Abstract
Ionic liquid 1-ethyl-3-methylimidazolium acetate ([EMIM]oAc) was employed for the pretreatment of sugarcane bagasse (SCB) and extraction of lignin, a potentially valuable by-product of the biofuel industry. Response surface methodology based on central composite design was exploited and thereby an empirical model, exhibiting a coefficient of determination, R2, of 0.9890, was established to optimize lignin recovery. In particular, a maximum lignin yield, equal to 90.1%, was calculated at the optimal pretreatment conditions, namely time: 120 min, temperature: 140 °C, and ionic liquid to bagasse ratio equal to 20:1 (wt/wt). The presence of guaiacyl and syringyl rings in lignin was confirmed by Fourier transform infrared spectroscopy (FTIR); whereas UV–Vis spectrophotometry showed that both p-coumaric acid and ferulic acid were contained in the lignin. Thermal analysis indicated a maximum decomposition rate of 2%/°C at 265 °C while Gel permeation chromatography analysis revealed that the molecular weight (Mw) of recovered lignin was equal to 1769 g/mol. Comparison of FTIR spectra of pretreated and untreated bagasse showed a negligible presence of lignin in the pretreated samples. Maximum delignification of bagasse after pretreatment was thus ensured. Thermal stability of the ionic liquid towards recyclability was proven by thermogravimetric analysis. The present study established adequate performance of neat and recycled ([EMIM]oAc) with regard to lignin recovery from SCB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignin, the most predominant plant-derived aromatic macromolecule, currently has the attention of researchers due to its availability, which may result in a huge and profitable production of lignin-based chemicals. Lignin is a biofuel industry by-product and its proper use and development into valuable products could lead to sustainable economic growth of biorefineries. The emerging areas for potential application of lignin are bioplastics, dispersants, (nano) composites, nanoparticles and carbon fiber manufacturing (Norgren and Edlund 2014). Lignin is a polymer constituted by three monolignols: coniferyl alcohol, p-coumeryl alcohol, and sinapyl alcohol. It has an amorphous, cross-linked structure (Boeriu et al. 2004). As lignin is one of the main components of lignocellulose, it is recovered and isolated from different kinds of biomass during pretreatment. Around 20–30% lignin can be found in the structure of sugarcane bagasse, which is the lignocellulosic fibrous material remaining after extraction of the juice of sugarcane (Canilha et al. 2012). Sugarcane is one of the most important crops cultivated in India and Brazil. The production of sugarcane was approximately 652 million metric tons during the 2013/14 harvest year in Brazil (Antunes et al. 2014) and 336.15 million tons in the 2012/13 harvest year in India (Solomon 2014). Sugarcane bagasse was proposed as a suitable substrate for bio-ethanol production (Chakraborty et al. 2012). Bagasse is a complex matrix of the polysaccharides cellulose and hemicelluloses, to which lignin is cross-linked. Lignin binds hemicelluloses by ester linkage (Moghaddam et al. 2014). Cellulose is the linear polymer of glucose sugar residues that are linked by β-1,4-glycosidic bonds; whereas, hemicelluloses are short, highly branched heteropolymers of pentose and hexose sugars (Balat 2011). Pretreatment of lignocellulose to rupture the strong linkage between lignin and hemicelluloses is essential to recover lignin as well as to disrupt the crystalline structure to make it accessible to the enzyme aimed at its bioconversion.
Among the different available pretreatment processes for biomass, room temperature ionic liquids (RTILs) have achieved some success due to their eco-friendliness, recyclability, high thermal stability, and non-volatile nature (Li et al. 2009). RTILs are organic salts composed of cation/anion pairs having a melting point below 100 °C (Huddleston et al. 1998). Green synthesis of RTILs, their use in lignocellulose processing, and their successful recycling turned the attention of researchers towards the exploitation of this novel biomass pretreatment. RTILs are eminent solvents for decrystallization and dissolution of carbohydrate and extraction of lignin (Weerachanchai et al. 2012). However, there are certain major limitations to use of ionic liquids in industry. These are represented by the high costs related to their production and to the handling and pumping of a very high viscosity solution. Such drawbacks can be overcome by using an aqueous ionic liquid (Fu and Mazza 2011), synthesizing ionic liquid and recycling it after each pretreatment step.
Recently, many research groups extracted lignin from lignocellulosic biomass using different ionic liquids. Saha et al. (2017) reviewed ionic liquid aided recovery of lignin from the different lignocellulosic material. Commercial imidazolium-based ionic liquids with acetate or chloride anions were identified as efficient solvents for lignocellulosic biomass dissolution, and aimed at recovering lignin in pure form. (Yuan et al. 2013; Casas et al. 2012). Kim et al. (2011) recovered almost 5.8% lignin while processing poplar wood with commercial [EMIM]oAc. Lignin yield increased when eucalyptus stem, a lignocellulosic biomass, was treated with organic solvent along with ionic liquid (Sun et al. 2013). Moghaddam et al. (2014) reported a recovery of 42% lignin, having a purity higher than 90%, while treating sugarcane bagasse with commercial 1-butyl-3-methylimidazolium chloride [BMIM]Cl or 1-butyl-3-methylimidazolium methylsulfonate [BMIM][CH3SO3] with hydrochloric acid as catalyst. Ionic liquids (Imidazolium) derived from a food additive were successfully applied for lignin extraction from wood, as well (Pinkert et al. 2011). Zhang et al. (2015) synthesized six different functional acidic ionic liquids having separate anions and applied the same to bring about ultrasound mediated corn stover fractionation at 70 °C. 60.48% of S-G-H-type lignin recovery, 92.55% cellulose conversion and 97.77% reducing sugar yield from cellulose fraction were observed when corn stover was treated with 1-ethyl-3-methylimidazolium chloride ([HMIM]Cl). Ma et al. (2016) investigated the efficiency of pyrrolidonium ionic liquid for extraction of lignin from corn stalk and achieved 19.07% lignin yield (85.94% of original corn stalk lignin) while pretreatment was carried out using laboratory synthesized N-methyl-2- pyrrolidonium chloride ([Hnmp]Cl). Rashid et al. (2016) synthesized pyridinium-based protic ionic liquids to study the dissolution of commercial cellulose and commercial kraft lignin in ionic liquids. Maximum lignin solubility of 70% was observed in the case of pyridinium formate at a temperature of 75 °C. Hydrogen bond basicity, long alkyl chain length and an increase in water content in ionic liquid decreased the solubility of lignin. The average molecular weight and Polydispersity index of regenerated lignin was lower than that of pure kraft lignin. Ionic liquid pretreatment not only recovered lignin but also enhanced the enzymatic digestibility of carbohydrate materials contained in biomass (Ninomiya et al. 2015). Barr et al. (2014) studied the effect of pretreatment temperature on poplar, switch grass and corn stover in terms of enzymatic hydrolysis and found maximum glucose conversion at 120, 110 and 100 °C for poplar, switch grass and corn stover, respectively. Lee et al. (2015) studied the solid acid-mediated saccharification of sago waste, following its pretreatment with 1-butyl-3-methylimidazolium chloride ([BMIM]Cl). Lignin was not separated but almost 60% of the ionic liquid was directly recovered from hydrolysate solution by a potassium phosphate mediated multi step aqueous biphasic system. Recycling of ionic liquid without losing its chemical structure, properties, and lignocelluloses processing activity demonstrated that the biomass pretreatment process is eco-friendly, as studied by Sun et al. (2013) and Pinkert et al. (2011). Prado et al. (2016) studied the recyclability of methylsulphate 1-butyl-3methylimidazolium ([Bmim][MeSO4]) for lignin extraction from Apple tree pruning fibers and found similar purity of lignin in three consecutive cycles. In the 4th cycle, significant structural changes of ionic liquid were reported.
Till date, there are very few reports on optimization of process parameters for pretreatment of lignocellulosic biomass by ionic liquids. Sidik et al. (2013) studied the optimization of lignin production from empty fruit bunch using [BMIM]Cl and obtained 26.6% lignin recovery at optimum condition i.e. 150.5 °C, 151 min, Empty fruit bunch (EFB): ionic liquid (IL)-1:3 and 4.73 wt% catalyst concentration. Another research group investigated the optimum conditions for sugarcane bagasse pretreatment by 1-ethyl-3-methylimidazolium acetate [EMIM]oAc in terms of enzymatic hydrolysis of recovered cellulosic material (Yoon et al. 2012). The literature has shown [EMIM]oAc to be the most appropriate solvent for sugarcane bagasse pretreatment as compared to other imidazolium-based ionic liquids and conventional acid and alkali pretreatments (Yoon et al. 2011). Several pretreatment conditions were applied to recover lignin, so optimization of process parameters is necessary to determine the required conditions to obtain the highest lignin yield. The conventional approach involves numerous sets of experiments, which make the process time-consuming; in addition, this procedure might not sufficient to determine the combined effects of all the experimental conditions. As an alternate, Response Surface Methodology (RSM) represents a significant tool for statistical modelling and can be used to investigate the optimum process conditions using Central Composite Design (CCD), thereby reducing the total number of tests that are to be performed (Timung et al. 2015). Response Surface Methodology is superior and quicker than conventional techniques as it describes both the influence of individual independent factors and the effect of their interaction (Trinh and Kang 2011). RSM based on CCD is the most relevant design used to fit second order model.
This study investigated sugarcane bagasse pretreatment using the ionic liquid [EMIM]oAc as this green solvent does not alter the chemical structure of lignin (Lee et al. 2009). The aim of the paper was to explore the influence of temperature, time and ionic liquid to biomass loading ratio on the recovery of lignin from sugarcane bagasse. Experimental conditions for lignin recovery were determined. Recovered lignin under the calculated set of optimal conditions were characterized by Thermogravimetric analysis (TGA), Gel permeation chromatography (GPC) and UV–Vis spectrophotometry and Fourier transform infrared spectroscopy (FTIR), this last was also used to characterize pretreated and untreated bagasse. The efficiency of recycled ionic liquid for lignin recovery was investigated, as well.
Materials and methods
Materials
Sugarcane bagasse (SCB) was collected as waste from a juice mill in Durgapur, West Bengal, India. First, SCB was washed to remove dust, sieved to an average size of 1–3 cm and dried at 60 °C for 24 h in a hot air oven. Dried bagasse was ground to a fine powder (Bajaj Twister Mixer Grinder), then screened into 250–500 µm particle size and stored at room temperature (30 °C) in an airtight container for pretreatment. Ionic liquid 1-ethyl-3-methylimidazolium acetate (>95%) was purchased from Io-li-tech, Germany, and acetone (>95%) was purchased from Sigma Aldrich (USA). The lignin content of sugarcane bagasse was determined as: 18% acid insoluble lignin and 4.2% acid soluble lignin by the National Renewable Energy Laboratory (NREL) standard procedure (Sluiter et al. 2012).
Recovery of lignin from SCB and regeneration of IL
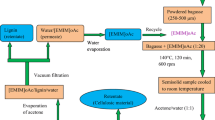
Pretreatment of sugarcane bagasse for recovery of lignin was conducted by charging air-dried bagasse powder and ionic liquid [EMIM]oAc (wt/wt) in a 30 ml culture vial. The reaction homogenization was performed by heating the culture vial using a hot plate cum magnetic stirrer set up (IKA® RCT Basis Safety Control, Germany) at various temperatures (100–140 °C), times (60–120 min) and ionic liquid to bagasse ratio (10:1–20:1) (wt/wt). This instrument features both temperature controller and magnetic stirrer. After completion of the reaction, the pretreated sample was cooled to room temperature. Acetone/deionized water (1:1 v/v) was added to the bagasse—ionic liquid solution in 10:1(v/v) ratio and stirred for 1 h so as to regenerate cellulosic material. Then, it was filtered using a 0.45 µm filter paper under vacuum (AXIVA) to recover cellulosic material as retentate. The cellulosic material was washed with acetone/deionized water (1:1) to remove the ionic liquid. Then it was dried overnight at 60 °C and kept in sealed container for analysis. The pretreated bagasse (cellulosic material) was analyzed by the NREL method. The content of acid insoluble lignin was equal to 1.4% and that of acid-soluble lignin was 0.9% at the determined conditions. After separating cellulose, acetone was evaporated from the remaining solution (filtrate) using a hot plate (IKA® RCT Basis Safety Control, Germany) at 60 °C. Lignin was precipitated as the acetone evaporated from the filtrate. The remaining solution was filtered to recover lignin by vacuum filtration and washed twice with deionized water. Lignin was dried overnight at 50 °C and kept in a sealed vial; lignin content was measured by gravimetry. For complete removal, water was evaporated from the permeate at 100 °C using a hot plate (IKA® RCT Basis Safety Control, Germany) and the ionic liquid was recycled. Although contact with water or moisture converts [EMIM]oAc to acetic acid, ethylimidazole and methyl acetate, after water evaporation the resulting ionic liquid performed equally as well as its original, pure form (Wendler et al. 2012; Maton et al. 2013). Lignin recovery process from sugarcane bagasse is sketched in Fig. 1. Lignin yield was determined according to Eq. 1 (Sun et al. 2013):
Analytical methods
The lignin sample was mixed with about 80–90% potassium bromide (KBr) and pressed to form a pellet for FTIR analysis to indicate its chemical structure (Ninomiya et al. 2015). FTIR spectra of lignin were recorded on an ‘IR Prestige- 21, SHIMADZU spectrometer (Japan) scanned at a wavelength range of 4000–400 cm−1 at a resolution of 4 cm−1. The semi-quantitative analysis of lignin was performed by Ultraviolet (UV) scanning. The sample was prepared according to the procedure described by Sidik et al. (2013). Five mg of lignin was dissolved in 10 ml of dioxane/water (9:1) solution. One millilitre of this aliquot was diluted to 10 ml in 1:1 dioxane/water solution and it was scanned from 260 to 400 nm in a UV spectrophotometer (CECIL, 7000 series, India). Different combustion phases of lignin were determined by thermogravimetric analysis (TGA). TGA, SHIMADZU, Japan instrument was used to study lignin thermal decomposition features. The analysis was carried out from room temperature to 700 °C at a 10 °C/min heating rate. Herein an inert atmosphere was maintained using a N2 flow at 25 ml/min. Fresh and recycled ionic liquid were characterized by TGA, wherein the temperature ranged from room temperature to 400 °C and the heating rate and nitrogen flow were the same as those of lignin. The molecular weight of extracted lignin was determined by GPC (Agilent Technologies 1260 infinity) equipped with PLgel 20 micron mixed-A column (300 × 7.5 mm) and RI detector. Mw, Mn and polydispersity (Mw/Mn) were determined from the chromatogram. Each sample was prepared at room temperature by dissolving 5 mg of lignin in 500 µl N,N-dimethylformamide (DMF). The operating conditions were: mobile phase: DMF, Column temperature: 30 °C; injection volume: 20 µl and flow rate: 1 ml/min. Calibration was performed by using known molecular weight polystyrene as standard.
Experimental design
Response Surface Methodology is a widely applied statistical tool that optimizes numerous chemical processes (Cao et al. 2014). Central Composite Design (CCD) of RSM was implemented by Design Expert Software to optimize the process conditions pertaining ionic liquid pretreatment. The effects of three independent process variables: temperature (A), time (B) and ionic liquid to bagasse ratio (C) were investigated on the response of lignin yield (Y). A total of 20 experimental runs were carried out applying an axial distance α value of 1.682 to optimize the operating conditions.
Table 1 represents the operational ranges as well as the coded values and the actual units of computation of the three independent variables considered in this study. The CCD, in the present case, comprised 8 factorial points, 6 axial points and 6 duplicates of center points, thus resulting in a total number of 20 treatment points, according to the equation “2m + 2 m + mc = M”, where m is the number of independent variables and M is the total number of experiments (Dasgupta et al. 2015). Center points validate data reproducibility by repeating certain sets of experiments and give an evaluation of the experimental error variation. Thus, center points are chosen to estimate the experimental design accuracy (Chakraborty et al. 2014). The exploratory design matrix consisting of 20 experimental sets along with the response yield was shown in Table 2.
The response surface model predicting the interaction between the input variables and their relationship with the output response was depicted by a second order polynomial equation:
where Y is the predicted response, Xi, Xj are the regressors values. Ao, Ai, Aii, Aij signify constant, linear, quadratic and interaction coefficient, respectively (Dasgupta et al. 2015). The regression coefficients of multiple regression model were assessed by data acquired from CCD. The analysis of variance (ANOVA) studies, response surfaces, curves, residual plots were created using Design-Expert® Version 8.0.6: Stat-Ease, Inc. software.
Results and discussion
The influence of important parameters, such as pretreatment temperature, time and solid loading (ionic liquid to bagasse ratio) on the yield of lignin recovery, was investigated through Response Surface Methodology.
Statistical analysis
The data, summarized in Table 2, were used to identify the regression coefficients by way of RSM. ANOVA was implemented to examine whether the established model was significant or not. Table 3 summarizes the results derived from ANOVA studies for the recommended model (Eq. 2).
The Model F-value of 100.24 implied that the model was significant at the 95% confidence level. The p value statistics were used to determine the significance of the linear, quadratic, and interaction effects of the model terms on response yield. Model terms are considered to be significant if their p value or “Prob > F” value is less than 0.05. In this model, the p value was less than 0.0001, which implied that the lignin yield was effectively satisfied by Eq. 2. The p values for each model term recommended that A, C, AB, and BC were significant model terms i.e. they had substantial effects on lignin recovery under 95% confidence level.
Overall, the linear effects of temperature and pretreatment time, interaction effects of temperature and pretreatment time, and interaction effect of pretreatment time and ionic liquid to bagasse ratio significantly influenced lignin yield. As the interaction AB is a significant term, the model should also include the main effects A and B, with the objective to maintain the hierarchy of the model (Nelder 1998). The final equation, after elimination of insignificant factors obtained in terms of coded factors fitting the observed data for the yield of lignin is:
where R denoted the response yield and A, B, C are coded factors of temperature, time, and ionic liquid to bagasse ratio. Figure 2 compares the experimental lignin yield (Y) with the statistically predicted response derived from the process model.
The figure demonstrated that the experimental and the predicted results were closely clustered along the line of best fit, thereby indicating reasonable consistency between observed and predicted values. In this study, the coefficient of determination (R2) for lignin yield was 0.9890 implying a 98.90% variability of response, which indicated a good relationship between predicted and experimental values.
Table 4 shows the value of standard deviation, mean value, Coefficient of variation, R-squared, Adj R-squared, Pred R-squared, adequate precision value of the developed model.
The Coefficient of Variation (CV) is the ratio of standard error of the estimate to the mean value of the response. This term is defined as a percentage and represents a factor of model reproducibility. When the value of CV is lower than 10%, the model can be considered as reproducible. The calculated value of CV in the present study was equal to 2.69%. Furthermore, the Adj R2 is considered as a precise assignment of regression model quality. It does not always increase with the addition of variables to the model. Rather, it may decrease when unnecessary terms are added to the model (Chakraborty et al. 2014). The “Predicted R2” value in the present study was 0.9470 which concurred adequately with “Adj R2” value of 0.9792 and R2 value of 0.9890. The “Adeq Precision” parameter, defined as the ratio between signal to noise, should have a value greater than 4. In this study 41.156 indicated an adequate signal. Thus this model could be used to adequately explore the design space, as represented by the considered variables. The STD DEV (% of error) values for all the parameters are: Temperature (A) 16.96, Pretreatment time (B) 25.43, and Ionic liquid to bagasse ratio (C) 4.24. Altogether, the statistical sets corroborated the efficiency and capability of the present regression model to represent data as well as providing a worthy explanation of the correlation between experimental process condition and response yield. The second order model adequately predicted the yield of lignin under several process conditions within the prescribed range of study.
Effect of pretreatment variables on lignin yield
On the basis of the obtained quadratic model, response surface graphs (Fig. 3) of three dimensions were developed to deduce the correlative effects of independent process variables on the response yield, by differing two variables over their defined ranges, keeping the third one constant at the center level (Fig. 3a) or a higher level (Fig. 3b). The resulting plots reveal the analytical nature of the interaction between the parameters and their influence on the response yield within the range of study.
Interaction effect of pretreatment time and temperature
The interactive relationship of the effects of temperature and time on lignin recovery at a constant bagasse to ionic liquid ratio of 1:15 is presented in the three-dimensional response surface plot shown in Fig. 3a. The interactive effects of temperature and time had a significant effect on response as its p value was less than 0.0001. Lignin recovery increased with an increase in temperature from 100 to 140 °C and 60–120 min showing consistency with an earlier report (Sidik et al. 2013), as reaction rate was proportional to temperature. The trend showed a better yield of lignin with the increase in the temperature at higher pretreatment time, thus resulting in higher bagasse delignification as well as in higher lignin recovery. Rising the temperature from 80 to 100 °C reduces the viscosity of [EMIM]oAc, which further boosts the mixing property (Zavrel et al. 2009). Nonetheless a temperature increase above 120 °C caused a 20% decomposition of ionic liquid as per this current study.
Interaction effects of pretreatment time and ionic liquid to bagasse ratio on lignin yield
Figure 3b revealed the interactive effects of pretreatment time (B) and ionic liquid to bagasse ratio (C) on lignin yield. Interaction of both the parameters (B × C) was significant as indicated by the ANOVA results, wherein the p value corresponding to (B × C) was less than 0.05. The trend showed an increase in lignin yield with increasing ionic liquid/bagasse ratio at a fixed time.
Imidazolium ionic liquid depolymerized lignin by cleaving β-O-4 ether linkages and forming guaiacol (Jia et al. 2010). Thus, it could be considered as a potential solvent that induced the disruption of lignocellulose molecular structure. Lignin solubility in ionic liquid solvent was promoted by a large amount of ionic liquid present in the reaction mixture (Sidik et al. 2013). With the increase in both IL/bagasse loading ratio and pretreatment time, the heating rate of the biomass increased, causing the bagasse to react with the ionic liquid and brought about sufficient delignification or efficient lignin extraction. The viscous nature of ionic liquid usually hinders its mixing with biomass and thus restricts mass diffusion. This was overcome by increasing ionic liquid content. This phenomenon enhanced lignin dissolution by inducing biomass collision on the surface of the ionic liquid (Tan and Lee 2012). Thus, higher IL/bagasse loading ratio along with prolonged reaction time gave a better yield of lignin as compared to that obtained using higher loading and relatively less reaction time. This phenomenon was validated in this study using the surface plot obtained.
Optimization of pretreatment conditions
Utilization of ionic liquid [EMIM]oAc in sugarcane bagasse pretreatment and subsequent lignin recovery is an expensive method for biomass treatment. Hence, optimization of pretreatment conditions is mandatory so as to employ the most feasible levels of the process parameters, in general, and ionic liquid, in particular, for bagasse dissolution and lignin recovery. Thus, in our present approach, based on the trend provided by statical analysis, higher temperature, higher reaction time and higher solid loading ratio were favoured in order to get maximum lignin yield. The complete statistical assessment confirmed that the established model was suitable enough to fit the experimental data and demonstrated a good relationship between process parameters and their effects on the obtained response. The acquired equation suggested a proper justification for the yield of lignin from sugarcane bagasse using ionic liquid [EMIM]oAc under different experimental conditions within the range of study.
In other work related to lignin recovery, a maximum yield of 26.6% lignin from empty fruit bunch under optimal conditions i.e. 150.5 °C temperature, 151 min and IL to EFB- 3:1 ratio was investigated by Sidik et al. (2013) while using [BMIM]Cl for pretreatment. The present experimental design showed higher lignin yield under optimal conditions using lower temperature but higher ionic liquid quantity than the observation of Sidik et al. (2013). Actually, our study used a higher quantity of ionic liquid, however, as this solvent could be recycled and reused up to three or four times, this process is potentially economically feasible.
In spite of the trend shown by our results, even higher temperature and longer time of pretreatment were not considered because, besides impairing the economy viability of the process, those conditions could cause the overcooking of biomass leading to burning of biomass surface and formation of other inhibitory components due to cellulose decomposition (Teramoto et al. 2008; Sun and Chen 2008).
FTIR analysis of recovered lignin
FTIR spectroscopy is a method to characterize lignin that demonstrates structural features of lignin recovered from sugarcane bagasse by [EMIM]oAc pretreatment. The spectrum was taken at middle infrared region (4000–400 cm−1) because organic compounds generally show fundamental vibrations in this region (Ghaffar and Fan 2013). The spectrum of lignin is shown in Fig. 4, allowng prediction of the chemical composition and lignin functional groups. The wide band at 3100–3500 cm−1 was recognized as O–H stretching and the presence of hydrogen bonds between the alcoholic/aliphatic hydroxyl groups and phenolic hydroxyl groups (Ghaffar and Fan 2013; Moghaddam et al. 2014). The infrared absorption bands at 2850–2936 cm−1 were attributed to the C–H asymmetric and symmetrical vibration in methyl/methylene groups and methoxyl groups, thereby indicating the presence of these groups in lignin (Kim et al. 2011; Moghaddam et al. 2014). The peak between 1599 and 1606 cm−1 and peak at 1502 cm−1 raised due to aromatic skeletal vibrations (Koo et al. 2011; Ghaffar and fan 2013; Sun et al. 2013). The characteristic peak at 1512.19 cm−1 was assigned as C–C stretching vibrations of aromatic skeletal (Kim et al. 2011; Moghaddam et al. 2014; Ninomiya et al. 2015). The bands at 1462 and 1425 cm−1 signified C–H asymmetric bending of methylene groups and aromatic ring vibration with C–H deformation in-plane (Sun et al. 2013). The peak at 1328 cm−1 was associated with syringyl ring breathing with C=O vibration and condensed guaiacyl absorption (Casas et al. 2012; Ninomiya et al. 2015). Stretching of C-O in ester groups occurred at the peak between 1165 and 1168 cm−1 (Ghaffar and fan 2013). Ether-O- was assigned to the absorption at 1125–1126 cm−1 (Ninomiya et al. 2015). A strong peak intensity between 1032 and 1034 cm−1 indicated aromatic C–H in-plane deformation and deformation of C–O in primary alcohol and unconjugated C=O vibration (Sun et al. 2013). The peak at 985 cm−1 possibly signified the C=N stretch, which might occur due to the presence of ionic liquid in lignin sample. The occurrence of C–H out-of-plane deformation in the 2nd and 6th position of syringyl unit was indicated by the strong intensity of the peak at 835 cm−1 (Casas et al. 2012). All the above-mentioned peaks showed that the process of lignin extraction from sugarcane bagasse using [EMIM]oAc was successful and the lignin was extracted almost in pure form.
Thermal analysis of recovered lignin
Thermal stability of extracted lignin was examined using thermogravimetric analysis (Fig. 5). DTG curve (Fig. 5b) revealed a maximum decomposition rate of 2%/ °C corresponding to a temperature of 265 °C. This temperature is denoted as Tmax as maximum lignin degradation occurs at this temperature. Casas et al. (2012) studied the thermal stability of commercial lignin (Indulin AT lignin from Sigma Aldrich) and found Tmax at 378 °C, and 10% weight loss, i.e. T10%, at 303 °C. Tmax of bagasse lignin in this study is much lower than observation for commercial lignin by Casas et al. (2012). In the current study, 10% weight loss was observed at 211 °C i.e. T10% = 211 °C indicating the maximum thermal stability of lignin up to 211 °C. The TGA curve (Fig. 5a) showed three steps of lignin decomposition; (1) 50–130 °C which is due to lignin dehydration, (2) 130–450 °C due to inter-unit link breakage, which gave rise to formation of phenolic, organic products, (3) slow decomposition above 450 °C occurred for the degradation of aromatic rings (Casas et al. 2012). Cracking of the methoxy group and release of methane occurred above 450 °C (Moghaddam et al. 2014). TGA results show almost 48% degradation of lignin at 400 °C and 55% degradation at 450 °C. The lower thermal stability resulted from higher fragmentation of lignin during the pretreatment process. Thermal stability increases with increasing Mw and decreases at lower Mw (Sun et al. 2000). This phenomenon was justified by GPC analysis of this study, which showed the low molecular weight of extracted lignin due to more fragmentation.
UV spectra of lignin
This semi-quantitatively spectroscopic UV analysis of lignin in Fig. 6 showed high absorbance indicating the presence of the most important functional group i.e. a free phenolic group in the extracted lignin, which agreed with the absorbance band obtained in FTIR analysis and the existence of aromatic rings conjugated by Cα = Cβ and C=C linkages. The UV analysis of commercial alkali lignin as observed by Sidik et al. (2013) showed maximum absorption at 213 nm and a low maxima at 280 nm. The UV analysis of the current study showed two maximum absorbances at 270–280 and 312 nm. This study mostly followed the lignin extraction process as depicted by Sidik et al. (2013). The peak at 270–280 nm which correlated with commercial alkali lignin (Sidik et al. 2013) signified the occurrence of the high content of etherified alcohol i.e. guaiacyl unit by indicating the electronic transition in the aromatic group present in the unconjugated phenolic unit. The absorbance at 312 nm corresponds to the electronic transition in extracted lignin comprising Cα = Cβ linkage and C=C bond, which was evidence of the existence of p-coumaric acid and ferulic acid in lignin (Li et al. 2010).
Average molecular weight of extracted lignin
Weight average molecular weight (Mw) and number average molecular weight (Mn) of lignin were determined by Gel permeation chromatography. The Mw of extracted lignin was 1769 g/mol and Mn was 1098 g/mol. This finding is lower than the observation of Moghaddam et al. (2014) but closely related to the observation of Sun et al. (2013) when they extracted low molecular weight lignin from eucalyptus using 1-butyl-3-methylimidazolium acesulfamate ([BMIM]Ace) due to high access of ionic liquid to the lignin in the cell wall. Obtaining lower molecular weight lignin from IL-treated sugarcane bagasse indicated the fragmentation of lignin polymer by cleavage of the β-O-4 linkage at the time of pretreatment (Moghaddam et al. 2014). The result confirms that ionic liquid [EMIM]oAc aided treatment is an efficient method for lignin fragmentation. The polydispersity index (PDI) was determined as Mw/Mn and calculated as 1.6 which is similar to the PDI of milled wood lignin as described by Kim et al. (2011). Extracted low molecular weight lignin can be used to synthesize polymers (Asada et al. 2015; Matsushita et al. 2011).
FTIR analysis of untreated and pretreated bagasse
Untreated bagasse and pretreated bagasse were also characterized by FTIR (Fig. 7) to determine the effect of ionic liquid pretreatment on sugarcane bagasse in terms of lignin removal and recovery. Lignin specified absorption peaks as discussed in lignin FTIR characterization were observed in untreated bagasse and disappeared in pretreated bagasse. The wide band at 3100–3500 cm−1 represented O–H stretching vibration between the alcoholic/aliphatic hydroxyl group and a phenolic hydroxyl group (Ghaffar and Fan 2013; Moghaddam et al. 2014). This wide band was present in untreated bagasse and after pretreatment, it diminished. Absorption peaks from 2852, 2925 to 2935 cm−1 were strongly found in the raw sample as lignin characteristics implied symmetric and asymmetric C–H stretching of CH3, CH2 and CH and the intensity reduced in the pretreated sample (da Costa Lopes et al. 2013). The aromatic skeletal vibration of lignin appeared at 1599–1606 cm−1. Syringyl ring and guaiacyl units of lignin are associated with absorption peaks at 1328 and 835 cm−1 which are present in the untreated sample and not in pretreated bagasse indicating depolymerization and reduction of lignin quantity after pretreatment. The characteristic peaks of cellulose occur in sugarcane bagasse both before and after pretreatment. The infrared absorption band at 1724–1734 cm−1 only in raw sugarcane bagasse implies ester linked acetyl groups in hemicelluloses and carboxylic group of p-coumaric acid and feruloyl group of lignin (Chirayil et al. 2014; da Costa Lopes et al. 2013; Sun et al. 2009). After IL treatment, decreases in C=C stretching vibration peaks at 1512–1514 and 1420–1430 cm−1 indicate less lignin in pretreated bagasse (Perez-Pimienta et al. 2015; da Costa Lopes et al. 2013; Su et al. 2015; Yoon et al. 2012). C–H bending vibrations of cellulose occurred in both untreated and pretreated sample at 1370–1376 cm−1 (da Costa Lopes et al. 2013; Ninomiya et al. 2015; Qiu et al. 2012). The adsorption at 1245–1251 cm−1 in both raw and IL-processed bagasse is assigned to acetyl groups in hemicellulose. Peaks attributed to native cellulose at 1161 and 1107 cm−1 indicate a C–O asymmetric band (da Costa Lopes et al. 2013). A high intensity peak at 2882 cm−1 in pretreated bagasse signifies C–H stretch in cellulose (Trinh et al. 2015). A significant peak at 1050–1056 cm−1 is the indication of C–O stretching in carbohydrate (Perez-Pimienta et al. 2015). The peak at 893–897 cm−1 appeared before and after pretreatment, which typically specifies β-glycosidic linkage, namely the major characteristic of glucose present in cellulose (Chirayil et al. 2014). This band indicates the more amorphous regions and thus the intensity increased after pretreatment. This phenomenon occurred due to a decrease in cellulose crystallinity after [EMIM]oAc pretreatment (Kuo and Lee 2009). Wang et al. (2011) also demonstrated the peak at 557–679 cm−1 as characteristics of carbohydrate. This absorption band appeared in untreated and [EMIM]oAc treated bagasse in this experiment. Thus the obtained FTIR spectrum manifested that [EMIM]oAc aided pretreatment is an efficient method to extract the maximum quantity of lignin from raw bagasse and to make the remaining cellulose more susceptible to enzymatic hydrolysis.
Recycling ionic liquid after pretreatments
Recycled ionic liquid was used to extract lignin from bagasse. To verify the pretreatment efficiency of recovered ionic liquid, UV–Vis spectroscopy analysis of extracted lignin and TGA analysis of neat and recycled ionic liquid were carried out. Lignin, extracted with recycled ionic liquid was analyzed by UV–Vis spectroscopy. This analysis was carried out in the same scanning range as it was previously done for lignin extracted with fresh ionic liquid. Figure 8a showed the absorbance at 282 and 312 nm, thus justifying the recyclability and reusability of [EMIM]oAc. Thermal stability of fresh and recycled ionic liquid was analyzed by TGA as shown in Fig. 8b. TGA analysis agrees with the previously reported result. The maximum mass loss occurred around 215 °C for neat [EMIM]oAc and 234 °C for recycled [EMIM]oAc. This study concurred with the observation made by Wendler et al. (2012). Degradation started at 100 °C in both fresh and recycled ionic liquid, which indicated the presence of moisture. The recycled ionic liquid was dried properly to make it moisture free prior to analysis. Thus, the degradation observed in the case of fresh sample was higher than that obtained apropos of recycled sample near 100 °C; in this case, the fresh sample was not dried before analysis. The presence of a small quantity of moisture was observed in the recycled sample in spite of the fact that the sample was dried prior to analysis.
Conclusion
The prime objective of the current study was to optimize ionic liquid aided pretreatment conditions to achieve a reasonably high degree of lignin extraction from sugarcane bagasse and to recover the extracted lignin for use in making value-added products. Additionally, the study also aimed at converting the pretreated bagasse to biofuel in subsequent stages. A good correlation was found between the experimental values and the predicted values of lignin yield, which indicated that the obtained model was well-fitted for this optimization study. Extracted lignin was characterized for its quality/purity and FTIR analysis confirmed the presence of essential functional groups. The low molecular weight of the extracted lignin, as revealed by GPC, was due to fragmentation of lignin which caused the lowering of its thermal stability. FTIR analysis of both untreated and pretreated bagasse verified that the removal of lignin from bagasse was enhanced by pretreatment. The feasibility of recovery and reusability of the ionic liquid was confirmed by TGA characterization of [EMIM]oAc, thus demonstrating the process to be economically feasible and eco-friendly. However, based on the current investigation, further study is required to enhance the cost-effectiveness of the pretreatment strategy of sugarcane bagasse by recycling [EMIM]oAc a greater number of times.
References
Antunes FAF, Chandel AK, Milessi TSS, Santos JC, Rosa CA, da Silva SS (2014) Bioethanol production from sugarcane bagasse by a novel brazilian pentose fermenting yeast Scheffersomyces shehatae UFMG-HM 52.2: evaluation of fermentation medium. Int J Chem Eng Article Id 180681:1–8
Asada C, Basnet S, Otsuka M, Sasaki C, Nakamura Y (2015) Epoxy resin synthesis using low molecular weight lignin separated from various lignocellulosic materials. Int J Biol Macromolec 74:413–419
Balat M (2011) Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Convers Manage 52:858–875
Barr CJ, Hanson BL, Click K, Perrotta G, Schall CA (2014) Influence of ionic-liquid incubation temperature on changes in cellulose structure, biomass composition, and enzymatic digestibility. Cellulose 21:973–982
Boeriu CG, Bravo D, Gosselink RJA, Van Dam JEG (2004) Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind Crop Prod 20:205–218
Canilha L, Chandel AK, dos Santos Milessi TS, Antunes FAF, da Costa Freitas WL, das Graças Almeida Felipe M, da Silva SS, (2012) Bioconversion of sugarcane biomass into ethanol: an overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J Biomed Biotechnol 2012:1–15
Cao J, Wu Y, Jin Y, Yilihan P, Huang W (2014) Response surface methodology approach for optimization of the removal of chromium (VI) by NH2-MCM-41. J Taiwan Inst Chem Eng 45:860–868
Casas A, Oliet M, Alonso MV, Rodriguez F (2012) Dissolution of Pinus radiata and Eucalyptus globulus woods in ionic liquids under microwave radiation: lignin regeneration and characterization. Sep Purif Technol 97:115–122
Chakraborty S, Aggarwal V, Mukherjee D, Andras K (2012) Biomass to biofuel: a review on production technology. Asia Pac J Chem Eng 7:S254–S262
Chakraborty S, Dasgupta J, Farooq U, Sikder J, Drioli E, Curcio S (2014) Experimental analysis, modeling and optimization of chromium (VI) removal from aqueous solutions by polymer-enhanced ultrafiltration. J Memb Sci 456:139–154
Chirayil CJ, Joy J, Mathew L, Mozetic M, Koetz J, Thomas S (2014) Isolation and characterization of cellulose nanofibrils from Helicteres isora plant. Ind Crops Prod 59:27–34
da Costa Lopes AM, João KG, Rubik DF, Bogel-Łukasik E, Duarte LC, Andreaus J, Bogel-Łukasik R (2013) Pre-treatment of lignocellulosic biomass using ionic liquids: wheat straw fractionation. Biores Technol 142:198–208
Dasgupta J, Singh M, Sikder J, Padarthi V, Chakraborty S, Curcio S (2015) Response surface-optimized removal of Reactive Red 120 dye from its aqueous solutions using polyethyleneimine enhanced ultrafiltration. Ecotox Environ Safe 121:271–278
Fu D, Mazza G (2011) Aqueous ionic liquid pretreatment of straw. Bioresour Technol 102:7008–7011
Ghaffar SH, Fan M (2013) Structural analysis for lignin characteristics in biomass straw. Biomass Bioenerg 57:264–279
Huddleston JG, Willauer HD, Swatloski RP, Visser AE, Rogers RD (1998) Room temperature ionic liquids as novel media for ‘clean’ liquid–liquid extraction. Chem Commun 99:1765–1766
Jia S, Cox BJ, Guo X, Zhang ZC, Ekerdt JG (2010) Cleaving the β-O-4 bonds of lignin model compounds in an acidic ionic liquid, 1-H-3-methylimidazolium chloride: an optional strategy for the degradation of lignin. Chem Sus Chem 3:1078–1084
Kim J-Y, Shin E-J, Eom I-Y, Won K, Kim YH, Choi D, Choi I-G, Choi J-W (2011) Structural features of lignin macromolecules extracted with ionic liquid from poplar wood. Bioresour Technol 102:9020–9025
Koo B-W, Park N, Jeong H-S, Choi J-W, Yeo H, Choi I-G (2011) Characterization of by-products from organosolv pretreatments of yellow poplar wood (Liriodendron tulipifera) in the presence of acid and alkali catalysts. J Ind Eng Chem 17:18–24
Kuo C-H, Lee C-K (2009) Enhancement of enzymatic saccharification of cellulose by cellulose dissolution pretreatments. Carbohyd Polym 77(1):41–46
Lee SH, Doherty TV, Linhardt RJ, Dordick JS (2009) Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol Bioeng 102:1368–1376
Lee KM, Ngoh GC, Chua ASM (2015) Ionic liquid-mediated solid acid saccharification of sago waste: kinetic, ionic liquid recovery and solid acid catalyst reusability study. Ind Crop Prod 77:415–423
Li Q, He YC, Xian M, Jun G, Xu X, Yang JM, Li LZ (2009) Improving enzymatic hydrolysis of wheat straw using ionic liquid 1-ethyl-3-methyl imidazolium diethyl phosphate pretreatment. Bioresour Technol 100:3570–3575
Li MF, Fan YM, Sun RC, Xu F (2010) Characterization of extracted lignin of Bamboo (Neosinocalamusaffinis) pretreated with sodium hydroxide/urea solution at low temperature. BioResources 5(3):1762–1778
Ma H-H, Zhang B-X, Zhang P, Li S, Gao Y-F, Hu X-M (2016) An efficient process for lignin extraction and enzymatic hydrolysis of corn stalk by pyrrolidonium ionic liquids. Fuel Process Technol 148:138–145
Maton C, De Vos N, Stevens CV (2013) Ionic liquid thermal stabilities: decomposition mechanisms and analysis tools. Chem Soc Rev 42(13):5963
Matsushita Y, Inomata T, Takagi Y, Hasegawa T, Fukushima K (2011) Conversion of sulfuric acid lignin generated during bioethanol production from lignocellulosic materials into polyesters with ɛ-caprolactone. J Wood Sci 57:214–218
Moghaddam L, Zhang Z, Wellard RM, Bartley JP, O’Hara IM, Doherty WOS (2014) Characterisation of lignins isolated from sugarcane bagasse pretreated with acidified ethylene glycol and ionic liquids. Biomass Bioenerg 70:498–512
Nelder JA (1998) The selection of terms in response surface models—how strong is the weak heredity principle. Am Stat 52:315–318
Ninomiya K, Inoue K, Aomori Y, Ohnishi A, Ogino C, Shimizu N, Takahashi K (2015) Characterization of fractionated biomass component and recovered ionic liquid during repeated process of cholinium ionic liquid-assisted pretreatment and fractionation. Chem Eng J 259:323–329
Norgren M, Edlund H (2014) Lignin: recent advances and emerging applications. Curr Opin Colloid Interfaceterface 19:406–419
Perez-Pimienta JA, Lopez-Ortega MG, Chavez-Carvayar JA, Varanasi P, Stavila V, Cheng G, Singh S, Simmons BA (2015) Characterization of agave bagasse as a function of  ionic liquid pretreatment. Biomass Bioenerg 75:180–188
Pinkert A, Goeke DF, Marsh KN, Pang S (2011) Extracting wood lignin without dissolving or degrading cellulose: investigations on the use of food additive-derived ionic liquids. Green Chem 13:3124–3136
Prado R, Erdocia X, Labidi J (2016) Study of the influence of reutilization ionic liquid on lignin extraction. J Clean Prod 111:125–132
Qiu Z, Aita GM, Walker MS (2012) Effect of ionic liquid pretreatment on the chemical composition, structure and enzymatic hydrolysis of energy cane bagasse. Biores Technol 117:251–256
Rashid T, Kait CF, Regupathi I, Murugesan T (2016) Dissolution of kraft lignin using protic ionic liquids and characterization. Ind Crop Prod 84:284–293
Saha K, Maheswari RU, Sikder J, Chakraborty S, da Silva SS, dos Santos JC (2017) Membranes as a tool to support biorefineries: applications in enzymatic hydrolysis, fermentation and dehydration for bioethanol production. Renew Sustain Energy Rev 74:873–890
Sidik DAB, Ngadi N, Amin NAS (2013) Optimization of lignin production from empty fruit bunch via liquefaction with ionic liquid. BioresourTechnol 135:690–696
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2012) Determination of structural carbohydrates and lignin in biomass: laboratory analytical procedure (LAP) (Revised August 2012). Issue Date: 4/25/2008 NREL/TP-510-42618, 18 pp
Solomon S (2014) Sugarcane Agriculture and Sugar Industry in India: at a Glance. Sugar Tech 16(2):113–124
Su Y, Du R, Guo H, Cao M, Wu Q, Su R, Qi W, He Z (2015) Fractional pretreatment of lignocellulose by alkaline hydrogen peroxide: characterization of its major components. Food Bioprod Process 94:322–330
Sun F, Chen H (2008) Enhanced enzymatic hydrolysis of wheat straw by aqueous glycerol pretreatment. Bioresour Technol 99:6156–6161
Sun RC, Tomkinson J, Jones GL (2000) Fractional characterization of ash-AQ lignin by successive extraction with organic solvents from oil palm EFB fibre. Polym Degrad Stab 68(1):111–119
Sun N, Rahman M, Qin Y, Maxim ML, Rodríguez H, Rogers RD (2009) Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem 11(5):646
Sun Y-C, Xu JK, Xu F, Sun R-C (2013) Efficient separation and physico-chemical characterization of lignin from eucalyptus using ionic liquid–organic solvent and alkaline ethanol solvent. Ind Crop Prod 47:277–285
Tan HT, Lee KT (2012) Understanding the impact of ionic liquid pretreatment on biomass and enzymatic hydrolysis. Chem Eng J183:448–458
Teramoto Y, Lee SH, Endo T (2008) Pretreatment of woody and herbaceous biomass for enzymatic saccharification using sulfuric acid-free ethanol cooking. Bioresour Technol 99:8856–8863
Timung R, Mohan M, Chilukoti B, Sasmal S, Banerjee T, Goud VV (2015) Optimization of dilute acid and hot water pretreatment of different lignocellulosic biomass: a comparative study. Biomass Bioenerg 81:9–18
Trinh TK, Kang LS (2011) Response surface methodological approach to optimize the coagulation–flocculation process in drinking water treatment. Chem Eng Res Des 89:1126–1135
Trinh LTP, Lee YJ, Lee J-W, Lee H-J (2015) Characterization of ionic liquid pretreatment and  the bioconversion of pretreated mixed softwood biomass. Biomass Bioenerg 81:1–8
Wang X, Li H, Cao Y, Tang Q (2011) Cellulose extraction from wood chip in an ionic liquid 1-allyl-3-methylimidazolium chloride (AmimCl). Biores Technol 102(17):7959–7965
Weerachanchai P, Leong SSJ, Chang MW, Ching CB, Lee J-M (2012) Improvement of biomass properties by pretreatment with ionic liquids for bioconversion process. Bioresour Technol 111:453–459
Wendler F, Todi L-N, Meister F (2012) Thermostability of imidazolium ionic liquids as direct solvents for cellulose. Thermochim Acta 528:76–84
Yoon LW, Ngoh GC, Chua ASM (2011) Comparison of ionic liquid, acid and alkali pretreatments for sugarcane bagasse enzymatic saccharification. J Chem Technol Biotechnol 86:134–138
Yoon LW, Ang TN, Ngoh GC, Chua ASM (2012) Regression analysis on ionic liquid pretreatment of sugarcane bagasse and assessment of structural changes. Biomass Bioenerg 36:160–169
Yuan TQ, You TT, Wang W, Xu F, Sun RC (2013) Synergistic benefits of ionic liquid and alkaline pretreatments of poplar wood. Part 2: characterization of lignin and hemicelluloses. Bioresour Technol 136:345–350
Zavrel M, Bross D, Funke M, Buchs J, Spiess AC (2009) High-throughput screening for ionic liquids dissolving (ligno-) cellulose. Bioresour Technol 100:2580–2587
Zhang P, Dong S-J, Ma H-H, Zhang B-X, Wang Y-F, Hu X-M (2015) Fractionation of corn stover into cellulose, hemicellulose and lignin using a series of ionic liquids. Ind Crop Prod 76:688–696
Acknowledgments
The authors would like to extend their sincerest gratitude to the Department of Biotechnology, Government of India for the financial support granted for this research work under the bilateral collaboration between India and Brazil (DBT- India and MCTI-CNPq-Brazil) vide no. DBT/In-Bz/2013-16/06 and CNPq process no: 401361/2013-6. Authors are thankful to the Research Council for the State of São Paulo (FAPESP) (Award Number 2014/27055-2) for financial support and Department of Science and Technology, Government of India (DST) for the grants under DST-FIST Program (SR/FST/ETI-204/2007) with which the infrastructure for the present research was developed. All the authors are grateful to Dr. Alfred D. French for his concern and valuable suggestions in improving the writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saha, K., Dasgupta, J., Chakraborty, S. et al. Optimization of lignin recovery from sugarcane bagasse using ionic liquid aided pretreatment. Cellulose 24, 3191–3207 (2017). https://doi.org/10.1007/s10570-017-1330-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1330-x