Abstract
In this study, we investigate the “chemical welding” of paper with the ionic liquid (IL) 1-ethyl-3-methylimidazolium acetate ([EMIM]OAc) using a two-step process. First, the IL is transported into the structure of the paper as a water solution. Then, partial dissolution is achieved by activation with heat (80–95 °C), where the water evaporates and the surfaces of the fibres partially dissolve. The activated paper is washed with water to remove IL, and dried to fuse fibre surfaces into each other. The “chemically welded” paper structure has both elevated dry and wet strength. The treatment conditions can be adjusted to produce both paper-like materials and films. The most severe treatment conditions produce films that are fully transparent and their oxygen and grease barrier properties are excellent. As an all-cellulose material, the “chemically welded” paper is fully biodegradable and is a potential alternative to fossil fuel-based plastics.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The consumption of packaging materials has continuously been rising due to increased transportation of goods and food materials around the world, which has been steadily increased due to online shopping and offshoring of manufacturing plants (da Cruz et al. 2014). This rise in material consumption has been met mainly by fossil fuel-based plastics and lignocellulose-based materials. Since packages are typically utilized only once, plastic materials, which have incomplete recycling cycles, cause environmental pollution, also harming animals and other living organisms, including, for example, human health issues related to the microplastics problem(Haward 2018). Consequently, the choice of packaging materials has been guided towards biodegradable, renewable materials. Thus, much of the growth in packaging material consumption has been covered by lignocellulosic materials, due to the renewability, recyclability, and abundance of wood fibre materials(Johansson et al. 2012). Among wood-based materials, cardboard, wrapping papers and films have been the most important materials used in packaging applications.
Wood fibres are mostly composed of cellulose, a linear polymer composed of glucose units connected by glycosidic bonds(Sjöström 1993). In paper and board, fibres are connected by interfibrillar hydrogen bonds forming layered sheets. Paper is strong when dry, but easily disintegrates in water, because water effectively breaks those bonds(Lindström et al. 2005). Therefore, in many paper applications, some wet strength additives are used to increase the water tolerance of paper via hydrophobization or forming chemical linkages between fibres(Lindström et al. 2005). Crystalline cellulose has an inherent water resistance due to the tight orientation and packing of cellulose chains, which is utilized in regenerated cellulose films and filaments(Fink et al. 2001). Regenerated cellulose materials are made by first dissolving cellulose in a suitable cellulose solvent system and then the dissolved cellulose is regenerated into a solid structure by using an antisolvent(Klemm 1998). A large variety of the suitable non-derivatizing and derivatizing dissolution systems have been reported. Non-derivatizing direct dissolution chemistries such as cupro, NMMO·H2O, and ILs are attractive since they do not alter the chemistry of cellulose, maintaining the excellent biodegradability of pure cellulose(Simon et al. 1998; Eichhorn et al. 2010; Liebert 2010). The most common process used to produce regenerated cellulose films is the cellophane process, where cellulose is regenerated from cellulose xanthate (“viscose”) by dilute sulfuric acid; but greener alternatives such as novel ILs have been proposed(Hao et al. 2004; Paunonen 2013). These regenerated films have excellent mechanical properties, and due to their pure cellulose structure they maintain the natural hydrophilicity of cellulose. Recent developments have targeted combining regenerated cellulose with intact cellulose fibres to manufacture high-performance all-cellulose materials(Eichhorn et al. 2001; Huber et al. 2011). With this concept, no additives or coatings are required to achieve excellent mechanical properties both in a dry and wet state, which is combined with good barrier properties and excellent biodegradability.
All-cellulose materials can be made by using composite or partial dissolution approaches(Huber et al. 2011). In the composite approach, cellulose fibres are embedded into a cellulose matrix, which is cast into the required shape, typically into a film. Comparatively, in partial dissolution, the premade paper is “chemically welded” by dissolving a thin layer on the fibre surface, allowing the fibre surfaces fuse together after regeneration. This approach has earlier been utilized with N-methylmorpholine-N-oxide (NMMO)(Johnson 1969), LiCl/DMAc(Nishino and Arimoto 2007), NaOH-urea(Atalla and Vanderhart 1984), and ionic liquids(Graenacher 1934). In these studies, pre-made paper sheets were directly immersed into the active dissolution solutions. The drawback of this approach is that if the cellulose solvent is viscous, dissolution mostly takes place only on the surfaces of the paper. We have shown that NMMO diluted with methanol can be utilized to more controllably “chemically weld” cellulose nanofibrils (CNF) film when the paper is first impregnated with NMMO in methanol, the methanol is evaporated off and the NMMO is activated with a hot calender(Orelma et al. 2017). The methanol for dilution has four functions: to lower the viscosity of cellulose solvent, to prevent dissolution of cellulose during the impregnation, to swell the paper structure in order to ease the permeation of the paper with the cellulose solvent, and to control the quantity of the active solvent added. The severity of the partial dissolution treatment can be controlled by the concentration of the cellulose solvent, treatment time and heat activation temperature. ILs have a special role in the preparation of all-lignocellulose material due to their claimed ability to dissolve all components of wood including cellulose, hemicellulose, lignin and even wood(Kilpeläinen et al. 2007). As an example, partial dissolution with 1-butyl-3-methylimidazolium chloride ([BMIM]Cl) has been utilized to produce an all-wood-fibre composite from hinoki wood(Shibata et al. 2013). In the same study, cotton fabric was also transformed into a solid all-cellulose material. Havershals et al. studied the “chemical welding” of hemp and cotton yarns with [EMIM]OAc(Haverhals et al. 2010). In that study, the yarns were placed in hot IL and then purified in water. The treatment causes gluing of the surface of yarns, and the toughness of the yarn was significantly increased. However, the authors present no industrially applicable conversion methods or attractive specified product applications.
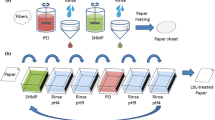
In this study, pre-made paper sheets were treated with 1-ethyl-3-methylimidazolium acetate ([EMIM]OAc) diluted by water, followed by heat activation of the solvent in the oven (Scheme 1). [EMIM]OAc was selected because it has been reported to tolerate water up to 15 wt% (Tan et al. 2016). After activation, the IL was removed from the paper by washing with water, and then the sheets were dried in an oven. Precipitation of the dissolved cellulose resulted in a fusion of the fibres of paper into a solid, uniform, high wet- and dry-strength material, with properties similar to thermoset plastics.
Experimental
Materials
Bleached pine kraft pulp was obtained from a Finnish pulp mill. Laboratory handsheets (size 150 × 150 mm2) with 100 g/m2 (relative humidity (RH) 50%) grammage were prepared according to ISO standard 5269-1:05. 1-Ethyl-3-methylimidazolium acetate ([EMIM]OAc, purity 95%) was purchased from IoLiTec GmbH, Heilbronn, Germany. [EMIM]OAc was not purified before its use in the “chemical welding” of paper. All other chemicals used in the study were of analytical grade. All water used in the study was purified with a Milli-Q device.
Methods
Partial dissolution of paper sheets by ionic liquid
Partial dissolution of handsheets was carried out with 1-ethyl-3-methylimidazolium acetate ([EMIM]OAc). [EMIM]OAc was diluted with water to 75 wt% concentration before the treatment. Dry paper sheets were dipped into the [EMIM]OAc solution either for 15 s or for 3 min to change the absorbed volume of [EMIM]OAc-water in the paper sheet. Keeping the sheet in the solution for more than 3 min significantly weakened the paper sheet, leading to handling issues. Impregnated paper sheets were placed on a plate covered by aluminium foil and heated in the oven, where water quickly evaporated and the fibres then partially dissolved. A series of samples (Fig. 1) were prepared with varying oven temperatures and treatment times: (a) a reference without any treatment; (b) 15 s impregnation, oven 80 °C, treatment time of 50 min; (c) 3 min impregnation, 80 °C, 50 min; (d) 3 min impregnation, 95 °C, 50 min; (e) 3 min impregnation, 95 °C, 16 h. The heat-treated paper sheets carrying [EMIM]OAc on aluminium foil were then placed into water. Water was changed four times to wash out completely all the remaining ionic liquid. Aluminium foil was carefully peeled from the treated sheet after the first rinsing. The rinsed sheets were then wet-pressed at 3.5 bar for 5 min (ISO 5269-1:05) and dried with a L&W Rapid Dryer (ABB Ab/Lorentzen & Wettre) at 135 °C for 5 min. Dried sheets were stored at the standard thermo- and hygrostated conditions of 23 °C and 50% RH.
Viscosity of aqueous IL solutions
The shear viscosity of IL-water solutions at concentrations of 100, 90, 75 and 50 wt% were measured using a rotational rheometer (Anton Paar Rheometer MCR301) with TruGap™ measurement geometry. The sample was placed between a fixed bottom plate and a moving upper cone with the gap at 0.051 mm. The upper cone geometry was CP50-2, i.e. diameter 50 mm and cone angle 2°. Temperature was precisely controlled at 23 °C. Viscosity was measured at three shear rates: 10, 100, and 500 1/s.
Gravimetrical analyses for impregnation and water evaporation
Impregnation of [EMIM]OAc into paper was measured gravimetrically by using a laboratory balance (Precisa H600). All tests were carried out by 75 wt% [EMIM]OAc-water solution. The sample size was approximately 14x14 cm. Paper samples were placed in the IL-water solution for different times (2,15, and 180 s.). The samples carrying the IL-water were drained in air for 30 s. The weight of the paper with IL-water was then measured, and the liquid uptake was calculated. All tests were repeated at least thrice.
The evaporation of water from [EMIM]OAc was estimated with a computer controlled gravimetric moisture analyser (Ohaus MB45, NJ, USA). The measurements were carried out with the water contents of 0, 10, 25, 50 wt%. The drying temperature was set to constant 95 °C. The sample size was approximately 7 × 7 cm2. The paper samples were impregnated in the given solutions for 3 min followed by draining in air for 30 s. Then the evaporation of the water from IL was measured. All measurements were at least duplicated.
Microscopy analyses
Scanning electron microscopy (SEM) imaging was carried out with a Merlin Field Emission (FE)-SEM (Carl Zeiss NTS GmbH, Germany) with applied gold sputter coatings (30 mA, 30 s). The electron gun voltage was a constant 3 kV with a grid current of 60 pA. The pixel resolution was 2048 × 1536.
Chemical analyses of IL-treated papers by solid state 13C CP/MAS NMR spectrometry
Changes in the crystallinity of the wood fibres after [EMIM]OAc treatment were characterized by using a 13C cross polarization magic angle spinning (CP-MAS) NMR spectrometer (Bruker AVANCE-III 400 MHz, Bruker BioSpin, Germany). For all samples, 20,000 scans were collected using an 8 kHz spinning frequency, 2-ms contact time, and a 5-second delay between pulses.
Mechanical properties of “chemically welded” papers
The apparent bulk density of the paper sheets with and without IL treatment was measured in accordance with ISO 5270:12, ISO 534:05. Gurley air resistance was measured in accordance with ISO 5270:12, ISO 5636-5:13. Tensile strength, tensile index, stretch at break, tensile energy absorption, TEA index, and tensile stiffness were measured in accordance with ISO 5270:12, ISO 1924-2:08. Tear strength was measured in accordance with ISO 5270:12, ISO 1974:12. Internal bonding strength (Scott bond) was measured in accordance with TAPPI T569:00. Wet tensile strength was measured at 10 min and 24 h immersion times in accordance with ISO 3781:11, SCAN-P 20:95.
Barrier properties
Air permeabilities of the samples were investigated using an L&W Air Permeance Tester, in accordance with ISO 5636-3:2013(E) Part 3: Bendtsen method. Measurements were repeated at least five times.
Water vapour permeabilities of the samples were determined gravimetrically using a modified ASTM-E-96B procedure “wet cup method”. Samples with a test area of 30 cm2 were mounted on circular aluminium cups (68-3000 Vapometer EZ-Cups; Thwing-Albert Instrument Company) containing distilled water (100% RH). The cups were stored in test conditions of 23 °C and 50% RH and weighed periodically until a constant rate of weight reduction was attained. Two parallel measurements were carried out. Results were normalized by the film thickness (in µm).
Oxygen permeabilities through the films were determined according to standard ASTM D3985 using an Oxygen Permeation Analyser Model 8001 (Systech Instruments Ltd, UK). Tests were carried out at 23 °C and 0 and 80% RH using 100% oxygen as a test gas. Two parallel measurements were carried out. Results were normalized by the film thickness (in µm).
Grease barrier properties were evaluated according to the method reported by Vähä-Nissi et al. (2016). The paper sheets with the size of 50 mm × 50 mm were placed on a transparent glass plate. The sample (same size) was put on it. A circular blotting paper (diameter 30 mm) was further put on the sample, and 200 µl of “Oil Red O” (1-([4-(xylylazo)xylyl]azo)-2-naphthol, 1-[2,5-dimethyl-4-(2,5-dimethylphenylazo)phenylazo]-2-naph-thol, Solvent Red 27, Sigma-Aldrich) dyed oil was pipetted onto the blotting paper. Then a 50-g weight (diameter 30 mm) was placed onto the blotting paper. Penetration of grease through the sample was detected by an image scanner (300 dpi, 24-bit colour) through the glass plate. 5 parallel measurements were carried out for 148 h with periodic image scanning.
Results
Processing paper sheets with aqueous IL solution
The paper samples were treated with the IL-water solution before heat activation in an oven. The role of the water in IL was to decrease the viscosity of the IL and swell paper so that the IL can better penetrate into its structure. Pure [EMIM]OAc with and without added water behaved as a Newtonian fluid without the shear rate response (Fig. 2a). The viscosity of the pure [EMIM]OAc was 169.5 mPa s (Fig. 2b). When a 10 wt% of water (water in IL mole ratio 1.1) was added, the viscosity decreased to 56.1 mPa s. The higher water content, 25 (mole ratio 3.2) and 50 (mole ratio 10) wt%, further decreased the viscosity value to 24.8 and 7.4 mPa s, respectively. This explains that with a 10 wt% water addition, the viscosity of [EMIM]OAc can be decreased sufficiently, easing processing. The observed viscosity decrease as a function of added water content was the same as reported earlier.(Quijada-Maldonado et al. 2012) The viscosity of the pure [EMIM]OAc has also a strong temperature dependence. However, if the temperature is used to decrease the viscosity of IL, the dissolution reaction propagates simultaneously(Kilpeläinen et al. 2007). In this study, we did not investigate temperature dependence, because the aim of our procedure was to prevent dissolution during the impregnation phase in order to enhance the controllability of the treatment process.
The amount of [EMIM]OAc in the paper could be controlled with the dilution of the IL and the impregnation time. The mass increase of the paper sheets during impregnation with a 75 w-% [EMIM]OAc solution was estimated gravimetrically (Fig. 3a). The impregnation process of the paper is rapid, and the plateau was reached after 30 s impregnation time. After 15 s the mass increase was approximately 2.5 g/g. After 30 s the mass did not significantly increase. From the processability point of view, this is good because the treatment time is short. In this, the process is analogous to surface sizing and chemical impregnation, which are common processes used in the paper industry to enhance the permanence of paper(Gurnagul et al. 1993). Besides IL concentration, the impregnation rate is influenced by the type of the cellulose fibres used and paper density.
Water prevents the dissolution of cellulose by ionic liquid. However, [EMIM]OAc is a special case among ionic liquids because it can tolerate water: it can dissolve microcrystalline cellulose (MCC) with water contents as high as 15 w-%(Tan et al. 2016). Water tolerance is desirable for a cellulose solvent, because water can be tightly bound to the IL and be difficult to completely remove by evaporation at atmospheric pressure(Cammarata et al. 2001). The rate of water evaporation from [EMIM]OAc at 95 °C was estimated with a gravimetric moisture analyser at water contents of 0, 10, 25, 50 wt% (Fig. 3b). For 25 m-% and above, mass loss was exponential, while for 50 m-%, it was partially linear. A plateau below 10 m-%, sufficiently low to dissolve cellulose, was reached in all cases after a 15-min heating time. Partial dissolution and diffusion of cellulose molecules begins only after evaporation is complete. This quantitative result confirms the visual observation that water is quickly evaporated from the tested paper samples in the oven.
Partial dissolution of paper with the IL-water system
The procedure used caused a controlled transformation of paper to permanent solid structures (Fig. 4). Visually, when treatment conditions were more severe, the transparency of the paper increased. The sample treated with the mildest treatment (Sample a, Fig. 1) still had a paper-like feel and appearance (Fig. 4a), but when the impregnated amount of the IL-water was increased at a constant heating temperature (sample b), the transparency of paper increased significantly (Fig. 4b). In the most severe treatment conditions (samples c and d), paper achieves a fully transparent structure (Fig. 4c, d), and these samples resemble plastic films. Moreover, the paper shrinks during treatment and drying, causing a remarkable increase of the apparent density of paper samples as a function treatment severity (Table 1). The evaporation of the water from the film structure causes the compaction of the film, which leads also to the vanishing of the pores and voids when the fibrillar structure is lost(Katekawa and Silva 2006). The apparent sheet density of the samples increases from 557 ± 16 kg/m3 (reference) up to 1192 ± 167 kg/m3 (sample 4), and simultaneously the paper is transformed into transparent film. The IL treatment of paper with [BMIM]Cl has been studied earlier elsewhere.(Ichiura et al. 2017) In the given study the paper sheets were treated by direct immersion in hot [BMIM]Cl. This treatment resulted in only a change of visual appearance because only the surface of the paper was treated.
In scanning electron microscopy (SEM), the reference sample shows a fibre structure typical for regular paper (Fig. 5a). Already the mildest IL treatment (sample a) caused the fibres to bind together tightly (Fig. 5b). When the volume of the IL in the paper was increased with a longer impregnation time (sample b), the structure of the fibre was loosened and fibres were mobilized (Fig. 5c). A higher activation temperature (sample c) caused the partial dissolution of the fibres, and they were embedded into a regenerated cellulose matrix (Fig. 5d). The sample contained also some air bubbles that were mostly formed by the evaporation of the water. Heat activation overnight, for 960 min (sample d), caused full dissolution of the paper’s fibrillar structure (Fig. 5e). Cross-sectional images verify the same observations (Fig. 5f–j). With this long treatment time, the dissolved cellulose on the fibre surface had enough time to diffuse and flow into a solid shape. Mild treatments retained the fibre structure mostly intact (sample a and b), but in severe treatment conditions (sample c and d), the transparency of the paper was increased and the fibrillar structure vanished.
13C CP/MAS NMR spectroscopy is an established tool used to distinguish between cellulose I and II polymorphs (Foston 2014)(Isogai et al. 1989). As expected, the reference paper consisted of crystalline cellulose I and non-crystalline cellulose (Fig. 6). After the mildest treatment (sample a), small changes in the NMR spectrum were observed indicating the formation of cellulose II (C6 moved to lower chemical shift) and loss of cellulose I structure (a decrease in the intensity of the crystalline C4 peak). In sample b, the change from the cellulose I to the cellulose II crystalline form was more drastic, but clearly there was still some cellulose I signal left. In the spectrum of sample c, the cellulose I signal vanished and was entirely replaced by the cellulose II signal. Treatment of wood pulp with N-methyl morpholine-N-oxide (NMMO) has been studied earlier,(Virtanen and Maunu 2014) and similar changes in the NMR spectrum were observed. The NMR results are in accordance with the visual (Fig. 4) and SEM (Fig. 5) characterizations: the paper became transparent, and simultaneously the fibrillar structure of the paper partially dissolved.
Mechanical properties of “chemically welded” paper sheets
The prepared samples were studied in detail with typical paper characterization methods (Table 1). The IL treatment significantly increased the mechanical strength of paper. The typical tensile index-strain curves, measured in dry condition, are shown in Fig. 7a. The curve profiles of reference, sample b, and sample c were similar to typical paper samples with strains between 3 and 5% (Borodulina et al. 2012). Whereas, the profiles of the both curves of sample a and sample d differenced from the reference. The sample a showed clear strengthening with significantly elevated strain. In pure paper, the fibres are kept together by hydrogen bonding, whereas after “chemo welding” the traditional hydrogen bonded fibre–fibre joints are replaced by regenerated cellulose–cellulose joints (Gardner and Tajvidi 2016). The reason why sample a exhibited higher strength and strain than samples b and c, was most probably due to uneven structures of samples b and c (Fig. 4). The profile of the curve of sample d was similar to that reported for cellophane (Zhang et al. 2015). With the mildest treatment conditions (sample a), the dry tensile index value more than tripled from 24.1 ± 0.8–78.1 ± 3.6 Nm/g (Fig. 7b). When more IL and a higher heat activation temperature was used (samples b, c, and d), the tensile index was slightly lower compared to that of sample a. However, this is only an apparent decrease because in the definition of the tensile index, the force is normalized with the grammage of the paper, which increased. The wet strength of the paper was drastically increased already with the mildest treatment conditions (sample 2, wet strength 8.36 ± 2.59 Nm/g, 24-h immersion in water). The highest wet strength values (18.23 ± 4.97 Nm/g) were achieved with sample b. The transparent samples (sample c and d) had slightly lower wet strength values. The wet tensile strength retention of sample a was 10%, and with more severe treatment conditions (samples b and d), it increased to above 20%. Sample c had a somewhat lower wet strength retention of 15%, which was mostly caused by the pores left behind by air bubbles formed during heat activation, as seen in SEM images (Fig. 5d). The wet strength retention of 20% is comparable to the value reported for regenerated cellulose films made from cotton linters by dissolution to and regeneration from NaOH/urea(Zhang et al. 2001). The wet strength is increased by the fusion of the fibre surfaces, forming a similar structure as seen in regenerated cellulose films. Earlier, paper has also been treated with undiluted molten [BMIM]Cl, resulting in the dry and wet strength values of 2.8 and 0.38 kN/m, respectively.(Ichiura et al. 2017) The values found in this this study (sample b 8.21 and 0.88 kN/m, respectively) were significantly higher. Direct dipping in pure IL mostly affects the surface of the paper, whereas dilution followed by cosolvent evaporation causes a more even fusion of fibre surfaces, because the IL can be transported evenly through the paper structure. This explains why both wet- and dry-strength properties were significantly increased.
Considering all the mechanical strength data (Table 1), we can conclude that the IL treatment increased the toughness of the paper significantly. This can be seen in the significant increases in the TEA index, the modulus of elasticity, and Scott bond values. However, the IL treatment does not increase the tear index of paper. Analogously, kraft pulp beating results in a paper with a denser structure and a higher tensile strength, but lower tear strength(Lindström et al. 2005).
Barrier properties of the IL-treated papers
Paper is one of the most common packaging materials, but it cannot fully replace plastic film materials due to its low water resistance and oxygen permeability. Thus, we tested if our “chemically welded” paper could have better barrier properties. For our samples, the air permeabilities were: reference (3880 ml/min), samples a (5720 ml/min), b (43.3 ml/min), c (7.6 ml/min), and d (0.265 ml/min). For the detailed water vapour and oxygen permeability and grease barrier characterizations, we selected sample d. As a reference, we measured commercial cellophane and LDPE films. The “chemically welded” paper (sample d) has a rather high water vapour permeability when compared to the commercial materials tested (Table 2). Cellulose is a hygroscopic material, which explains why also cellophane shows a rather high water permeability value compared to LDPE. The oxygen permeability of sample d was significantly lower compared to commercial references. In the grease barrier test of sample d, the grease did not leak through the sample at all. In summary, the IL-treated paper has good oxygen and grease barrier properties, but it transmits water vapour significantly. Therefore, it may be used in applications in packaging where a water-resistant material with good oxygen barrier properties is required, but a water vapour barrier is not required(Siracusa et al. 2008). Furthermore, as an additional benefit, as an all-cellulose structure that is fully biodegradable, the accumulation of the prepared all-cellulose material in nature is prevented.
Conclusions
In this study, we investigated the “chemical welding” of paper by IL by using a two-step process where the IL is transported into the paper structure with water. Dissolution is achieved by heat activation where water evaporates and fibre surfaces are dissolved in the IL. The solvated cellulose can diffuse as a liquid, embedding the still undissolved parts of fibres into a matrix of regenerated cellulose. We conducted a full set of industry-standard paper tests to evaluate the industrially relevant properties of the material. Both the dry and the wet strength of the paper structure is improved. The treatment conditions can be adjusted to produce paper-like materials or regenerated cellulose films. The most severe conditions produce films that are fully transparent and have excellent oxygen and grease barrier properties. As an all-cellulose composite, the “chemically welded paper” can be a biodegradable alternative to fossil fuel-based plastics in applications that can accommodate its high water vapour permeability.
References
Atalla RH, Vanderhart DL (1984) Native cellulose: a composite of two distinct crystalline forms. Science 223(80):283–285
Borodulina S, Kulachenko A, Galland S, Nygårds M (2012) Stress-strain curve of paper revisited. Nord Pulp Pap Res J 27:318
Cammarata L, Kazarian SG, Salter PA, Welton T (2001) Molecular states of water in room temperature ionic liquids. Phys Chem Chem Phys 3:5192–5200. https://doi.org/10.1039/B106900D
da Cruz NF, Ferreira S, Cabral M et al (2014) Packaging waste recycling in Europe: Is the industry paying for it? Waste Manag 34:298–308. https://doi.org/10.1016/j.wasman.2013.10.035
Eichhorn SJ, Baillie CA, Zafeiropoulos N et al (2001) Review: current international research into cellulosic fibres and composites. J Mater Sci 36:2107–2131. https://doi.org/10.1023/A:1017512029696
Eichhorn SJ, Dufresne A, Aranguren M et al (2010) Review: current international research into cellulose nanofibres and nanocomposites. J Mater Sci 45:1–33
Fink H-P, Weigel P, Purz H, Ganster J (2001) Structure formation of regenerated cellulose materials from NMMO-solutions. Prog Polym Sci 26:1473–1524. https://doi.org/10.1016/S0079-6700(01)00025-9
Foston M (2014) Advances in solid-state NMR of cellulose. Curr Opin Biotechnol 27:176–184
Gardner D, Tajvidi M (2016) Hydrogen bonding in wood-based materials: an update. Wood Fiber Sci 48:234–244
Graenacher C (1934) Cellulose solutions. Patent US1943176
Gurnagul N, Howard RC, Zou X et al (1993) The mechanical permanence of paper: a literature review. J Pulp Pap Sci 19:J160–J160
Hao W, Chen Z, Wang J, Liu X (2004) Modeling of protein adsorption in membrane affinity chromatography. Anal Lett 37:1319–1338
Haverhals LM, Reichert WM, De Long HC, Trulove PC (2010) Natural fiber welding. Macromol Mater Eng 295:425–430. https://doi.org/10.1002/mame.201000005
Haward M (2018) Plastic pollution of the world’s seas and oceans as a contemporary challenge in ocean governance. Nat Commun 9:667. https://doi.org/10.1038/s41467-018-03104-3
Huber T, Müssig J, Curnow O et al (2011) A critical review of all-cellulose composites. J Mater Sci 47:1171–1186. https://doi.org/10.1007/s10853-011-5774-3
Ichiura H, Hirose Y, Masumoto M, Ohtani Y (2017) Ionic liquid treatment for increasing the wet strength of cellulose paper. Cellulose 24:3469–3477. https://doi.org/10.1007/s10570-017-1340-8
Isogai A, Usuda M, Kato T et al (1989) Solid-state CP/MAS carbon-13 NMR study of cellulose polymorphs. Macromolecules 22:3168–3172. https://doi.org/10.1021/ma00197a045
Johansson C, Bras J, Mondragon I et al (2012) Renewable fibers and bio-based materials for packaging applications–a review of recent developments. BioResources 7:2506–2552
Johnson DL (1969) Strengthening swellable fibrous material with an amine oxide. US Patent US3447956A
Katekawa ME, Silva MA (2006) A Review of Drying Models Including Shrinkage Effects. Dry Technol 24:5–20. https://doi.org/10.1080/07373930500538519
Kilpeläinen I, Xie H, King A et al (2007) Dissolution of Wood in Ionic Liquids. J Agric Food Chem 55:9142–9148. https://doi.org/10.1021/jf071692e
Klemm D (1998) Comprehensive cellulose chemistry, vol 1. Fundamentals and analytical methods. Wiley, Weinheim
Liebert T (2010) Cellulose solvents —remarkable history, bright future. In: Liebert T (ed) Cellulose solvents: for analysis, shaping and chemical modification. American Chemical Society, Washington, pp 1–3
Lindström T, Wågberg L, Larsson T (2005) On the nature of joint strength in paper-A review of dry and wet strength resins used in paper manufacturing. In: 13th fundamental research symposium. The Pulp and Paper Fundamental Research Society Cambridge, UK, pp 457–562
Nishino T, Arimoto N (2007) All-cellulose composite prepared by selective dissolving of fiber surface. Biomacromol 8:2712–2716. https://doi.org/10.1021/bm0703416
Orelma H, Korpela A, Kunnari V et al (2017) Improving the mechanical properties of CNF films by NMMO partial dissolution with hot calender activation. Cellulose. https://doi.org/10.1007/s10570-017-1229-6
Paunonen S (2013) Strength and barrier enhancements of cellophane and cellulose derivative films: a review. BioResources 8:3098–3121
Quijada-Maldonado E, van der Boogaart S, Lijbers JH et al (2012) Experimental densities, dynamic viscosities and surface tensions of the ionic liquids series 1-ethyl-3-methylimidazolium acetate and dicyanamide and their binary and ternary mixtures with water and ethanol at T = (298.15–343.15 K). J Chem Thermodyn 51:51–58. https://doi.org/10.1016/j.jct.2012.02.027
Shibata M, Teramoto N, Nakamura T, Saitoh Y (2013) All-cellulose and all-wood composites by partial dissolution of cotton fabric and wood in ionic liquid. Carbohydr Polym 98:1532–1539. https://doi.org/10.1016/j.carbpol.2013.07.062
Simon J, Müller HP, Koch R, Müller V (1998) Thermoplastic and biodegradable polymers of cellulose. Polym Degrad Stab 59:107–115. https://doi.org/10.1016/S0141-3910(97)00151-1
Siracusa V, Rocculi P, Romani S, Rosa MD (2008) Biodegradable polymers for food packaging: a review. Trends Food Sci Technol 19:634–643. https://doi.org/10.1016/j.tifs.2008.07.003
Sjöström E (1993) Wood chemistry: fundamentals and applications. Academic Press, San Diego
Tan X, Li X, Chen L, Xie F (2016) Solubility of starch and microcrystalline cellulose in 1-ethyl-3-methylimidazolium acetate ionic liquid and solution rheological properties. Phys Chem Chem Phys 18:27584–27593. https://doi.org/10.1039/C6CP04426C
Vähä-Nissi M, Laine C, Rautkoski H, Pitkänen M, Vartiainen J, Ohra-Aho T, Sneck A, Gestranius M, Ketoja J (2016) Test methods for evaluating grease and mineral oil barriers. In: TAPPI Place Conference, 10–13 April, Fort Worth, TX, USA
Virtanen T, Maunu SL (2014) NMR spectroscopic studies on dissolution of softwood pulp with enhanced reactivity. Cellulose 21:153–165. https://doi.org/10.1007/s10570-013-0144-8
Zhang L, Ruan D, Zhou J (2001) Structure and properties of regenerated cellulose films prepared from cotton linters in NaOH/urea aqueous solution. Ind Eng Chem Res 40:5923–5928. https://doi.org/10.1021/ie0010417
Zhang B, Azuma J, Uyama H (2015) Preparation and characterization of a transparent amorphous cellulose film. RSC Adv 5:2900–2907. https://doi.org/10.1039/C4RA14090G
Acknowledgments
This study was carried out in the CellFi (Conversion of cellulose to plastic) project funded by Business Finland and Finnish industries companies (Metsä Fibre Ltd, Metsä Board Ltd, Stora Enso Ltd, FL Pipe Ltd, Pölkky Ltd, and Versoul Ltd). Mika Vähä-Nissi is acknowledged for his comments on the barrier measurements, Heikki Pajari is thanked for performing viscosity analyses, and Tommi Virtanen is thanked for performing the NMR analyses. Professor Ilkka Kilpeläinen (University of Helsinki) is thanked for active participation in the CellFi project.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all the authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, A., Khakalo, A., Hauru, L. et al. Conversion of paper to film by ionic liquids: manufacturing process and properties. Cellulose 25, 6107–6119 (2018). https://doi.org/10.1007/s10570-018-1944-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-1944-7