Abstract
In this paper, a new method is introduced for producing multi-functional cellulose nanofibers in order to achieve the biodegradable materials for various applications with a minimal amount of potentially toxic materials. Cellulose nanofibers (CNFs) were fabricated by electrospinning cellulose acetate solution followed by deacetylation. The CNFs were then treated with silver nitrate, ammonia, and sodium hydroxide and subsequently with dopamine as reducing and adhesive agent. Ag ions on the CNF surface were photo-reduced to Ag nanoparticles (NPs) using UVA irradiation to produce a dense layer of silver nanoparticles on the nanofibers. This is based on the simultaneous formation of polydopamine and Ag NPs on CNFs. Overall, this is a fast, simple, and efficient procedure that takes place in a conventional method at ambient temperature. The crystalline structure of CNFs decorated with AgNPs was studied by X-ray diffraction. Field-emission scanning electron microscopy and energy-dispersive X-ray patterns showed uniform distribution of silver nanoparticles on the CNF surface. Incorporation of AgNPs on the CNF surface via dopamine improved the electrical conductivity and also the tensile strength of the nanomat. The CNFs decorated with AgNPs exhibited a low electrical resistivity around 35 KΩ/square and a tensile strength of 87% higher than untreated CNFs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last few decades, a huge interest in the field of cellulose nanofibers has appeared because of wide variety of potential applications such as green composites, energy storage devices, separator membranes, electrical devices, electrochemical actuators, drug delivery systems, solar cells, biosensors, and gas sensors (Jung et al. 2015; Ge et al. 2010; Jonoobi et al. 2015; Jabbour et al. 2013). Cellulose, as the most abundant renewable biopolymer on earth, has exceptionally good mechanical performance at comparable low weight (Lu and Hsieh 2012; Zhang et al. 2013). In addition, formation of cellulose fibers in nanoscale leads to high surface area, large volume-to-mass ratio and high porosity (Tungprapa et al. 2007). Electrospinning of cellulose acetate (CA) with subsequent deacetylation is still the main process for producing cellulose nanofibers (Konwarh et al. 2013; Ohkawa 2015). Cellulose acetate has been electrospun under a wide variety of conditions and straightforward conversion to cellulose (Frey 2008). Liu and Hsieh reported that acetone:dimethylacetamide (2:1) (DMAc) is the most versatile solvent for fabrication of continuous CA nanofibers that can be deacetylated to cellulose using NaOH solution (Liu and Hsieh 2002). Silver nanoparticles represent one of the most commonly studied among metal nanoparticles due to unique optical, electrical, catalytic, and thermal properties (Dastjerdi and Montazer 2010). There are many known methods for synthesis of Ag nanoparticles on cellulosic materials, including hydrothermal reduction (Li et al. 2015), chemical reduction (Jung et al. 2009), microwave irradiation (Li et al. 2011), sonochemical reduction (Perelshtein et al. 2008), laser ablation (Koshy et al. 2016), gamma irradiation (Thi et al. 2014), photochemical (Son et al. 2006), and biological synthesis methods (El-Rafie et al. 2012). Examples of recent reports on this field are presented in Table 1. Numerous research papers and patents are available and significant number of research works is in progress for producing cellulose nanofibers containing silver nanoparticles as ecofriendly materials for various applications (Jung et al. 2015; Martins et al. 2012). For instance, Nogi et al. (2015) produced optically transparent conductive paper with a low sheet resistance of 39 Ω/square using cellulose nanofibers and silver nanowires for lightweight and portable electronic devices. Jang et al. prepared cellulose-based nanofibers with catalytic activity by electrospinning of CA solution with 0–5 wt% AgNO3, UV irradiation and post-deacetylation under alkali condition. They produced cellulose nanofibers with different Ag content and phase showed the important role of Ag ions in catalytic reduction of methylene blue (Jang et al. 2014).
Among the various synthesis methods for the preparation of silver nanoparticles, chemical reduction by using a predominant salt precursor, reducing agent, and stabilizing agent is the most common method (Ravindra et al. 2010). Therefore, the environmental issues associated with the manufacture of silver nanoparticles and usage of the safe material in the synthesis processing is an important subject (Tolaymat et al. 2010).
Dopamine (3, 4-dihydroxyphenethylamine), a mussel-inspired catecholamine, has recently attracted considerable interest as an effective adhesive compound at slightly alkali pH and in the presence of atmospheric oxygen, weak alkali, and oxidants (Xu et al. 2011; Sa et al. 2014). The oxidative self-polymerization of dopamine forms a polydopamine (PDA) layer on a broad range of surfaces including metal, nonmetal, ceramics, and polymers (Zhang et al. 2012; Hao et al. 2016; Li et al. 2012). PDA coating have been frequently reported to be thin, surface-adherent, and robust enough to use for variety of applications including electrochemical systems, biosensors, drug delivery, and tissue engineering (Zhu et al. 2012; Lee et al. 2016). Moreover, the self-polymerization and self-adhesive nature of polydopamine layer leads to spontaneously reduce noble metal ions to metal nanoparticles (Peng et al. 2016; Liao et al. 2010; Rastegar et al. 2016). Su et al. (2015) successfully synthesized monodisperse gold nanorods using dopamine hydrochloride as an effective reducing agent. They reported the action of dopamine as additive and generation of polydopamine as a binding agent for controlling the size and shape of nanoparticles. Wang et al. prepared a highly conductive silver-plated polyester (PET) fiber with a bio-inspired polydopamine coating and subsequently electroless plating of silver on the surface of PET-PDA fiber. They suggested the action of dopamine as an adhesive and reducing agent for synthesis of a silver layer and also decreased resistivity of the silver-plated PET as low as 0.4 m Ω cm (Wang et al. 2012). Hao et al. prepared carbon nanotubes decorated with silver nanoparticles through PDA surface functionalization and UV irradiation. CNT surface was first functionalized by PDA layer and then silver nanoparticles immobilized onto CNTs-PDA surface by the weak reduction performance of PDA layer on CNTs and the high energy of UV light. The as-prepared nanocomposites demonstrated excellent electrical conductivity with a surface conductivity of 340 S/cm (Hao et al. 2016). Su et al. fabricated high performance transparent conductive cellulose with enhanced mechanical robustness and long-term durability based on polydopamine functionalized nanofibrillated cellulose substrate incorporating with silver nanowires network. They showed tightly binding between PDA coating and silver nanowires produced continuous conductive network layer and simultaneously welded the wire to wire. This dramatically decreased the sheet resistance to 14.2 Ω/square and increased the mechanical robustness and chemical stability in order to use in green flexible electronics (Su et al. 2017).
In this research, we explored cellulose nanofibers decorated with Ag nanoparticles manufactured through combination of electrospinning technique and nanofinishing of nanofibers. Therefore, bioinspired dopamine as a reducing and adhesive agent was employed to reduce Ag ions to nanoparticles and simultaneously polymerized and formed polydopamine layer on the surface of CNFs. The coating of PDA on CNFs mechanically strengthen the nanofibers structure and chemically modified nanofibers surface led to easier electron transfer. In addition, UV irradiation accelerates the dopamine-quinone oxidation and generation of Ag nanoparticles. Also, incorporation of Ag nanoparticles on the surface of CNFs causes enhanced mechanical and electrical properties. The tensile strength, electrical resistivity, weight changes, and the structural characteristics of the cellulose nanofibers decorated with silver nanoparticles were also examined.
Materials and methods
Materials
Silver nitrate (AgNO3, extra-pure >99.8%), sodium hydroxide, aqueous ammonia (25%), acetone (99.9%), dimethylacetamide (DMAc, 99.9%) were purchased from Merck Co. (Darmstadt, Germany). Cellulose acetate (CA, Mn = 30,000, 39.8% acetyl groups) and dopamine were supplied from Sigma Aldrich Co (Germany). All the chemicals were used without further purification.
Electrospinning
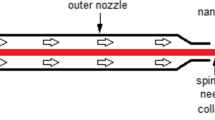
A 17 wt% sample of CA was dissolved in mixture of acetone/DMAc in the volume ratio of 2:1 and stirred for 5 h to achieve the homogeneous solution which is finally considered as a precursor solution for electrospinning. The CA solutions were electrospun at a positive voltage of 20 kV, a needle tip-to-collector distance of 15 cm and a solution flow rate of 3.0 mL/h. Electrospinning was performed at 20 ± 2 °C and relative humidity 45–60%.
Deacetylation of CA nanofibers
Electrospun CA fibrous mats were deacetylated in 0.1 M NaOH solution for 48 h to regenerate into cellulose. The fibrous mats were then rinsed with distilled water to neutralize the pH and dried at ambient conditions.
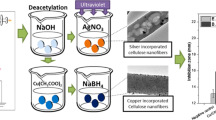
Preparation of cellulose nanofibers decorated with Ag nanoparticles
Silver nitrate was dissolved in ammonia under stirring to produce a transparent solution and then 1 N sodium hydroxide was added to the solution. Electrospun cellulose nanofiber mats were immersed in the solution and then 2 g/L dopamine was added dropwise to initiate the reduction reaction. The samples were then taken out and irradiated under UVA (320 nm) for 2 h at room temperature. The silver coated cellulose nanofibers were finally rinsed with distilled water and dried at room temperature.
Characteristics
The X-ray diffraction (XRD) study was carried out with X-ray diffractometer (model EQuniox3000, INEL, Artenay, France) having nickel-filtered Cu Kα radiation to investigate the crystalline size and phase of the synthesized silver nanoparticles on the cellulose nanofibers. Field emission scanning electron microscopy (FESEM SIGMA VP-500, Germany) was used to observe the surface morphology of the treated electrospun nanofibers and synthesized Ag nanoparticles. Prior to taking FE-SEM pictures, a gold layer was placed on the fabric surface to make the final image much clearer. X-ray mapping images and Energy-dispersive spectroscopy (EDX) (Oxford Instrument, England) patterns was also applied for elemental composition analysis.
Fourier transform infrared (FTIR) spectra of the cellulose/Ag nanofiber were taken on FTIR Microscope (Thermo Nicolet Corporation, Madison, WI, USA) in the range of 4000–675 cm−1 to characterize the changes appeared in functional groups on the cellulose acetate nanofibers after deacetylation and cellulose nanofibers after treatment with dopamine. The electrical resistivity was measured by two-point probe instrument. The tensile strength of the samples before and after treatment was determined using Instron with a gauge length of 2 cm and an extension rate of 5 mm/min. The weight change percentage (\(\Delta W\%\)) of various samples was also determined according to Eq. (1):
where W 1 and W 2 are the weight of samples before and after treatment, respectively.
Result and discussions
The surface modification of CNFs by coating with silver nanoparticles and simultaneous dopamine polymerization was carried out in three main steps. The first step involved fabricating CNFs by electrospinning of cellulose acetate solution and subsequent deacetylation with NaOH solution. The second step was the complex reaction for immobilization of Ag nanoparticles on the CNF surface by the reduction performance of dopamine. The third step consisted of the photo reduction of the residual silver ions to silver nanoparticles and the deposition of the nanoparticles onto the CNFs using UV irradiation. The above mentioned steps are shown in reaction r1 to r8 and each step is described in details.

At first, Ag ions are adsorbed by the catechol and nitrogen groups in dopamine and then reduced to silver nanoparticles. Catechol groups have a moderate reduction capability with a redox potential of −530 mV versus normal hydrogen electrode (NHE) (Son et al. 2013). Oxidative polymerization of dopamine causes the oxidation of catechol group in dopamine and loses two electrons that generates enough reducing capability to reduce Ag+ to metallic Ag0 (Son et al. 2013). Therefore, silver nanopaticles were deposited on the CNF surface through simultaneously formation of polydopamine layer while providing binding sites for the reduced silver nanoparticles. On the other hand, the high energy of UV light leads to photoreduction of the residual silver ions to silver nanoparticles and further diffusion and aggregation on the cellulose nanofibers (Jang et al. 2014).
FTIR
The FTIR method was employed for analysis of the changes appeared in chemical structure of CA after deacetylation and CNFs after treatment with dopamine. The infrared spectrum of the pure cellulose acetate fibers (Fig. 1a, b) shows the characteristic peaks attributed to stretching vibrations of the acetate substituent around 1745 cm−1 (C=O), 1375 cm−1 (C–CH3), and 1235 cm−1 (C–O–C). After deacetylation, the intensity of characteristic peaks ascribed to -OH at 3479 cm−1 increased, while acetate groups disappeared. This proves the formation of pure cellulose fiber as a result of deacetylation with NaOH. After cellulose nanofibers were treated with dopamine, the intensity of absorption peak at 3497 cm−1 assigned to the asymmetric stretching vibration of aromatic O–H increased and also widened. This indicates the successful deposition of polydopamine and formation of hydroxyl groups on the CNF surface.
XRD
Analysis of X-ray diffraction patterns is a suitable method to investigate the purity, crystalline nature, and unit cell dimensions of crystalline compounds. The XRD patterns of the silver-coated cellulose nanofibers are illustrated in Fig. 2. Five obvious peaks located at 37.6°, 43.5°, 63.6°, 76.1°, and 80.6° related to (111), (200), (220), (311), and (222) planes, which are reflections of face-centered cubic (fcc) silver (JCPDS, silver file 4-0783) (Montazer and Allahyarzadeh 2013). No characteristic peaks were observed in this sample corresponded to impurities such as silver halide or silver oxide. Therefore, synthesized pure silver metal with fcc symmetry on the cellulose nanofibers leads to conductive properties on the cellulose nanofibers.
The crystallite size was calculated around 18 nm through Scherer’s Eq. (2):
where K = 0.9 is the shape factor, λ = 1.54 is the wavelength of X-ray of Cu radiation, θ is Bragg’s angle and FWHM is full width at half maximum of the peak (Montazer and Allahyarzadeh 2013).
FE-SEM
The surface morphology of the treated and untreated CNFs samples was observed by FE-SEM and the images are illustrated in Fig. 3. The FE-SEM images of regenerated cellulose nanofibers with two different magnifications (1000 and 2000×) illustrated the smooth surface with the fiber diameters between 90 and 350 nm (Fig. 3a, b). The FE-SEM images of the treated cellulose nanofibers with 0.1 and 0.2 w% AgNO3 are illustrated in Fig. 3c–f, respectively. The average size was estimated around 26.5 and 206 nm for nanoparticles and nanofibers diameter, respectively.
The presence of Ag nanoparticles on CNFs was also confirmed by EDX patterns (Fig. 4). Nitrogen relates to polydopamine formation, and Ag is the extra element on the treated nanofibers compared to untreated nanofibers. The presence of Au in the EDX pattern is due to shielding of a gold layer on the sample before FE-SEM observation. Mapping images (Fig. 5) of Ag indicated a uniform distribution of Ag nanoparticles with no agglomerated particles on the CNF surface. Dopamine with an aromatic structure acts not only as a reducing agent, but also as an additive for controlling the size and shape of Ag nanoparticles (Su et al. 2015). Nanoparticles are kept in suspension by repulsive electrostatic forces between the particles owing to adsorbed dopamine.
Weight change
The weight changes of the treated samples are shown in Table 2. An increase in the weight of cellulose nanofibers (6%) after treatment with dopamine under alkali conditions indicated polydopamine formation (PDA) on the fiber surface. UV light accelerates both the dopamine polymerization and PDA layer formation on the nanofiber surface (Du et al. 2014). This also leads to a thicker PDA layer formation on the fiber surface, which consequently enhances the nanofibers weight by about 11%. Physical interlocking and chemical bonding of Ag NPs with hydroxyl and amine groups of formed PDA on the cellulose nanofibers leads to more weight gain comparing with other treated samples. Therefore, increasing silver nitrate to 0.2 w% in the presence of dopamine and UV irradiation causes more synthesis of Ag NPs on the fiber surface. There is negligible weight change on the treated samples at higher concentration of silver salt (0.3 w%) because of the blocking of the functional groups in PDA and cellulosic nanofibers. It is safe to infer that the polymerization of dopamine and adsorption of AgNPs on the nanofibers results in weight gain.
Tensile strength
The results of tensile strength [tensile strain (%), maximum load (cN), and stress at maximum (MPa)] for treated and untreated CNFs are presented in Table 2. The treated CNFs with dopamine showed higher tensile strain compared to raw CNFs. Dopamine can be oxidized and cyclized through covalently bonding via aryl–aryl linkages and also strong noncovalent forces including charge transfer, π–π stacking, and hydrogen bonding to form spontaneous deposition of an adherent polydopamine layer (Zhang et al. 2012; Son et al. 2013). Polydopamine coating on CNFs generates a highly stable polymer layer that enhances tensile strain of the modified CNFs from 10.88 to 13.40. In addition, cellulose nanofibers treated with dopamine and also UV irradiation for 2 h showed higher tensile strength compared with the same sample kept in dark (6.12 MPa). Du et al. reported that polymerization and formation of polydopamine layer on the solid surfaces can be accelerated by UV light as a trigger. Generation of reactive oxygen species (ROS), including singlet oxygen (1O2), superoxide radicals (O −·2 ), or hydroxyl radicals (·OH) under UV irradiation play a key role for initiating the dopamine polymerization due to oxidation-induced process of polydopamine formation. UV-triggered dopamine polymerization leads to thicker polydopamine on the solid surface in short time and higher tensile strength (Du et al. 2014). On the other hand, the samples treated with silver nitrate in dopamine media indicated higher tensile strength than the untreated fibers. Polydopamine plays a key role in immobilization of Ag nanoparticles on CNF surface due to metal-binding ability of catechol and nitrogen-containing groups presented in the structure (Su et al. 2017; Fu et al. 2014). Introducing Ag nanoparticles into the structure of cellulose nanofibers improved the tensile strength by interaction with hydroxyl groups of CNs creating in the alkali condition (Su et al. 2017). Increasing AgNO3 to 0.2 w% causes more Ag nanoparticles that provides more interfacial interactions and higher tensile strength. Further increase in AgNO3 has no influence on the tensile strength, indicating the surface coating of silver nanoparticles are complete and nanoparticles connect with each other form a continuous layer.
Electrical conductivity
The electrical conductivity of silver coated CNFs was determined by two probe resistance measurement. The results in Table 2 demonstrated that resistivity of silver coated nanofibers decreases with increasing silver nitrate concentration due to formation of a continuous and compact silver nanoparticles coating and closely connected Ag nanoparticles. The electrical resistivity reaches to 100 kΩ/square with 0.1 w% AgNO3; however, a further increase in AgNO3 to 0.2 w% significantly reduces the electrical resistivity to 35 kΩ/square. There is no obvious change in the resistivity when silver nitrate exceeds to 0.2 w%. The highly adhesive PDA coating on NCFs surface uniformly connected Ag nanoparticles and PDA as a welding material simultaneously bridges the discrete Ag nanoparticles by covering the void spaces, strengthening the electrical contact between neighboring nanoparticles, providing more conductive pathways for electron hence improving the overall electrical conductivity.
Conclusion
Here, we made cellulose nanofibers decorated with AgNPs by synthesizing AgNPs with uniform distribution on the nanofiber surface assisted by simultaneous polydopamine layer formation and UV irradiation. PDA coating with catechol and amine functional groups was used as an effective reducing agent to generate Ag NPs, as well as a chemisorption site for the silver nanoparticles and adhesive layer between CNFs and AgNPs. Also, dopamine acts as an additive for controlling the size and shape of AgNPs while polydopmine formed on nanofibers surface improves the electrical conductivity by providing more conductive pathways for electron and tensile strength by generating highly stable polymer layer on the fibers surface. UV irradiation increases polydopamine formation rate and reduction of Ag ions to Ag nanoparticles in short time and then enhances the tensile strength and electrical conductivity. FE-SEM, EDX, and XRD results verified the presence of silver nanoparticles in the metallic state. The cellulose nanofibers decorated with Ag nanoparticles exhibited higher tensile strength and electrical conductivity around 111 cN and 35 KΩ/square in comparison to untreated cellulose nanofibers. This simple method can be used to prepare a variety of metal/CNFs composites for various applications.
References
Dastjerdi R, Montazer M (2010) A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids Surf B Biointerfaces 79:5–18. doi:10.1016/j.colsurfb.2010.03.029
Du X, Li L, Li J, Yang C et al (2014) UV-triggered dopamine polymerization: control of polymerization, surface coating, and photopatterning. Adv Mater 26:8029–8033. doi:10.1002/adma.201403709
El-Rafie MH, Shaheen TI, Mohamed AA, Hebeish A (2012) Bio-synthesis and applications of silver nanoparticles onto cotton fabrics. Carbohydr Polym 90:915–920. doi:10.1016/j.carbpol.2012.06.020
Frey MW (2008) Electrospinning cellulose and cellulose derivatives. Polym Rev 48:378–391. doi:10.1080/15583720802022281
Fu Y, Liu L, Zhang L, Wang W (2014) Highly conductive one-dimensional nano fibers: silvered electrospun silica nano fibers via poly (dopamine) functionalization. ACS Appl Mater Interfaces 6:5105–5112. doi:10.1021/am5002663
Ge D, Ru X, Hong S et al (2010) Coating metals on cellulose-polypyrrole composites: a new route to self-powered drug delivery system. Electrochem Commun 12:1367–1370. doi:10.1016/j.elecom.2010.07.022
Hao M, Tang M, Wang W, Tian M, Zhang L, Lu Y (2016) Silver-nanoparticle-decorated multiwalled carbon nanotubes prepared by poly(dopamine) functionalization and ultraviolet irradiation. Compos Part B Eng 95:395–403. doi:10.1016/j.compositesb.2016.03.084
Jabbour L, Bongiovanni R, Chaussy D, Gerbaldi C, Beneventi D (2013) Cellulose-based Li-ion batteries: a review. Cellulose 20:1523–1545. doi:10.1007/s10570-013-9973-8
Jang KH, Kang YO, Park WH (2014) Functional cellulose-based nanofibers with catalytic activity: effect of Ag content and Ag phase. Int J Biol Macromol 67:394–400. doi:10.1016/j.ijbiomac.2014.03.052
Jonoobi M, Oladi R, Davoudpour Y et al (2015) Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: a review. Cellulose 22:935–969. doi:10.1007/s10570-015-0551-0
Jung R, Kim Y, Kim H-S, Jin H-J (2009) Antimicrobial properties of hydrated cellulose membranes with silver nanoparticles. J Biomater Sci Polym Ed 20:311–324. doi:10.1163/156856209X412182
Jung YH, Chang T-H, Zhang H et al (2015) High-performance green flexible electronics based on biodegradable cellulose nanofibril paper. Nat Commun. doi:10.1038/ncomms8170
Konwarh R, Karak N, Misra M (2013) Electrospun cellulose acetate nanofibers: the present status and gamut of biotechnological applications. Biotechnol Adv 31:421–437. doi:10.1016/j.biotechadv.2013.01.002
Koshy O, Thankappan A, Vibin B, Thomas S, Mujeeb A (2016) Naked eye detection of hydrogen peroxide by laser ablated silver nanoparticle coated flexible paper. J Bionanoscience 10:377–380. doi:10.1166/jbns.2016.1395
Lee H, Han T, Cho KY, Ryou MH, Lee YM (2016) Dopamine as a novel electrolyte additive for high-voltage lithium-ion batteries. ACS Appl Mater Interfaces 8:21366–21372. doi:10.1021/acsami.6b06074
Li SM, Jia N, Ma MG, Zhang Z, Liu QH, Sun RC (2011) Cellulose-silver nanocomposites: microwave-assisted synthesis, characterization, their thermal stability, and antimicrobial property. Carbohydr Polym 86:441–447. doi:10.1016/j.carbpol.2011.04.060
Li Q, Tian M, Liu L, Zou H, Zhang L, Wang WC (2012) Facile preparation of α-Fe2O3@Ag core-shell structured nanoparticles. Electrochim Acta 91:114–121. doi:10.1016/j.electacta.2012.12.137
Li R, He M, Li T, Zhang L (2015) Preparation and properties of cellulose/silver nanocomposite fibers. Carbohydr Polym 115:269–275. doi:10.1016/j.carbpol.2014.08.046
Liao Y, Wang Y, Feng X, Wang W, Xu F, Zhang L (2010) Antibacterial surfaces through dopamine functionalization and silver nanoparticle immobilization. Mater Chem Phys 121:534–540. doi:10.1016/j.matchemphys.2010.02.019
Liu H, Hsieh YL (2002) Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J Polym Sci, Part B: Polym Phys 40:2119–2129. doi:10.1002/polb.10261
Lu P, Hsieh YL (2012) Multiwalled carbon nanotube (MWCNT) reinforced cellulose fibers by electrospinning. ACS Appl Mater Interfaces 2:2413–2420. doi:10.1021/am1004128
Martins NCT, Freire CSR, Pinto RJB et al (2012) Electrostatic assembly of Ag nanoparticles onto nanofibrillated cellulose for antibacterial paper products. Cellulose 19:1425–1436. doi:10.1007/s10570-012-9713-5
Montazer M, Allahyarzadeh V (2013) Electroless plating of silver nanoparticles/nanolayer on polyester fabric using AgNO3/NaOH and ammonia. Ind Eng Chem Res 25:8436–8444. doi:10.1021/ie400804n
Nogi M, Karakawa M, Komoda N, Yagyu H, Nge TT (2015) Transparent conductive nanofiber paper for foldable solar cells. Nat Publ Gr 5:1–7. doi:10.1038/srep17254
Ohkawa K (2015) Nanofibers of cellulose and its derivatives fabricated using direct electrospinning. Molecules 20:9139–9154. doi:10.3390/molecules20059139
Peng L, Guo R, Lan J, Jiang S, Lin S (2016) Microwave-assisted deposition of silver nanoparticles on bamboo pulp fabric through dopamine functionalization. Appl Surf Sci 386:151–159. doi:10.1016/j.apsusc.2016.05.170
Perelshtein I, Applerot G, Perkas N, Guibert G et al (2008) Sonochemical coating of silver nanoparticles on textile fabrics (nylon, polyester and cotton) and their antibacterial activity. Nanotechnology 19:245705. doi:10.1088/0957-4484/19/24/245705
Rastegar L, Montazer M, Gaminian H (2016) Clean low-temperature in situ synthesis of durable silver nanoparticles along with aminolysis of polyester fabric using dopamine hydrochloride. Clean Technol Environ Policy. doi:10.1007/s10098-016-1127-x
Ravindra S, Murali Mohan Y, Narayana Reddy N, Mohana Raju K (2010) Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via “Green Approach”. Colloids Surf A Physicochem Eng Asp 367:31–40. doi:10.1016/j.colsurfa.2010.06.013
Sa R, Yan Y, Wei Z, Zhang L, Wang W, Tian M (2014) Surface modification of aramid fibers by bio-inspired poly(dopamine) and epoxy functionalized silane grafting. ACS Appl Mater Interfaces 6:21730–21738. doi:10.1021/am507087p
Son WK, Youk JH, Park WH (2006) Antimicrobial cellulose acetate nanofibers containing silver nanoparticles. Carbohydr Polym 65:430–434. doi:10.1016/j.carbpol.2006.01.037
Son HY, Ryu JH, Lee H, Nam YS (2013) Silver-polydopamine hybrid coatings of electrospun poly(vinyl alcohol) nanofibers. Macromol Mater Eng 298:547–554. doi:10.1002/mame.201200231
Su G, Yang C, Zhu JJ (2015) Fabrication of gold nanorods with tunable longitudinal surface plasmon resonance peaks by reductive dopamine. Langmuir 31:817–823. doi:10.1021/la504041f
Su Y, Zhao Y, Zhang H, Feng X, Shi L, Fang J (2017) Polydopamine functionalized transparent conductive cellulose nanopaper with long-term durability. J Mater Chem C. doi:10.1039/C6TC04928A
Thi T, Van Phu D, Thi N, Anh L, Duyen D, Quoc N (2014) Gamma irradiation of cotton fabrics in AgNO3 solution for preparation of antibacterial fabrics. Carbohydr Polym 101:1241–1248. doi:10.1016/j.carbpol.2013.10.069
Tolaymat TM, El Badawy AM, Genaidy A, Scheckel KG, Luxton TP, Suidan M (2010) An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papers. Sci Total Environ 408:999–1006. doi:10.1016/j.scitotenv.2009.11.003
Tungprapa S, Puangparn T, Weerasombut M et al (2007) Electrospun cellulose acetate fibers: effect of solvent system on morphology and fiber diameter. Cellulose 14:563–575. doi:10.1007/s10570-007-9113-4
Wang W, Cheng W, Tian M, Zou H, Li L, Zhang L (2012) Preparation of PET/Ag hybrid fibers via a biomimetic surface functionalization method. Electrochim Acta 79:37–45. doi:10.1016/j.electacta.2012.06.063
Xu H, Shi X, Ma H, Lv Y, Zhang L, Mao Z (2011) The preparation and antibacterial effects of dopa-cotton/AgNPs. Appl Surf Sci 257:6799–6803. doi:10.1016/j.apsusc.2011.02.129
Zhang Y, Thingholm B, Goldie KN, Ogaki R, Städler B (2012) Assembly of poly (dopamine) films mixed with a non- ionic polymer. Langmuir 28:1–3
Zhang Y, Nypelö T, Salas C, Arboleda J, Hoeger IC, Rojas OJ (2013) Cellulose nanofibrils. J Renew Mater 1:195–211. doi:10.7569/JRM.2013.634115
Zhu L, Lu Y, Wang Y, Zhang L, Wang W (2012) Preparation and characterization of dopamine-decorated hydrophilic carbon black. Appl Surf Sci 258:5387–5393. doi:10.1016/j.apsusc.2012.02.016
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaminian, H., Montazer, M. Decorating silver nanoparticles on electrospun cellulose nanofibers through a facile method by dopamine and ultraviolet irradiation. Cellulose 24, 3179–3190 (2017). https://doi.org/10.1007/s10570-017-1343-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1343-5