2.5, 5.0, 7.5, 10.0, and 12.5 g/L). Shida et al. (2012) used glucose (2 g/L) as a substrate for mesophilic (30 °C) hydrogen production in an AFBR to evaluate the effect of HRTs from 8 to 1 h and observed glucose conversions higher than 91%.
However, a lower consumption of xylose was observed in other studies that performed the fermentation of glucose and xylose as co-substrates similarly to AFBR-GX (Ren et al. 2008; Luo et al. 2017). Luo et al. (2017) performed the fermentation of glucose and xylose as co-substrates (total sugar concentration of 30 g/L) for the mesophilic (37 °C) production of butyric acid (HBu) in batch experiments and observed that at a 1:1 xylose:glucose ratio, xylose was only consumed after the total consumption of glucose in the medium. Zhao et al. (2018) used an anaerobic sequencing batch reactor (ASBR) for thermophilic (60 °C) H2 production from the co-substrates glucose and xylose (total sugar concentration of 10 g/L) and observed a glucose conversion of 90% and xylose conversion of 66% at a substrate ratio of 1:1.
Performance of hydrogen production in the thermophilic AFBRs
The hydrogen content (%H2) and HPR values of the AFBR-GX and AFBR-C are shown in Figs. 3 and 4, respectively. No methane was detected during the operation of both reactors, demonstrating the effectiveness of the strategies adopted to suppress methanogenic archaea, such as sludge thermal treatment and pH control below 5.0. In the AFBR-GX, the %H2 remained stable in the range between 55.0 ± 2.2 and 65.0 ± 3.0%, and the HPR increased from 76.0 ± 14.0 to 279.9 ± 38.8 mL H2/h L as the HRT decreased from 16 to 0.5 h. In the AFBR-C, the %H2 remained in the range between 41.6 ± 8.8 and 58.8 ± 3.8%, and the HPR increased from 5.5 ± 1.9 to 115.7 ± 20.1 mL H2/h L as the HRT decreased from 24 to 8 h.
Kongjan and Angelidaki (2010) investigated the production of H2 from wheat straw hydrolysate (total sugar concentration of 3.9 g/L) under extreme thermophilic conditions (70 °C) in the HRT of 24 h and 12 h and observed %H2 values equal to 32.6%, 41.5% and 43.2% for anaerobic filter (AF), CSTR and UASB reactors, respectively. Toledo-Cervantes et al. (2018) used an ASBR (37 °C) for H2 production from agave tequilana bagasse hydrolysate (total sugar concentration of 8.0 g COD/L) and observed a %H2 value of 49.3% at an HRT of 24 h. Kirli and Karapinar (2018) used an anaerobic packed bed reactor (APBR) for mesophilic (37 °C) H2 production from wheat residues (total sugar concentration of 5 g/L) and observed a %H2 increase from 55 to 67% by decreasing the HRT from 13 to 2 h. These results with complex lignocellulosic biomass hydrolysates from the literature are similar to the %H2 observed in AFBR-C using hydrolyzed microcrystalline cellulose, indicating the reliable application of this substrate as a model for lignocellulosic biomass.
Simulating a cellulosic hydrolysate with the mixture of glucose and xylose in the ratio of 1:1 (total sugar concentration of 5 g/L), Hniman et al. (2011) observed a %H2 value of 21% in batch experiments under thermophilic conditions (60 °C). The %H2 values observed by the authors were lower than those of the AFBR-GX. Li et al. (2010) also performed thermophilic (55 °C) hydrogen production in batch experiments from the mixture of glucose and xylose (total sugar concentration of 10 g/L) and observed %H2 values between 26 and 49%. Zhao et al. (2018) used an ASBR for thermophilic (60 °C) H2 production from the glucose (8 g/L) and xylose (2 g/L) mixture while decreasing the HRT from 24 to 6 h and observed a maximum %H2 value of 61% at the HRT of 12 h.
The increase in HPR may be attributed to increased substrate availability because of the increase in the organic loading rate (OLR) from 2.0 to 6.0 kg COD/m3 day in the AFBR-C and from 6.0 to 192.0 kg COD/m3 day in the AFBR-GX. This phenomenon was noted by other studies that observed an increase in the hydrogen production with increasing OLR (Arriaga et al. 2011; Alexandropoulou et al. 2018; Wang et al. 2019). Contreras-Dávila et al. (2017) used agave tequilana bagasse hydrolysate in mesophilic (37 °C) H2 production in a CSTR and reported an increase in the HPR from 82.5 to 105.4 mL H2/h L as the OLR increased from 39.9 to 52.2 kg COD/m3 day. Alexandropoulou et al. (2018), using food residues (21.3 g COD/L) for mesophilic (35 °C) H2 production in a CSTR reported that increasing the OLR from 42.6 to 127.8 kg COD/m3 day, by decreasing the HRT from 12 to 4 h, increased the HPR from 172.1 to 449.6 mL H2/h L.
Metabolic products and selectivity
The soluble metabolite concentrations and the hydrogen yield (HY) observed in the AFBR-GX and AFBR-C are shown in Figs. 5 and 6, respectively. In the AFBR-GX, the predominant metabolite at HRTs from 16 to 4 h was HLa, corresponding to the range between 20.3% and 44.6% of the total metabolites produced. By decreasing the HRT to 2 h, the HLa molar fraction decreased to 10.7%. At this HRT there was an increase in EtOH production, which corresponded to 56.7% of the metabolites produced. When the HRT was decreased to 1 h and 0.5 h, the molar fraction of EtOH was 44.0 and 50.2%, respectively. The HY, standardized in terms of hexose by the sum of glucose with its xylose equivalent (0.833 mol of glucose), remained constant (between 0.3 ± 0.1 and 0.4 ± 0.04 mol H2/mol hexose) as the HRT decreased from 16 to 4 h in the AFBR-GX. By further decreasing the HRT to 0.5 h, the HY decreased to 0.1 ± 0.01 mol H2/mol hexose.
In the AFBR-C, the predominant metabolites were acetic acid (HAc) (50.5–84.2%) and HBu (6.6–41.2%). As the HRT decreased from 12 to 8 h, the HAc molar fraction decreased from 84.2 to 15.9%, and the EtOH molar fraction increased from 5.1 to 58.9%. In the AFBR-C, the HY values were 0.6 ± 0.1, 0.6 ± 0.2, 0.4 ± 0.1 and 0.3 ± 0.1 mol H2/mol hexose at the HRTs of 24 h, 20 h, 16 h and 12 h, respectively. When the HRT decreased to 8 h, the HY increased to 1.1 ± 0.02 mol H2/mol hexose.
Silva et al. (2018) studied the production of bioproducts from xylose (1.29 g/L), glucose (1.06 g/L) and cellulose (1.18 g/L) in batch experiments using thermophilic (55 °C) UASB sludge from Usina São Martinho sugarcane mill (Pradópolis, São Paulo, Brazil). When using simple carbohydrates, such as glucose and xylose, EtOH production was favored in the first 8 h of the batch essays with concentrations of 1.22 g EtOH/L (glucose) and 1.39 g EtOH/L (xylose). On the other hand, the conversion of a complex lignocellulosic biomass (cellulose) favored the production of HAc (1.81 g/L). These results are similar to findings for AFBR-GX and AFBR-C. The main metabolite produced in AFBR-GX at HRTs of 2 h, 1 h and 0.5 h was EtOH, while in AFBR-C the HAc was favored in the HRT of 24 h.
In the AFBR-C, HAc and HBu molar fractions accounted for 82.3%, 92.8%, 91.6% and 95.0% of the total metabolites produced at the HRTs of 24 h, 20 h, 16 h and 12 h, respectively. At these HRTs, the concentrations of HAc remained between 0.16 and 0.58 g/L, and the concentrations of HBu remained between 0.08 and 0.19 g/L. In these conditions, the concentrations of furfural and 5-hydroxymethylfurfural were 1 μg/L and 7 μg/L, respectively. It is important to avoid high concentrations of these components because of their inhibitory effect to hydrogen production (Pattra et al. 2008). Similarly, Pattra et al. (2008) observed as the predominant metabolites HAc (1.46 g COD/L) and HBu (6.77 g COD/L) in mesophilic (37 °C) batch experiments for H2 production from sugarcane bagasse hydrolysate (4.86 g/L of glucose and 5.34 g/L of xylose) with a maximum HY of 1.7 mol H2/mol hexose. The authors attributed the result to the low concentration of furfural.
Despite the inhibitory effect of furfural and 5-hydroxymethylfurfural on hydrogen production in acidogenic reactors, Haroun et al. (2016) concluded that the adaptation of the reactor biomass may increase tolerance to these compounds. The authors used glucose (10 g/L) and xylose (10 g/L) separately for mesophilic (37 °C) H2 production in a CSTR and observed constant values for the production of HAc (1.5–2.6 g/L using glucose and 1.6–2.6 g/L using xylose) and HBu (2.1–2.5 g/L using glucose and 1.8–2.6 g/L using xylose) while increasing the concentrations of furfural and 5-hydroxymethylfurfural from 0.25 to 4.0 g/L.
Contreras-Dávila et al. (2017) observed that HAc (1.5–3.1 g/L) and HBu (1.4–4.5 g/L) were the predominant metabolites at OLRs from 17.3 to 52.9 kg COD/m3 day in the mesophilic (37 °C) H2 production from agave tequilana bagasse hydrolysate in a CSTR. Jamali et al. (2016) studied the thermophilic (60 °C) H2 production from the mixture of xylose and glucose (total sugar concentration of 55.03 g COD/L) in batch experiments and observed an HY of 1.8 mol H2/mol carbohydrates, coinciding with the predominance of HAc (1.12 g/L) and HBu (1.86 g/L). Zhao et al. (2018) studied the effect of HRT (6–24 h) on thermophilic (60 °C) H2 production from the mixture of 8 g glucose/L and 2 g xylose/L in an ASBR and observed a predominance of HAc (1.17–3.02 g/L) and HBu (1.22–2.16 g/L) under all operational conditions. However, in the AFBR-GX, the HAc concentrations (0.18–0.25 g/L) were lower than the HLa concentrations (0.29–0.65 g/L) at HRTs from 16 to 4 h, indicating a predominance of HLa over HAc.
At higher HRTs, there is greater exposure of the substrates to the biomass, causing an accumulation of pyruvate and triggering the production of HLa (Baghchehsaraee et al. 2009). The fermentative production of HLa can be initiated according to the CO2 levels of the system and does not affect the HY (d’Ippolito et al. 2014). This phenomenon explains the stability of the H2 production at HRTs ranging from 12 to 4 h in the AFBR-GX. Under these the operational conditions the higher concentrations of HLa were observed. Baghchehsaraee et al. (2009) reported that following the catabolism of HLa, there is an increase in NADH levels because of lactate accumulation, which can subsequently act as a precursor to hydrogen production. Alexandropoulou et al. (2018) studied the mesophilic (35 °C) H2 production from food residues (total sugar concentration of 12.43 g/L) in a CSTR and observed HAc concentrations (1.83 and 1.57 g/L) that were lower than the HLa concentrations (2.89 and 3.03 g/L), with maximum HY values of 2.2 and 2.5 mol H2/mol carbohydrates at the HRTs of 6 h and 4 h, respectively.
Obligate heterolactic microorganisms can convert pentoses [Eq. (1)] and hexoses [Eq. (2)] to HLa and HAc. When no pentoses are available as carbon sources, hexoses can also be converted into HLa and EtOH [Eq. (3)] (Cubas-Cano et al. 2018). This process may explain why HAc and HLa make up the largest molar fraction in the AFBR-GX at HRTs from 16 to 8 h, where the xylose conversion remained between 26.5 and 56.7%. At HRTs from 2 to 0.5 h, the xylose conversion remained between 7.3 and 11.1%, and EtOH became the predominant metabolite.
Because the EtOH concentration increased to 0.46 g/L and the HY was constant with a value of 0.2 mol H2/mol hexose at the HRT of 2 h, the results of this study cannot exclude the potential occurrence of ethanol-type fermentation, in which there is the simultaneous production of H2, EtOH and HAc [Eq. (4)] (Barros and Silva 2012).
Wang et al. (2013) evaluated the mesophilic (25 °C) H2 production from sugarcane molasses (8 g COD/L) in a CSTR and observed an increase in the EtOH concentration from 1.49 to 3.82 g COD/L, the simultaneous production of HAc (0.44–0.66 g COD/L) and an increase in the HPR to 0.31 mL H2/h L by decreasing the HRT from 10 to 5 h. Anzola-Rojas et al. (2016) evaluated the mesophilic (25 °C) hydrogen production from sucrose in an anaerobic downflow structured bed reactor (ASTBR) and observed an increase in the EtOH molar fraction from 29 to 61% and a constant HAc molar fraction (21 and 24%) as the OLR increased from 12.0 to 96.0 kg COD/m3 day. The authors indicated that the pH of 4.5 favored ethanol-type fermentation.
Ethanol-type fermentation is favored in pH values between 4.0–4.5 and can preserve the balance between NAD+ and NADH, restrict the production of other metabolites and promote stable hydrogen productivity (Ren et al. 2007). In the AFBR-GX, this pathway was possibly present at HRTs of 2 h, 1 h and 0.5 h, where the pH was maintained between 4.2 and 4.5. The pH of 4.0 in AFBR-C favored the production of EtOH (0.48 g/L; 58.9%) and HAc (0.17 g/L; 15.9%) as the HRT was decreased from 12 to 8 h. This metabolic pathway resulted in a higher HY of 1.1 mol H2/mol hexose.
Barros and Silva (2012) studied mesophilic (23 °C) H2 production from glucose (4 g/L) in an AFBR with a pH maintained between 3.40 and 3.99 and observed that EtOH was the predominant metabolite. Additionally, the decrease in the HRT from 8 to 2 h increased the HY from 0.7 to 1.3 mol H2/mol hexose and increased the EtOH concentration from approximately 0.7–1.3 g/L. Similarly, Zhao et al. (2013) performed the fermentation of 10 g/L of glucose and 10 g/L of xylose as co-substrates in a hyperthermophilic (70 °C) UASB reactor with a pH maintained between 5 and 6. The authors observed that a decrease in the HRT from 24 to 12 h increased the HY from 0.2 to 0.6 mol H2/mol hexose and increased the EtOH concentration from 2.23 to 3.71 g/L.
Qiu et al. (2017) evaluated the effect of pH and substrate concentration on the hyperthermophilic (70 °C) H2 production from xylose in batch experiments and observed that the maximum HY of 1.1 mol H2/mol hexose from 7.5 g xylose/L in the pH 7.0 coincided with the predominance of EtOH in the liquid phase (2.82–3.61 g/L). Reis and Silva (2014), using glucose (3.5 g/L) for mesophilic (25 °C) H2 production in an AFBR, observed a predominance of EtOH in the liquid phase (31–57% molar fraction) and an increase in the HY from 1.2 to 2.2 mol H2/mol hexose by decreasing the HRT from 8 to 1 h.
Table 1 presents a comparison of the main results for hydrogen production from the reactors in the present study and other studies that used continuous reactors for the fermentation of glucose and xylose as mono-substrates and as co-substrates, including hydrolyzed biomass.
Despite the low concentration of the influent substrate to the AFBR-C, the HY and HPR observed in this reactor were higher than those observed in the study by Kumar et al. (2018) using hydrolyzed algae in a CSTR (35 °C) (Table 1). The superior HPR and HY of AFBR-C can be explained by the maintenance of the temperature in the thermophilic range. High temperatures lead to higher selectivity for the hydrogen-producing microorganisms, resulting in higher hydrogen productivity in the thermophilic range in comparison to the mesophilic range (Dessì et al. 2018). Gadow et al. (2012) studied the production of H2 from cellulose in a CSTR and observed HY values equal to 0.1, 2.7 and 3.4 mol H2/mol hexose at temperatures of 37, 55 and 80 °C, respectively.
Additionally, Dessì et al. (2018) used a fluidized bed reactor (FBR) under thermophilic conditions (55 °C) for H2 production from xylose mono-fermentation (7.5 g/L) and observed a HY of 1.0 mol H2/mol hexose, which is superior to the value obtained with the AFBR-GX at the HRT of 0.5 h (0.1 mol H2/mol hexose). However, at the HRTs of 6 and 0.5 h, the HPRs in the study of Dessì et al. (2018) and the AFBR-GX were equal to 282.1 mL H2/h L and 279.9 mL H2/h L, respectively. In addition to the similarity between the HPRs in these two studies, the use of glucose and xylose as co-substrates may be advantageous over mono-fermentation because of the total energy gain from the simultaneous production of EtOH and H2 in AFBR-GX.
In practical terms, the effect of decreasing the HRT on continuous hydrogen production was similar when using hydrolyzed microcrystalline cellulose (AFBR-C) and the mixture of glucose and xylose (AFBR-GX) as model substrates for hydrolyzed lignocellulosic biomass. The results observed in AFBR-C and AFBR-GX were also similar to literature studies using different lignocellulosic materials for hydrogen production. This indicates that the results of AFBR-GX and AFBR-C can be used as a comparative base for studies using hydrolyzed lignocellulosic materials as substrates for continuous hydrogen production. The decrease in HRT favored the selection of metabolic pathways for H2 and EtOH production using mixed cultures, which are easier to apply in full-scale anaerobic reactors.
Energetic evaluation
Using the data on the EtOH and H2 production, energy productivity (EP) calculations were performed for the AFBR-GX and AFBR-C according to Han et al. (2012). The calculations were based on the heat of combustion values for hydrogen (286 kJ/mol) and EtOH (1366 kJ/mol). The AFBR-C reached a maximum EP of 7.4 kJ/h L at the HRT of 8 h. The AFBR-GX achieved a maximum EP of 47.7 kJ/h L at the HRT of 0.5 h and the lowest EP of 0.91 kJ/h L at the HRT of 12 h.
The EP values of the AFBR-GX in the present study were superior to those of other studies that used acidogenic cultures (Table 2), and the highest EP values were observed at the lowest HRTs of 0.5 h for AFBR-GX and 8 h for AFBR-C. In these operational conditions, the maximum values of HPR were also observed in each reactor. Zhao et al. (2013) observed a maximum EP of 10.7 kJ/h L at an HRT of 12 h by using glucose and xylose (10 g/L each) as co-substrates for the simultaneous production of EtOH and H2 in a hyperthermophilic (70 °C) UASB reactor. Han et al. (2012) observed a maximum EP of 31.2 kJ/h L in the mesophilic (35 °C) fermentation of molasses (10 g COD/L) for the simultaneous production of EtOH and H2 in a CSTR. Santos et al. (2014) observed a maximum EP of 4.9 kJ/h L at an HRT of 1 h when using sugarcane vinasse (5 g COD/L) for thermophilic (55 °C) H2 production in an AFBR.
Conclusion
The results of this study indicate the technical feasibility of continuous H2 and EtOH production from lignocellulosic biomass in AFBR using cellulosic hydrolysate and the mixture of glucose and xylose as model substrates. The simultaneous production of H2 and EtOH was confirmed in both reactors with the decrease in the HRT from 16 to 0.5 h in AFBR-GX and from 24 to 8 h in AFBR-C. The decrease in HRT applied favored EtOH production in both reactors, observing high EtOH molar fractions of 58.9% in AFBR-C and 50.2% in AFBR-GX in the HRT of 8 h and 0.5 h, respectively. However, each reactor exhibited different effects of HRT on HY during solventogenesis. In the AFBR-GX, the HY decreased from 0.4 to 0.1 mol H2/mol hexose as the HRT decreased from 16 to 0.5 h. On the other hand, the HY in AFBR-C increased from 0.3 to 1.1 mol H2/mol hexose by decreasing the HRT from 24 to 8 h. The maximum HPR observed in AFBR-GX was 279 mL H2/h L, coincident with the highest EP (47.7 kJ/h L) and an EtOH concentration of 0.22 g/L at the lowest HRT applied (0.5 h). In the AFBR-C, the highest HPR of 115 mL H2/h L and the highest EP (7.4 kJ/h L) were also observed at the lowest HRT applied (8 h), with an EtOH concentration of 0.48 g/L. Regarding the comparative approach between the results of the present study with model substrates it is fair to affirm that the fermentative performance of AFBR-GX and AFBR-C can be used in future researches of biohydrogen production from most diverse lignocellulosic biomass.
References
Alexandropoulou M, Antonopoulou G, Trably E et al (2018) Continuous biohydrogen production from a food industry waste: influence of operational parameters and microbial community analysis. J Clean Prod 174:1054–1063. https://doi.org/10.1016/j.jclepro.2017.11.078
APHA (2005) Standards methods for the examination of water and wastewater, twenty-first ed. American Public Health Association/American Water Works Association/Water Environmental Federation, Washington, DC
Araújo DJC, Machado AV, Vilarinho MCLG (2018) Availability and suitability of agroindustrial residues as feedstock for cellulose-based materials: Brazil case study. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-018-0291-0
Arriaga S, Rosas I, Alatriste-Mondragón F, Razo-Flores E (2011) Continuous production of hydrogen from oat straw hydrolysate in a biotrickling filter. Int J Hydrog Energy 36:3442–3449. https://doi.org/10.1016/j.ijhydene.2010.12.019
Baghchehsaraee B, Nakhla G, Karamanev D, Margaritis A (2009) Effect of extrinsic lactic acid on fermentative hydrogen production. Int J Hydrog Energy 34:2573–2579. https://doi.org/10.1016/j.ijhydene.2009.01.010
Barros AR, Silva EL (2012) Hydrogen and ethanol production in anaerobic fluidized bed reactors: performance evaluation for three support materials under different operating conditions. Biochem Eng J 61:59–65. https://doi.org/10.1016/j.bej.2011.12.002
Cao G, Ren N, Wang A et al (2009) Acid hydrolysis of corn stover for biohydrogen production using Thermoanaerobacterium thermosaccharolyticum W16. Int J Hydrog Energy 34:7182–7188. https://doi.org/10.1016/j.ijhydene.2009.07.009
Contreras-Dávila CA, Méndez-Acosta HO, Arellano-García L et al (2017) Continuous hydrogen production from enzymatic hydrolysate of Agave tequilana bagasse: effect of the organic loading rate and reactor configuration. Chem Eng J 313:671–679. https://doi.org/10.1016/j.cej.2016.12.084
Cubas-Cano E, González-Fernández C, Ballesteros M, Tomás-Pejó E (2018) Biotechnological advances in lactic acid production by lactic acid bacteria: lignocellulose as novel substrate. Biofuels Bioprod Biorefin 12:290–303. https://doi.org/10.1002/bbb.1852
d’Ippolito G, Dipasquale L, Fontana A (2014) Recycling of carbon dioxide and acetate as lactic acid by the hydrogen-producing bacterium Thermotoga neapolitana. Chemsuschem 7:2678–2683. https://doi.org/10.1002/cssc.201402155
del Anzola-Rojas MP, Zaiat M, De Wever H (2016) Improvement of hydrogen production via ethanol-type fermentation in an anaerobic down-flow structured bed reactor. Bioresour Technol 202:42–49. https://doi.org/10.1016/j.biortech.2015.11.084
Dessì P, Porca E, Waters NR et al (2018) Thermophilic versus mesophilic dark fermentation in xylose-fed fluidised bed reactors: biohydrogen production and active microbial community. Int J Hydrog Energy 43:5473–5485. https://doi.org/10.1016/j.ijhydene.2018.01.158
dos Reis CM, Silva EL (2014) Simultaneous coproduction of hydrogen and ethanol in anaerobic packed-bed reactors. Biomed Res Int 2014:1–10. https://doi.org/10.1155/2014/921291
Dubois M, Gilles KA, Hamilton JK et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Gadow SI, Li YY, Liu Y (2012) Effect of temperature on continuous hydrogen production of cellulose. Int J Hydrog Energy 37:15465–15472. https://doi.org/10.1016/j.ijhydene.2012.04.128
Gouveia ER, Do Nascimento RT, Souto-Maior AM, de Rocha GJM (2009) Validação de metodologia para a caracterização química de bagaço de cana-de-açúcar. Quim Nova 32:1500–1503. https://doi.org/10.1590/s0100-40422009000600026(in Portuguese)
Han W, Chen H, Jiao A et al (2012) Biological fermentative hydrogen and ethanol production using continuous stirred tank reactor. Int J Hydrog Energy 37:843–847. https://doi.org/10.1016/j.ijhydene.2011.04.048
Han W, Hu Y, Li S et al (2017) Simultaneous dark fermentative hydrogen and ethanol production from waste bread in a mixed packed tank reactor. J Clean Prod 141:608–611. https://doi.org/10.1016/j.jclepro.2016.09.143
Haroun BM, Nakhla G, Hafez H, Nasr FA (2016) Impact of furfural on biohydrogen production from glucose and xylose in continuous-flow systems. Renew Energy 93:302–311. https://doi.org/10.1016/j.renene.2016.02.072
Hniman A, Prasertsan P, O-Thong S (2011) Community analysis of thermophilic hydrogen-producing consortia enriched from Thailand hot spring with mixed xylose and glucose. Int J Hydrog Energy 36:14217–14226. https://doi.org/10.1016/j.ijhydene.2011.05.087
IBGE (2018) Brazilian institute of geography and statistics. In: Syst. Surv. Agric. Prod. https://www.ibge.gov.br. Accessed 11 Dec 2018 (in Portuguese)
Jamali NS, Md Jahim J, Wan Isahak WNR (2016) Biofilm formation on granular activated carbon in xylose and glucose mixture for thermophilic biohydrogen production. Int J Hydrog Energy 41:21617–21627. https://doi.org/10.1016/j.ijhydene.2016.05.092
Kim DH, Han S-K, Kim S-H, Shin H-S (2006) Effect of gas sparging on continuous fermentative hydrogen production. Int J Hydrog Energy 31:2158–2169. https://doi.org/10.1016/j.ijhydene.2006.02.012
Kirli B, Karapinar I (2018) The effect of HRT on biohydrogen production from acid hydrolyzed waste wheat in a continuously operated packed bed reactor. Int J Hydrog Energy 43:10678–10685. https://doi.org/10.1016/j.ijhydene.2018.01.175
Kongjan P, Angelidaki I (2010) Extreme thermophilic biohydrogen production from wheat straw hydrolysate using mixed culture fermentation: effect of reactor configuration. Bioresour Technol 101:7789–7796. https://doi.org/10.1016/j.biortech.2010.05.024
Kumar G, Sivagurunathan P, Anburajan P et al (2018) Continuous biogenic hydrogen production from dilute acid pretreated algal hydrolysate using hybrid immobilized mixed consortia. Int J Hydrog Energy 43:11452–11459. https://doi.org/10.1016/j.ijhydene.2017.06.050
Li S, Lai C, Cai Y et al (2010) High efficiency hydrogen production from glucose/xylose by the ldh-deleted Thermoanaerobacterium strain. Bioresour Technol 101:8718–8724. https://doi.org/10.1016/j.biortech.2010.06.111
Liu H, Hu H, Jin Y et al (2017) Co-fermentation of a mixture of glucose and xylose to fumaric acid by Rhizopus arrhizus RH 7-13-9. Bioresour Technol 233:30–33. https://doi.org/10.1016/j.biortech.2017.02.035
Luo G, Zhang L, Chen T et al (2017) Butyric acid fermentation in xylose and glucose by Clostridium tyrobutyricum. BioResources 12:2930–2940. https://doi.org/10.15376/biores.12.2.2930-2940
Nielsen F, Zacchi G, Galbe M, Wallberg O (2017) Sequential targeting of xylose and glucose conversion in fed-batch simultaneous saccharification and co-fermentation of steam-pretreated wheat straw for improved xylose conversion to ethanol. BioEnergy Res 10:800–810. https://doi.org/10.1007/s12155-017-9841-8
Nissilä ME, Li Y-C, Wu S-Y et al (2012) Hydrogenic and methanogenic fermentation of birch and conifer pulps. Appl Energy 100:58–65. https://doi.org/10.1016/j.apenergy.2012.06.015
Novy V, Brunner B, Nidetzky B (2018) l-Lactic acid production from glucose and xylose with engineered strains of Saccharomyces cerevisiae: aeration and carbon source influence yields and productivities. Microb Cell Fact 17:59. https://doi.org/10.1186/s12934-018-0905-z
Pattra S, Sangyoka S, Boonmee M, Reungsang A (2008) Bio-hydrogen production from the fermentation of sugarcane bagasse hydrolysate by Clostridium butyricum. Int J Hydrog Energy 33:5256–5265. https://doi.org/10.1016/j.ijhydene.2008.05.008
Qiu C, Zheng Y, Zheng J et al (2016) Mesophilic and thermophilic biohydrogen production from xylose at various initial pH and substrate concentrations with microflora community analysis. Energy Fuels 30:1013–1019. https://doi.org/10.1021/acs.energyfuels.5b02143
Qiu C, Shi P, Xiao S, Sun L (2017) Effects of pH and substrate concentrations on dark fermentative biohydrogen production from xylose by extreme thermophilic mixed culture. World J Microbiol Biotechnol 33:7. https://doi.org/10.1007/s11274-016-2178-1
Ren N, Xing D, Rittmann BE et al (2007) Microbial community structure of ethanol type fermentation in bio-hydrogen production. Environ Microbiol 9:1112–1125. https://doi.org/10.1111/j.1462-2920.2006.01234.x
Ren N, Cao G, Wang A et al (2008) Dark fermentation of xylose and glucose mix using isolated Thermoanaerobacterium ihermosaccharolyticum W16. Int J Hydrog Energy 33:6124–6132. https://doi.org/10.1016/j.ijhydene.2008.07.107
Santos SC, Rosa PRF, Sakamoto IK et al (2014) Continuous thermophilic hydrogen production and microbial community analysis from anaerobic digestion of diluted sugar cane stillage. Int J Hydrog Energy 39:9000–9011. https://doi.org/10.1016/j.ijhydene.2014.03.241
Saripan AF, Reungsang A (2014) Simultaneous saccharification and fermentation of cellulose for bio-hydrogen production by anaerobic mixed cultures in elephant dung. Int J Hydrog Energy 39:9028–9035. https://doi.org/10.1016/j.ijhydene.2014.04.066
Shida GM, Sader LT, de Amorim ELC et al (2012) Performance and composition of bacterial communities in anaerobic fluidized bed reactors for hydrogen production: effects of organic loading rate and alkalinity. Int J Hydrog Energy 37:16925–16934. https://doi.org/10.1016/j.ijhydene.2012.08.140
Silva V, Ratti RP, Sakamoto IK et al (2018) Biotechnological products in batch reactors obtained from cellulose, glucose and xylose using thermophilic anaerobic consortium. Renew Energy 125:537–545. https://doi.org/10.1016/j.renene.2018.02.124
Sittijunda S, Tomás AF, Reungsang A et al (2013) Ethanol production from glucose and xylose by immobilized Thermoanaerobacter pentosaceus at 70 °C in an up-flow anaerobic sludge blanket (UASB) reactor. Bioresour Technol 143:598–607. https://doi.org/10.1016/j.biortech.2013.06.056
Toledo-Cervantes A, Arreola-Vargas J, Elias-Palacios SV et al (2018) Evaluation of semi-continuous hydrogen production from enzymatic hydrolysates of Agave tequilana bagasse: insight into the enzymatic cocktail effect over the co-production of methane. Int J Hydrog Energy 43:14193–14201. https://doi.org/10.1016/j.ijhydene.2018.05.134
Walker M, Zhang Y, Heaven S, Banks C (2009) Potential errors in the quantitative evaluation of biogas production in anaerobic digestion processes. Bioresour Technol 100:6339–6346. https://doi.org/10.1016/j.biortech.2009.07.018
Wang B, Li Y, Ren N (2013) Biohydrogen from molasses with ethanol-type fermentation: effect of hydraulic retention time. Int J Hydrog Energy 38:4361–4367. https://doi.org/10.1016/j.ijhydene.2013.01.120
Wang Y, Joshee N, Cao W et al (2019) Continuous hydrogen production by dark and photo co-fermentation using a tubular multi-cycle bio-reactor with Pawlonia biomass. Cellulose. https://doi.org/10.1007/s10570-019-02468-z
Wu K-J, Chang C-F, Chang J-S (2007) Simultaneous production of biohydrogen and bioethanol with fluidized-bed and packed-bed bioreactors containing immobilized anaerobic sludge. Process Biochem 42:1165–1171. https://doi.org/10.1016/j.procbio.2007.05.012
Wu XB, Huang GF, Bai LP et al (2014) Enhanced hydrogen production from xylose and bamboo stalk hydrolysate by overexpression of xylulokinase and xylose isomerase in Klebsiella oxytoca HP1. Int J Hydrog Energy 39:221–230. https://doi.org/10.1016/j.ijhydene.2013.10.078
Zhao C, Lu W, Wang H (2013) Simultaneous hydrogen and ethanol production from a mixture of glucose and xylose using extreme thermophiles II: effect of hydraulic retention time. Int J Hydrog Energy 38:9701–9706. https://doi.org/10.1016/j.ijhydene.2013.05.041
Zhao L, Guo W-Q, Guo X-C et al (2018) Continuous hydrogen production from glucose/xylose by an anaerobic sequential batch reactor to maximize the energy recovery efficiency. RSC Adv 8:20712–20718. https://doi.org/10.1039/c8ra02991a
Acknowledgments
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico—Brasil (CNPq) (process number 306455/2015-3), and Fundação de Amparo à Pesquisa do Estado de São Paulo—Brasil (FAPESP) (Grant No. 15/06246-7).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lopes, H.J.S., Ramos, L.R., de Menezes, C.A. et al. Simultaneous hydrogen and ethanol production in a thermophilic AFBR: a comparative approach between cellulosic hydrolysate single fermentation and the fermentation of glucose and xylose as co-substrates. Cellulose 27, 2599–2612 (2020). https://doi.org/10.1007/s10570-020-03000-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03000-4