Abstract
Self-standing and flexible regenerated cellulose membranes were fabricated by using a facile ZnCl2/CaCl2/cellulose dissolution system. The filtration experiments of the membranes were conducted by using methyl orange and Congo red as water contaminations. To improve the selectivity and stability, the crosslinkers of glutaraldehyde and polyvinyl alcohol were introduced to the membrane network. The best performance was observed over glutaraldehyde and polyvinyl alcohol crosslinked cellulose (P-G-RC) membrane with a Congo red rejection rate of 99.9% and a methyl orange rejection rate of 93.5%. The suitable pore radius (0.67 nm), low root-mean-square roughness (2.9 nm) and abundant hydroxyl groups were considered as the primary reasons contributed to the separation behavior. Furthermore, the P-G-RC membrane displayed excellent stability and reusability during the filtration tests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The dye industry in China possesses more than 70% world production and generates a large amount of wastewater (Chen and He 2017; Gu et al. 2018; Vergis et al. 2018; Zhao et al. 2018; Khoshsang et al. 2018). Among the various methods for dye-containing wastewater treatment, membrane-based technology has attracted great attention and is emerging as the most promising alternative to adsorption, precipitation and chemical degradation methods. However, the charge and molecular size of dyes will influence the permeability and selectivity of membranes in the process of filtration/separation (Feng et al. 2017; Pan et al. 2018; Park and Oh 2017; Robati et al. 2016a, b, c). Conventional ceramic membranes are fragile and also have high molecular weight cut-off, which may lead to a lower rejection of small molecular weight dyes and limit their practical application (Xia et al. 2018).
Cellulose is a well-known biopolymer and is promising in constructing membrane materials (Zhang et al. 2017, 2018b; Khalili et al. 2018; Koli et al. 2018). The growing interest of cellulose for membrane fabrication lies on its bio-compatibility, flexibility, abundant availability and chemical modifiability (Chen et al. 2018; Moon et al. 2011). Particularly, the regenerated cellulose membranes are promising alternatives to polymeric (e.g. polyethersulfone, polyimide and Pebax) membranes used for treating wastewater (Kong et al. 2014; Lv et al. 2018; Puspasari et al. 2018; Štefelová et al. 2017). Weng and co-workers fabricated regenerated cellulose membrane via cellulose/ionic liquid system and the membrane has a Congo red rejection rate of 99.7% (Weng et al. 2017). However, the dyes will be adsorbed/deposited on the membrane surface in practical application, resulting in the decrease of flux and selectivity. Peinemann et al. crosslinked cellulose with glutaraldehyde and the prepared membrane has a sucrose rejection rate of 80% (Puspasari et al. 2015). They also found that a dense selective layer of membrane was constructed to achieve a high rejection of dyes.
Recently, an inorganic salt system (zinc chloride/calcium chloride) was reported for dissolving cellulose to fabricate cellulose membranes for gas separation (Xu et al. 2016). Herein, we prepared self-standing regenerated cellulose membranes from cellulose/inorganic salt system. To improve the rejection rate of membranes, glutaraldehyde and polyvinyl alcohol was used to crosslink cellulose and optimize the pore sizes and stability. Glutaraldehyde has a superior crosslink ability and polyvinyl alcohol exhibits abundant hydroxyl groups. The resulting membranes also exhibited excellent flexibility, good transparency and high mechanical strength.
Experimental
Materials
In this study, α-cellulose was bought from Aladdin Chemical Reagent Company. ZnCl2 (99%) and CaCl2 (99%) were supplied by Sinopharm Chemical Reagent Company. Glutaraldehyde, polyvinyl alcohol (PVA, DP 1750 ± 50), methyl orange (MO, Mw = 327.3 Da, λmax = 505 nm), and Congo red (CR, Mw = 696.7 Da, λmax = 498 nm) were obtained from Sinopharm Chemical Reagent Company. The chemicals were used as received without further purification.
Preparation of regenerated cellulose membranes
The regenerated cellulose membrane without crosslinker was fabricated according to the phase-inversion method described previously with a slight modification (Zhang et al. 2018b). In brief, 9.87 g of ZnCl2 and 0.2 g of CaCl2 were dissolved in deionized water (3.63 g) at 65 °C for 15 min. Simultaneously, 0.45 g of α-cellulose and 1.05 g of deionized water were mixed to obtain cellulose suspension. Then, the ZnCl2/CaCl2 solution was poured into the cellulose suspension and stirred for another 45 min. The solution was degassed under vacuum for 30 min to remove bubbles and casted by a knife (200 μm) over a glass plate. The glass plate was fully immersed into 500 mL of ethanol for 30 min to separate the membrane from the plate. The obtained membrane was fixed on a metallic frame and dried at 25 °C. The membrane was cut into rectangular strips (8 × 5 cm2) for tests and denoted as RC.
To change the pore sizes and surface properties of regenerated cellulose membrane, the crosslinkers of glutaraldehyde and PVA were introduced. The RC membrane was soaked into glutaraldehyde solution (5 wt%) at 70 °C for 2 h and dried at 25 °C. The inorganic salts were removed at the same time. The resulting membrane was denoted as G-RC. The PVA/α-cellulose membrane was prepared by the same procedure as that of RC membrane except the addition of PVA solution (PVA was dissolved in hot water at 90 °C for 60 min) in α-cellulose suspension in advance (the mass ratio of PVA to α-cellulose is 1:30). The resulting PVA/α-cellulose membrane was further immersed into glutaraldehyde solution to obtain P-G-RC membrane.
Membrane characterization
The hydrophilicity of the membranes was determined by water contact angle (Kruss DSA100). X-ray diffraction (XRD) experiments were conducted by Rigaku Ultima IV with Cu Kα radiation (λ = 0.1542 nm) at 40 kV and 30 mA. ATR characterization of the membranes was carried out on a Brucker Vertex-80 V spectrometer collected over the range of 600–4000 cm−1. The tensile tests were measured by a tensile tester (SANS CMT4000) at a crosshead speed of 30 mm/min. The specimen gauge lengths were 30 mm and the specimen widths were 10 mm. The surface and cross-section morphologies were observed by field emission scanning electron microscope (SEM, JSM-7600F, JEOL Ltd., Japan). Atomic force microscopy (AFM) images were recorded by Nanoscope IIIa controller (Dimension Edge, Bruker, Germany) operated in tapping mode with a silicon nitride cantilever tip.
The porosity of the membranes was determined via gravimetric method as expressed in the following Eq. (1) (Shen et al. 2016; Wang et al. 2014).
where Ww and Wd are the weight (g) of membranes in wet and dry state, respectively. ρH is the density of water (0.998 g/cm3). ρc is the density of cellulose (1.5 g/cm3).
The mean pore radius (rf) of the membranes was calculated by employing Eq. (2), following the straight through cylindrical pore model (Hamid et al. 2011; Sirus et al. 2014).
where η is the water viscosity (8.9 × 10−4 Pa s), L is the membrane thickness (m), J is the permeation flux (m3/(m2 s)) and ΔP is the load pressure (Pa).
Filtration tests
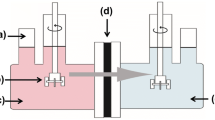
Water flux and dye rejection measurements were performed by a dead end filtration cell system (Fig. 1) with an effective membrane area of 19.63 cm2 at 0.2 MPa and 25 °C. MO and CR (10 mg/L) solutions were tested as water pollutants. Prior to tests, the membranes were pre-pressured at 0.25 MPa for 30 min with deionzed water to reach a steady state. After that, dye solution was added for the filtration process. The permeate flux (J) was calculated as Eq. (3).
where J is the permeate flux (L/m2 h), ΔV is the permeate volume (L) at the time interval of Δt (1 h), A is the effective area of the membrane (m2).
The rejection rate (R) was calculated as Eq. (4).
where Cp (mg/L) and Cf (mg/L) are the concentrations of the permeate and the feed solutions (Puspasari et al. 2015; Zhao and Wang 2017). The concentration of water-soluble dyes was measured by a UV–Vis spectrophotometer (UV-2600, Shimadzu, Japan). The pH value of 10 mg/L Congo red solution was adjusted to 6.0, and the full wavelength scanning curve was carried out in the range of 200–800 nm. The maximum absorption wavelength of the Congo red solution was measured to be 498 nm. By adjusting the pH of 10 mg/L methyl orange solution to 5.0, the maximum absorption wavelength of methyl orange solution was determined to be 505 nm. The solution concentration was obtained by substituting the absorbance value into the standard curve.
Continuous filtration and stability measurement
The stability of the membranes was conducted by continuous filtration tests. First, deionzed water was filtered through the membrane to reach a stabilization. Before filtration process, pure water was permeated across the membrane for about 1 h and the averaged flux was calculated as \(J_{w0}\). The feed solution was then changed to CR or MO solutions and kept for 6 h. The final flux was denoted as Jp. The stability was analyzed by testing the change in the flux of membranes. The total flux loss (\(J_{tot}\)) was calculated as Eq. (5).
Results and discussions
Characterization of membranes
As illustrated in Fig. 2, self-standing regenerated cellulose membranes exhibit high transparency and good flexibility. The colors of G-RC and P-G-RC membranes are slight yellow because of the addition of glutaraldehyde. The water contact angles were measured to explore the surface hydrophilicity of the membranes. The inset in Fig. 2a indicates that the pristine RC membrane is hydrophilic with a contact angle of 34.6°. After glutaraldehyde modification, G-RC membrane shows a higher water contact angle of 77.9° (Fig. 2b, inset). This is due to the hydrophilic hydroxyl groups are partly cross-linked by the aldehyde groups of glutaraldehyde. However, the addition of PVA reduces the water contact angle of P-G-RC to 48.4° (Fig. 2c, inset), which can be ascribed to the abundant hydrophilic hydroxyl groups in PVA molecules. According to prior studies, the hydrophilicity properties could influence the water flux and rejection rates of the membranes during filtration tests (Ning et al. 2017).
The crystalline structure of membranes was characterized by XRD analysis (Fig. 3a). The pristine α-cellulose powder corresponds to cellulose I topology, presenting typical characteristic peaks at 15.2°, 16.7° and 23.1° (Yang et al. 2017b) (details in Fig. S1). The RC membrane displays peaks at approximately 12.0° and 22.5°, indicating the crystal transformation from cellulose I to cellulose II during the regeneration process (French 2014; Gupta and Jiang 2015; Tanaka et al. 2018; Xu et al. 2018a). The inorganic salt system (ZnCl2/CaCl2 solution) employed in this study is effective to dissolve cellulose. The intensity and peak locations of G-RC and P-G-RC are very close to RC, proving that the crosslink reaction exerts no significant influence on the crystal patterns (Xu et al. 2018b, c).
To explore the effects of glutaraldehyde and PVA on the bonding configuration of cellulose, ATR experiments were conducted. As presented in Fig. 3b, RC membrane exhibits a strong peak around 3300–3350 cm−1, which is assigned to the O–H stretching. The peak centered at 2890 cm−1 is attributed to the C–H stretching. The spectrum of G-RC membrane has a higher peak intensity at 1050–1150 cm−1, which is ascribed to C–O stretching (Zhang et al. 2018a). This proves that glutaraldehyde is linked to cellulose chains and the double bonds (C=O) are open. Previous literature also reported that glutaraldehyde is appropriate to crosslink polysaccharide (Luo et al. 2018; Yang et al. 2017a). The linkages occurred between the reactive C-6 cellulosic hydroxyl groups of cellulose and the hydrated regions of glutaraldehyde (Lin et al. 2017; Pan et al. 2018). After PVA addition, the peak near 1047 cm−1 over P-G-RC became weaker compared with G-RC, indicating the re-formation of hydrogen bonds (Cai et al. 2018).
In addition, all samples display high tensile strength and strain-to-failure values (Fig. 3c). Sample RC possesses a tensile strength of 24.9 MPa and a strain-to-failure value of 2.0%. The tensile strength of G-RC and P-G-RC membrane increase from 24.9 to 53.2 MPa and 66.3 MPa, respectively. Meanwhile, the strain-to-failure values change from 2.0 to 1.9% and 2.6% for G-RC and P-G-RC, respectively. The change of mechanical property is associated with the crosslink interactions. Based on the characterization results, the link reactions occurred during the membrane formation was proposed (Fig. 4).
The smoothness of the crosslinked membranes was examined by AFM analysis (Fig. 5), giving a quite low root-mean-square roughness of 3.7 nm (G-RC) and 2.9 nm (P-G-RC). The low roughness of membranes is very important for inhibiting the deposition and adsorption of pollutants (Bian et al. 2018; Guo et al. 2013; Huang et al. 2013). The calculated values of mean pore radius for the RC, G-RC and P-G-RC samples are 0.88, 0.62 and 0.67 nm, respectively. This proves that the glutaraldehyde molecules connect the cellulose chains and thus decrease the pore sizes. With the addition of PVA, the abundant –OH groups conjugate with –OH groups in cellulose and weaken the effects of aldehyde groups, thus enlarging the pore radius a little. The pore sizes have a decisive effect on the filtration performance. The surface and fracture morphology of the crosslinked membranes were examined by SEM (details in supporting information, Fig. S2). Both G-RC and P-G-RC membrane exhibit a smooth surface without any significant defects and voids. The membrane thickness measured by the cross-section images were ca. 15 μm.
Separation performance
To determine the permeation and rejection performance of the membranes, filtration tests were explored by using anionic Congo red (CR, 10 mg/L) and anionic methyl orange (MO, 10 mg/L) solutions as model wastewater. After 1 h filtration, the permeate samples were collected for measurements. The final valves were acquired from the averaged numbers of three parallel experiments. As depicted in Fig. 6a, the change of solution color is clearly observed. The water permeation flux of pristine RC membrane was 22.4 L/m2 h for CR solution. When crosslinked by glutaraldehyde, the flux was decreased to 10.5 L/m2 h. The addition of PVA slightly improved the water permeation flux from 10.5 to 12.5 L/m2 h. The water permeance is mainly associated with the pore radius and the calculated pore radius was in the order: RC (0.88 nm) > P-G-RC (0.67 nm) > G-RC (0.62 nm). In general, a small pore size results in a low water permeance. The same tendency can be found in the filtration process of MO solution (Fig. 6b). The water permeance followed the order: RC (21.2 L/m2 h) > P-G-RC (12.7 L/m2 h) > G-RC (10.7 L/m2 h).
The rejection values of CR and MO over pristine RC membrane were 90.2% and 27.9%, respectively. The rejection value over G-RC membrane increased substantially to 98.6% (CR) and 90.1% (MO). This is due to a denser network was established after the crosslink reactions between glutaraldehyde and cellulose (Lin et al. 2017; Wang et al. 2018). The optimal performance was observed over P-G-RC with a CR rejection rate of 99.9% and a MO rejection rate of 93.5%. Compared with G-RC, P-G-RC had more –OH groups and provided more reactive sites to prevent the permeation of dyes.
Further experiments were carried out to exclude the influence of adsorption. The concentration of the retained solution increased from 10 to 17.4 mg/L after 6 h filtration test. Negligible amount of dyes was deposited on the membrane surface. By directly immersing the P-G-RC membrane into the MO solution (10 mg/L) for 6 h, the concentration of the dye solution had no obvious change (details in Fig. S3). Above-mentioned tests indicated that the separation of dyes by P-G-RC membrane was mainly through membrane separation (rejection) rather than adsorption. The negligible adsorption effects can be ascribed to the smooth membrane surface as indicated by the AFM analysis (Hu et al. 2018; Yang et al. 2018).
Stability is an important indicator of membrane in practical application. Figure 7 shows the result of 6 h flat-sheet long time operation plotted as a function of normalized flux versus time. The deionized water passed through the membranes initially until the flux is stable (~ 1 h). With the extension of time, RC membrane displayed an obvious decline in water flux in the process of Congo red filtration. This indicated that regenerated cellulose membrane was sensitive to water molecules under long-time tests as water could interact with the cellulose chains and narrowed the pore sizes. The introduction of glutaraldehyde to the membrane network reduced the hydrophilicity and improved the long time stability. The addition of PVA further modify the hydrophilicity and pore sizes, thus the flux of P-G-RC exhibited best stability and just decreased by 3% after 6 h test. The hydroxyl groups of PVA prefer to interact with water molecule and form a water layer on membrane surface, thus reducing the block effects of dyes on the pores. Because of MO has a lower molecular weight than that of CR, its flux reduction rates were also lower than the filtration for CR (Fig. 7). The reusability of the P-G-RC membrane was also explored by simply washing the membranes (after 6 h filtration tests) with 200 mL deionized water and dried at room temperature. The filtration performance just showed a slight loss (less than 5%) during the repeated use.
Conclusion
In this study, regenerated cellulose membranes were developed by crosslinking cellulose with glutaraldehyde and polyvinyl alcohol. The resulting membranes were characterized by a combination of XRD, ATR, SEM and AFM. The filtration tests were performed by using methyl orange and Congo red as water pollutants. The results demonstrated that the introduction of glutaraldehyde could enhance the selectivity and polyvinyl alcohol could contribute to stability. The membranes display high permeation flux and sufficient rejections for organic dyes in the water purification. The optimal performance was observed over P-G-RC sample with suitable pore radius (0.67 nm), low root-mean-square roughness (2.9 nm) and abundant hydroxyl groups. Apart from providing a new cellulose-derived membrane for efficient removal of dyes, the successful adjustment of selectivity and stability through crosslink strategy is expect to speed up the pace to practical applications.
References
Bian H, Wei L, Lin C, Ma Q, Dai H, Zhu JY (2018) Lignin-containing cellulose nanofibril-reinforced polyvinyl alcohol hydrogels. ACS Sustain Chem Eng 6:4821–4828
Cai Q, Fan Z, Chen J, Guo W, Ma F, Sun S, Hu L, Zhou Q (2018) Dissolving process of bamboo powder analyzed by FT-IR spectroscopy. J Mol Struct 1171:639–643
Chen Y, He C (2017) High salt permeation nanofiltration membranes based on NMG-assisted polydopamine coating for dye/salt fractionation. Desalination 413:29–39
Chen W, Yu H, Lee SY, Wei T, Li J, Fan Z (2018) Nanocellulose: a promising nanomaterial for advanced electrochemical energy storage. Chem Soc Rev 47:2837–2872
Feng Y, Wang Y, Liu S, Qiu J, Yao J (2017) Facile fabrication of ZIF-8 embedded millimeter-sized porous polyethersulfone beads for selective dye removal. Polym Compos 39:3896–3902
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896
Gu J, Hu C, Zhang W, Dichiara AB (2018) Reagentless preparation of shape memory cellulose nanofibril aerogels decorated with Pd nanoparticles and their application in dye discoloration. Appl Catal B Environ 237:482–490
Guo B, Chen W, Yan L (2013) Preparation of flexible, highly transparent, cross-linked cellulose thin film with high mechanical strength and low coefficient of thermal expansion. ACS Sustain Chem Eng 1:1474–1479
Gupta KM, Jiang J (2015) Cellulose dissolution and regeneration in ionic liquids: a computational perspective. Chem Eng Sci 121:180–189
Hamid NAA, Ismail AF, Matsuura T, Zularisam AW, Lau WJ, Yuliwati E, Abdullah MS (2011) Morphological and separation performance study of polysulfone/titanium dioxide (PSF/TiO2) ultrafiltration membranes for humic acid removal. Desalination 273:85–92
Hu M, Hu T, Li Z, Yang Y, Cheng R, Yang J, Cui C, Wang X (2018) Surface functional groups and interlayer water determine the electrochemical capacitance of Ti3C2Tx MXene. ACS Nano 12:3578–3586
Huang J, Zhu H, Chen Y, Preston C, Rohrbach K, Cumings J, Hu L (2013) Highly transparent and flexible nanopaper transistors. ACS Nano 7:2106–2113
Khalili MS, Zare K, Moradi O, Sillanpää M (2018) Preparation and characterization of MWCNT-COOH-cellulose-MgO NP nanocomposite as adsorbent for removal of methylene blue from aqueous solutions: isotherm, thermodynamic and kinetic studies. J Nanostruct Chem 8:103–121
Khoshsang H, Ghaffarinejad A, Kazemi H, Wang Y, Arandiyan H (2018) One-pot synthesis of S-doped Fe2O3/C magnetic nanocomposite as an adsorbent for anionic dye removal: equilibrium and kinetic studies. J Nanostruct Chem 8:23–32
Koli PB, Kapadnis KH, Deshpande UG, Patil MR (2018) Fabrication and characterization of pure and modified Co3O4 nanocatalyst and their application for photocatalytic degradation of eosine blue dye: a comparative study. J Nanostruct Chem 8:453–463
Kong L, Zhang D, Shao Z, Han B, Lv Y, Gao K, Peng X (2014) Superior effect of TEMPO-oxidized cellulose nanofibrils (TOCNs) on the performance of cellulose triacetate (CTA) ultrafiltration membrane. Desalination 332:117–125
Lin Q, Gao M, Chang J, Ma H (2017) Highly effective adsorption performance of carboxymethyl cellulose microspheres crosslinked with epichlorohydrin. J Appl Polym Sci 134:44363–44369
Luo H, Dong J, Yao F, Yang Z, Li W, Wang J, Xu X, Hu J, Wan Y (2018) Layer-by-layer assembled bacterial cellulose/graphene oxide hydrogels with extremely enhanced mechanical properties. Nano Micro Lett 10:52–61
Lv Y, Du Y, Chen ZX, Qiu WZ, Xu ZK (2018) Nanocomposite membranes of polydopamine/electropositive nanoparticles/polyethyleneimine for nanofiltration. J Membr Sci 545:99–106
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–3994
Ning R, Wu CN, Takeuchi M, Saito T, Isogai A (2017) Preparation and characterization of zinc oxide/TEMPO-oxidized cellulose nanofibril composite films. Cellulose 24:4861–4870
Pan Y, Shi X, Cai P, Guo T, Tong Z, Xiao H (2018) Dye removal from single and binary systems using gel-like bioadsorbent based on functional-modified cellulose. Cellulose 25:2559–2575
Park J, Oh M (2017) Construction of flexible metal-organic framework (MOF) papers through MOF growth on filter paper and their selective dye capture. Nanoscale 9:12850–12854
Puspasari T, Pradeep N, Peinemann KV (2015) Crosslinked cellulose thin film composite nanofiltration membranes with zero salt rejection. J Membr Sci 491:132–137
Puspasari T, Huang T, Sutisna B, Peinemann KV (2018) Cellulose-polyethyleneimine blend membranes with anomalous nanofiltration performance. J Membr Sci 564:97–105
Robati D, Mirza B, Ghazisaeidi R, Rajabi M, Moradie O, Agarwalg S, Gupta VK (2016a) Adsorption behavior of methylene blue dye on nanocomposite multi-walled carbon nanotube functionalized thiol (MWCNT-SH) as new adsorbent. J Mol Liq 216:830–835
Robati D, Rajabi M, Moradi O, Najafi F, Tyagi I, Agarwal S, Gupta VK (2016b) Kinetics and thermodynamics of malachite green dye adsorption from aqueous solutions on graphene oxide and reduced graphene oxide. J Mol Liq 216:259–263
Robati D, Mirza B, Rajabi M, Moradi O, Tyagi I, Agarwal S, Gupta VK (2016c) Removal of hazardous Dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chem Eng J 284:687–697
Shen L, Yang Y, Zhao J, Wang X (2016) High-performance nanofiltration membrane prepared by dopamine-assisted interfacial polymerization on PES nanofibrous scaffolds. Desalin Water Treat 57:9549–9557
Sirus Z, Zinatizadeh AA, Rahimi M, Vatanpour V, Zangeneh H (2014) Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J Membr Sci 453:292–301
Štefelová J, Slovák V, Siqueira G, Olsson RT, Tingaut P, Zimmermann T, Sehaqui H (2017) Drying and pyrolysis of cellulose nanofibers from wood, bacteria, and algae for char application in oil absorption and dye adsorption. ACS Sustain Chem Eng 5:2679–2692
Tanaka A, Khakalo A, Hauru L, Korpela A, Orelma H (2018) Conversion of paper to film by ionic liquids: manufacturing process and properties. Cellulose 25:6107–6119
Vergis BR, Krishna RH, Kottam N, Nagabhushana BM, Sharath R, Darukaprasad B (2018) Removal of malachite green from aqueous solution by magnetic CuFe2O4 nano-adsorbent synthesized by one pot solution combustion method. J Nanostruct Chem 8:1–12
Wang X, Ye T, Wang Z, Yang R, Wang R, Ma H, Xiao B, Chu B (2014) Nanofiltration membranes prepared by interfacial polymerization on thin-film nanofibrous composite scaffold. Polymer 55:1358–1366
Wang DC, Yu H, Fan X, Gu J, Ye S, Yao J, Ni Q (2018) High aspect ratio carboxylated cellulose nanofibers crosslinked to robust aerogels for superabsorption-flocculants: paving way from nano-scale to macro-scale. ACS Appl Mater Interfaces 10:20755–20766
Weng R, Chen L, Xiao H, Huang F, Lin S, Cao S, Huang L (2017) Preparation and characterization of cellulose nanofiltration membrane through hydrolysis followed by carboxymethylation. Fiber Polym 18:1235–1242
Xia L, Ren J, Wen M, McCutcheon JR (2018) Ceramic-supported thin film composite membrane for organic solvent nanofiltration. J Membr Sci 563:857–863
Xu Q, Chen C, Rosswurm K, Yao T, Janaswamy S (2016) A facile route to prepare cellulose-based films. Carbohydr Polym 149:274–281
Xu H, Bronner T, Yamamoto M, Yamane H (2018a) Regeneration of cellulose dissolved in ionic liquid using laser-heated melt-electrospinning. Carbohydr Polym 201:182–188
Xu Z, Jiang X, Zhou H, Li J (2018b) Preparation of magnetic hydrophobic polyvinyl alcohol (PVA)-cellulose nanofiber (CNF) aerogels as effective oil absorbents. Cellulose 25:1217–1227
Xu Z, Zhou H, Tan S, Jiang X, Wu W, Shi J, Chen P (2018c) Ultralight super-hydrophobic carbon aerogels based on cellulose nanofibers/poly(vinyl alcohol)/graphene oxide (CNFs/PVA/GO) for highly effective oil-water separation. Beilstein J Nanotechnol 9:508–519
Yang L, Mukhopadhyay A, Jiao YC, Yong Q, Chen L, Xing YJ, Hamel J, Zhu HL (2017a) Ultralight, highly thermally insulating and fire resistant aerogel by encapsulating cellulose nanofibers with two-dimensional MoS2. Nanoscale 9:11452–11462
Yang W, Jiao L, Min D, Liu Z, Dai H (2017b) Effects of preparation approaches on optical properties of self-assembled cellulose nanopapers. RSC Adv 7:10463–10468
Yang X, Sun H, Pal A, Bai Y, Shao L (2018) Biomimetic silicification on membrane surface for highly efficient treatments of both oil-in-water emulsion and protein wastewater. ACS Appl Mater Interfaces 10:29982–29991
Zhang XF, Feng Y, Huang C, Pan Y, Yao J (2017) Temperature-induced formation of cellulose nanofiber film with remarkably high gas separation performance. Cellulose 24:5649–5656
Zhang XF, Feng Y, Wang Z, Jia M, Yao J (2018a) Fabrication of cellulose nanofibrils/UiO-66-NH2 composite membrane for CO2/N2 separation. J Membr Sci 568:10–16
Zhang XF, Hou T, Chen J, Feng Y, Li B, Gu X, He M, Yao J (2018b) Facilitated transport of CO2 through the transparent and flexible cellulose membrane promoted by fixed-site carrier. ACS Appl Mater Interfaces 10:24930–24936
Zhao S, Wang Z (2017) A loose nano-filtration membrane prepared by coating HPAN UF membrane with modified PEI for dye reuse and desalination. J Membr Sci 524:214–224
Zhao Q, Zhu X, Chen B (2018) Stable graphene oxide/poly(ethyleneimine) 3D aerogel with tunable surface charge for high performance selective removal of ionic dyes from water. Chem Eng J 334:1119–1127
Acknowledgments
The authors are grateful for financial supports from the Natural Science Key Project of the Jiangsu Higher Education Institutions (15KJA220001), the Natural Science Foundation of Jiangsu Province Youth Fund (BK20180766, BK20170919), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The authors also thank the testing services from Advanced Analysis and Testing Center of Nanjing Forestry University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hou, T., Guo, K., Wang, Z. et al. Glutaraldehyde and polyvinyl alcohol crosslinked cellulose membranes for efficient methyl orange and Congo red removal. Cellulose 26, 5065–5074 (2019). https://doi.org/10.1007/s10570-019-02433-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02433-w