Abstract
Cotton fabric was bleached at a pilot-plant scale with the activated peroxide system based on N-[4-(triethylammoniomethyl) benzoyl] caprolactam chloride (TBCC). The performance of the TBCC-activated peroxide system on low-temperature bleaching of cotton fabric was evaluated by measuring the degree of whiteness, degree of polymerization, water absorbency, extractable contents, and dyeing properties of bleached cotton fabrics. For comparison purpose, cotton fabric was also bleached at the same pilot-plant scale with a traditional hydrogen peroxide system using a standard recipe. It was found that the pilot-plant bleaching with the TBCC-activated peroxide system resulted in a comparable degree of whiteness and a slightly lower water absorbency of cotton fabric but no apparent fiber damage. The bleached cotton fabric could meet requirements for trichromatic reactive dyeing. The investigation on resource utilization revealed that the pilot-plant bleaching of cotton fabric with the TBCC-activated peroxide system could save 60% water, 38% steam and 27% electric power in comparison with the traditional hydrogen peroxide system. These pilot-plant results are of great importance for further scaling up the TBCC-activated peroxide system to full-scale commercial production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton fabric is traditionally bleached with alkaline hydrogen peroxide at boiling temperature (Brooks and Moore 2000). In spite of delivery of a cotton fabric with satisfactory whiteness, the traditional hydrogen peroxide system causes problems such as high energy and water consumptions and severe fiber damage. To overcome these problems, it is proposed in the textile industry that cotton fabric can be bleached through low-temperature bleaching process which is approached using catalyzed peroxide systems and activated peroxide systems. A catalyzed peroxide system is formed by adding a transition-metal complex into a hydrogen peroxide solution, in which hydrogen peroxide is catalyzed by the transition-metal complex for low-temperature bleaching of cotton fabric (Hage and Lienke 2006; Topalovic et al. 2007; Qin et al. 2012). Though the catalyzed peroxide system has the advantages of saving energy and reducing fiber damage, more investigations need to be carried out for its application in industrial textile bleaching, especially to find an applicable catalyst. In comparison with catalyzed peroxide systems, activated peroxide systems are attracting more attention from the textile industry. An activated peroxide system is formed by adding a so-called bleach activator into a hydrogen peroxide solution, in which the bleach activator is converted into a peracid by reacting with hydrogen peroxide (Hofmann et al. 1992). The generated peracid has a higher oxidative potential and thereby allows bleaching to be conducted at low temperatures (Cai et al. 2001; Scarborough and Mathews 2000; Wang and Washington 2002). So far, a variety of bleach activators have been reported for use in low-temperature bleaching of cotton fabric, among which cationic bleach activators attract more interest (Abdel-Halim and Al-Deyab 2013; Cai and Evans 2007; Lee et al. 2010; Long et al. 2013; Wang et al. 2014b).
Cationic bleach activators with the generic name of N-[4-(triethylammoniomethyl) benzoyl] lactam chloride (TBLC) have a positive charge which makes them soluble in water as well as affinitive to cotton fibers. As shown in Scheme 1, in a hydrogen peroxide solution, TBLC can be cleaved by perhydroxyl anions (HOO−) to produce 4-(triethylammoniomethyl) perbenzoic acid (TPA) which is responsible for low-temperature bleaching of cotton fibers. The affinity of cationic bleach activators to cotton fibers enables TPA to be produced in close proximity to the colored impurities and as such enhances the bleaching effectiveness (Xu et al. 2010).
N-[4-(triethylammoniomethyl) benzoyl]caprolactam chloride (TBCC) is often used as a prototype of cationic bleach activators (i.e. n = 3 for TBLC). Recent research has shown that the TBCC-activated peroxide system can be applied for low-temperature bleaching of cotton fabric under near-neutral pH conditions, and the bleached cotton fabric exhibits a desired whiteness as well as wettability (Wang et al. 2014a). In our previous work, the TBCC-activated peroxide system was investigated by a fractional factorial screening design of experiments for identifying the effects of thirteen factors on low-temperature bleaching of cotton fabric (Fei et al. 2015), and the low-temperature bleaching performance was optimized by using response surface methodology (Luo et al. 2015). In this work, the TBCC-activated peroxide system will be scaled up to a pilot-plant scale for low-temperature bleaching of cotton fabric, of which the performance will be evaluated by testing the properties of the bleached cotton fabric and the utilization of resources such as water, steam and electric power.
Experimental
Materials
100% single jersey circular-knitted cotton greige fabric (175 g/m2) was provided by Cotton Incorporated (USA). TBCC was synthesized in our lab with 98% purity according to the procedure previously reported (Wei et al. 2014). Hydrogen peroxide (H2O2, 35% w/w) was provided by Sigma-Aldrich (USA). Levafix CA dyes (Yellow, Red and Blue) were provided by DyStar (China). Nonionic wetting agent (Sultafon D, provided by Stockhausen), peroxide stabilizer (Prestogen N-D, provided by BASF), and catalase (Invazyme CAT, provided by Huntsman) were used as bleaching auxiliaries. Lubricating agent (Multiplus NB-100, provided by BASF), chelating agent (Dekol N-SN, provided by BASF) and soaping agent (Domosperse 2000, recommended by Cotton Incorporated) were used as reactive dyeing auxiliaries. Potassium iodide starch test paper provided by Sargent Welch was used for testing the residual H2O2 in the bleaching bath. All the other chemicals and auxiliaries used in bleaching and dyeing were of commercial grade unless otherwise stated. The pilot-plant experiments were carried out in Cotton Incorporated, and the filtered tap water from the pilot plant was used through the investigation.
Pilot-plant bleaching of cotton fabric
A batch of cotton fabric (20 kg) was bleached with the TBCC-activated peroxide system on a jet dyeing machine (Sclavos, Greece) at a liquor-to-goods ratio of 15:1. The bleaching bath was prepared with the addition of the chemicals and auxiliaries as shown in Table 1. The bleaching process was carried out according to the temperature–time profile shown in Fig. 1a. The temperature was raised to 55 °C with a ramp rate of 2.2 °C/min and maintained for 45 min. During the bleaching process, a sample of cotton fabric (around 5 g) was cut from the fabric batch every 15 min to monitor the bleaching progress by measuring the degree of whiteness of cotton fabric. When bleaching was completed, the residual H2O2 in the bleaching bath was tested by potassium iodide starch test paper. If a positive test result was observed, Invazyme CAT was added to the bleaching bath at 55 °C to decompose the residual H2O2 for 10 min. Otherwise, Invazyme CAT was excluded from the bleaching bath. The bleaching bath was then drained off and the bleached cotton fabric was rinsed with cool water at a liquor-to-goods ratio of 15:1. After extraction in a centrifugal extractor, the bleached cotton fabric was dried through a conveyor belt infrared dryer.
Temperature–time profiles for bleaching of cotton fabric with a the TBBC-activated peroxide system and b the traditional hydrogen peroxide system (The code numbers have been defined in Table 1)
For comparison purpose, a batch of cotton fabric (20 kg) was also bleached with the traditional hydrogen peroxide system using a standard recipe on the Sclavos jet dyeing machine at a liquor-to-goods ratio of 15:1. The bleaching bath was prepared with the addition of the chemicals and bleaching auxiliaries as shown in Table 1. The bleaching process was carried out according to the temperature–time profile shown in Fig. 1b. The temperature was raised to 107 °C with a ramp rate of 5.5 °C/min and maintained for 25 min. When bleaching was completed, the bleaching bath was cooled down to 71 °C with a ramp rate of 2.8 °C/min and drained off. The bleached cotton fabric was consecutively treated by rinse with warm water at 50 °C for 3 min, neutralization with acetic acid at 71 °C for 5 min, and decomposition of hydrogen peroxide with Invazyme CAT at 50 °C for 10 min at a liquor-to-goods ratio of 15:1. Finally, the bleached cotton fabric was rinsed with fresh water for 10 min at a liquor-to-goods ratio of 15:1. The bleached cotton fabric was then sent for extraction in a centrifugal extractor and dried through an infrared conveyor belt dryer.
The utilization of resources such as water, steam and electric power was recorded by the monitors equipped on the machine.
Reactive dyeing of bleached cotton fabric
A total of 1.5 kg of cotton fabrics bleached with the TBCC-activated peroxide system and the commercial hydrogen peroxide system were dyed in one bath with Levafix CA dyes. Table 2 shows the recipes for trichromatic dyeing of cotton fabric in gray colors (light, medium and dark). The dyeing bath was prepared with the addition of the dyes and auxiliaries as given in Table 2. The dyeing process was carried out on an OPTILAB laboratory dyeing machine (Rousselet Robatel, France) at a liquor-to-goods ratio of 15:1. Figure 2 shows the temperature–time profile for reactive dyeing of cotton fabric. When the dyeing was completed, the dyed cotton fabric was treated with soaping agent and rinsed with fresh water, and then dried in a tumble dryer (Whirlpool, USA) at 105 °C.
Temperature–time profiles for trichromatic dyeing of bleached cotton fabric with Levafix CA dyes (The code numbers have been defined in Table 2)
Testing
The low-temperature bleaching performance of the TBCC-activated peroxide system was evaluated by testing the properties of the bleached cotton fabric. Table 3 shows the methods and apparatuses used for tests. In Table 3, the fluidity (F) of a dispersion of cotton fabric in cupriethylenediamine solution was conversed to the degree of polymerization (DP) as given by Eq. (1). DP was used as an indicator of chemical damage that cotton fibers underwent during the bleaching process.
Results and discussion
Pilot-plant bleaching performance
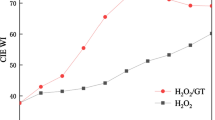
The TBCC-activated peroxide system works by reaction of TBCC with H2O2, from which TPA is generated in situ for low-temperature bleaching of cotton fabric and then converted to 4-(triethylammoniomethyl) benzoic acid (TBA) as shown in Scheme 2 (Wang et al. 2014a). In the recipe for pilot-plant bleaching process as shown in Table 1, the TBCC-activated peroxide system was conducted by using equimolar amounts of TBCC and H2O2, to which a slight excess of NaHCO3 was added for neutralizing TBA and as such maintaining the bleaching bath at near-neutral pH (Fei et al. 2015; Luo et al. 2015). As can be seen from Fig. 3, the degree of whiteness of cotton fabric was quickly improved at the stage of ramp heating. This indicates that the TBCC-activated peroxide system was much effective at low temperatures. At the bleaching stage, the degree of whiteness of cotton fabric was continuously improved as the bleaching time was extended, and an optimal degree of whiteness was reached within 45 min. Therefore, the TBCC-activated peroxide system could build a quick bleaching process for cotton fabric.
Table 4 shows the properties of cotton fabric bleached with the TBCC-activated peroxide system in comparison with the traditional hydrogen peroxide system. It can be seen that the TBCC-activated peroxide system was comparable to the traditional hydrogen peroxide system in improving the degree of whiteness of cotton fabric. The degree of polymerization of cotton fabric bleached with the TBCC-activated peroxide system was almost the same as that of the greige fabric, but relatively higher than that of cotton fabric bleached with the traditional hydrogen peroxide system. This indicates that the TBCC-activated peroxide system resulted in no apparent chemical damage to cotton fibers. The water absorbency of cotton fabric was greatly improved by the TBCC-activated peroxide system, indicating that the hydrophobic materials (e.g. waxes and fats) in cotton fibers could be removed to some extent during the bleaching process (Wang et al. 2014a). However, the water absorbency of cotton fabric bleached with the TBCC-activated peroxide system was slightly lower than that of cotton fabric bleached with the traditional hydrogen peroxide system. This is because the hydrophobic materials in cotton fibers are more readily removed under alkaline pH conditions at a high temperature rather than under near-neutral pH conditions at a low temperature.
It is necessary for the bleaching process to remove the impurities in cotton fibers as much as possible so as to provide a uniform substrate for further dyeing. Therefore, the residuals of impurities on the bleached cotton fabric were tested as given in Table 5. It was found that the cotton fabric bleached with the TBCC-activated peroxide system had a higher extractable content than that bleached with traditional hydrogen peroxide system. The water extract from the cotton fabric bleached with the TBCC-activated peroxide system can be partly ascribed to the natural minerals in cotton fibers which could not be adequately removed by bleaching under near-neutral pH conditions at a low temperature. Additionally, the cotton fabric bleached with the TBCC-activated peroxide system may also contain the residuals of sodium salts (e.g. TBA and NaHCO3) which could not be completely removed by rinsing once with cool water. The NaHCO3 residual could be the main reason for the high pH of water-extract and total alkali founded on the cotton fabric bleached with TBCC-activated peroxide system. The hexane extract of the cotton fabric bleached with TBCC-activated peroxide system indicates a higher residual content of hydrophobic materials (e.g. waxes and fats) in cotton fibers that would be the main reason for the lower water absorbency shown in Table 4.
Reactive dyeing of bleached cotton fabric
The cotton fabrics bleached with the TBCC-activated peroxide system and the traditional hydrogen peroxide system were dyed in one bath with reactive dyes into gray colors using the trichromatic recipes as given in Table 2. The color appearance of the two bleached cotton fabrics and their color difference are shown in Table 6. The two bleached cotton fabrics would be dyed into the same gray colors and exhibited an insignificant color difference providing that the difference in the properties of the two bleached cotton fabrics had no apparent effect on reactive dyeing. As shown in Table 6, compared to the cotton fabric bleached with the traditional hydrogen peroxide system, the cotton fabric bleached with the TBCC-activated peroxide system was dyed into a slightly duller and redder shade of gray. This may be ascribed to a slightly lower degree of whiteness (as shown in Table 4) and a higher extractable content (as shown in Table 5) of the cotton fabric bleached with the TBCC-activated peroxide system. However, the color differences between the cotton fabrics bleached with the two methods were found to be less than 0.6 that is generally thought to be of insignificance. This indicates that the cotton fabric bleached with the TBCC-activated peroxide system can meet the requirement for reactive dyeing.
Table 7 shows the colorfastness of the dyed cotton fabric to crocking, light and laundering. As can be seen, the cotton fabric bleached with the TBCC-activated peroxide system exhibited almost the same colorfastness as the cotton fabric bleached with the traditional hydrogen peroxide system. This indicates that, in spite of containing some impurity residuals (Table 5), the cotton fabric bleached with the TBCC-activated peroxide system could be dyed with reactive dyes for satisfactory colorfastness.
Utilization of resources in pilot-scale bleaching
To evaluate the resource utilization, the traditional hydrogen peroxide system was applied for bleaching of cotton fabric by using a standard method as presented in Fig. 2b, of which the full procedure consisted of high-temperature bleaching, neutralization with acetic acid, H2O2 decomposition with catalase, and necessary rinses with water. The resource utilization in the pilot-scale bleaching process is given in Table 8. It can be seen that the consumptions of water, steam and electric power in bleaching with the TBCC-activated peroxide system were much lower than in bleaching with the traditional hydrogen peroxide system. In the bleaching of cotton fabric with the TBCC-activated peroxide system, NaHCO3 was used as alkaline agent to maintain the bleaching bath at a near-neutral pH (Xu et al. 2015), and therefore neutralization with acetic acid was bypassed in the bleaching process. In addition, due to the fact that the TBCC-activated peroxide system was conducted by using equimolar amounts of TBCC and H2O2, there was no residual H2O2 observed in the bleaching bath when bleaching was completed. Hence, H2O2 decomposition with catalase was bypassed as well in the bleaching process. Consequently, bleaching of cotton fabric with the TBCC-activated peroxide system resulted in a 60% water saving in comparison with the traditional hydrogen peroxide bleaching. Besides the water saving, bleaching with the TBCC-activated peroxide system could also save 38% steam and 27% electric power, which was ascribed to the lower temperature and the shorter process. This indicates that the TBCC-activated peroxide system had the advantage over the traditional hydrogen peroxide system in reducing energy consumption.
Conclusions
The TBCC-activated peroxide system could be applied at a pilot-plant scale for bleaching of cotton fabric. Compared with the traditional hydrogen peroxide system, the TBCC-activated peroxide system provided cotton fabric with a comparable degree of whiteness and a slightly lower water absorbency, but caused no apparent damage to cotton fibers. In spite of a relatively high content of impurity residuals which were ascribed to natural materials (e.g. minerals, waxes and fats) in cotton fibers and sodium salts resulting from the bleaching bath, the cotton fabric bleached with TBCC-activated peroxide system could meet requirements for trichromatic reactive dyeing. The TBCC-activated peroxide system had advantages over the traditional hydrogen peroxide system in reducing resource utilization, i.e. saving 60% of water, 38% of steam and 27% of electric power.
References
Abdel-Halim ES, Al-Deyab SS (2013) One-step bleaching process for cotton fabrics using activated hydrogen peroxide. Carbohydr Polym 92:1844–1849
Brooks RE, Moore SB (2000) Alkaline hydrogen peroxide bleaching of cellulose. Cellulose 7:263–286
Cai JY, Evans DJ (2007) Guanidine derivatives used as peroxide activators for bleaching cellulosic textiles. Color Technol 123:115–118
Cai JY, Evans DJ, Smith SM (2001) Bleaching of natural fibers with TAED and NOBS activated peroxide systems. AATCC Rev 1(12):31–34
Fei X, Yao J, Du J, Sun C, Xiang Z, Xu C (2015) Analysis of factors affecting the performance of activated peroxide systems on bleaching of cotton fabric. Cellulose 22:1379–1388
Hage R, Lienke A (2006) Applications of transition-metal catalysts to textile and wood-pulp bleaching. Angew Chem Int Ed 45:206–222
Hofmann J, Just G, Pritzkow W, Schmidt H (1992) Bleaching activators and the mechanism of bleaching activation. J Prakt Chem Chem ZTG 334:293–297
Lee JJ, Hinks D, Lim SH, Hauser P (2010) Hydrolytic stability of a series of lactam-based cationic bleach activators and their impact on cellulose peroxide bleaching. Cellulose 17:671–678
Long X, Xu C, Du J, Fu S (2013) The TAED/H2O2/NaHCO3 system as an approach to low-temperature and near-neutral pH bleaching of cotton. Carbohydr Polym 95:107–113
Luo X et al (2015) Performance modelling of the TBCC-activated peroxide system for low-temperature bleaching of cotton using response surface methodology. Cellulose 22:3491–3499
Qin X, Song M, Ma H, Yin C, Zhong Y, Zhang L, Mao Z (2012) Low-temperature bleaching of cotton fabric with a binuclear manganese complex of 1,4,7-trimethyl-1,4,7-triazacyclononane as catalyst for hydrogen peroxide. Color Technol 128:410–415
Scarborough SJ, Mathews AJ (2000) Using T.A.E.D. in bleaching fiber blends to improve fiber quality. Text Chem Color Am Dyest Rep 32(3):33–37
Topalovic T, Nierstrasz V, Bautista L, Jocic D, Navarro A, Warmoeskerken MCG (2007) Analysis of the effects of catalytic bleaching on cotton. Cellulose 14:385–400
Wang J, Washington NM (2002) Hydrophobic bleach systems and textile preparation: a discontinuity in fabric care. AATCC Rev 2(6):21–24
Wang M, Long X, Du J, Sun C, Fu S, Xu C (2014a) X-ray photoelectron spectroscopy analysis of cotton treated with the TBCC/H2O2/NaHCO3 system. Text Res J 84:2149–2156
Wang S, Li S, Zhu Q, Yang CQ (2014b) A novel low temperature approach for simultaneous scouring and bleaching of knitted cotton fabric at 60 °C. Ind Eng Chem Res 53:9985–9991
Wei D, Sun C, Wang M, Du J, Xu C (2014) Synthesis of N-[4-(dimethylalkylammoniomethyl) benzoyl] caprolactam chlorides as cationic bleach activators for low-temperature bleaching of cotton fabric under near-neutral pH conditions. Color Technol 130:432–436
Xu C, Hinks D, Shamey R (2010) Bleaching cellulosic fibers via pre-sorption of N-[4-(triethylammoniomethyl)-benzoyl]-butyrolactam chloride. Cellulose 17:849–857
Xu C, Hinks D, Sun C, Wei Q (2015) Establishment of an activated peroxide system for low-temperature cotton bleaching using N-[4-(triethylammoniomethyl) benzoyl] butyrolactam chloride. Carbohydr Polym 119:71–77
Acknowledgments
The work was supported by the National Natural Science Foundation of China (Grant No. 21276106), the Major Research and Development Program of Jiangsu Province (Grant No. BE201596), and the Prospective Joint Research Project of Jiangsu Province (Grant No. BY2015019-09). We also greatly appreciate Cotton Incorporate for providing the pilot-plant bleaching and dyeing equipment.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yu, J., Shao, D., Sun, C. et al. Pilot-plant investigation on low-temperature bleaching of cotton fabric with TBCC-activated peroxide system. Cellulose 24, 2647–2655 (2017). https://doi.org/10.1007/s10570-017-1276-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1276-z