Abstract
The inhibiting ability of chitosan alone and in combination with KI for St37 steel in 15% H2SO4 solution has been studied using potentiodynamic polarization (PDP), electrochemical impedance spectroscopy (EIS), dynamic electrochemical impedance spectroscopy(DEIS), and weight loss (WL) complemented by surface morphological examination with the aid of scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS). The effect of immersion time on inhibition efficiency has been examined for 15 h and the influence of temperature studied over the temperature range of 25–60 °C. Results obtained from all the applied methods portray chitosan as a moderate inhibitor for St37 steel in the studied acid environment. Addition of KI is found to remarkably enhance the inhibition efficiency of the polymer above 92%. DEIS results show that the adsorbed chitosan-KI film on the metal surface is more stable at longer immersion time and performs more effectively. The inhibition efficiency of chitosan decreases with increasing temperature, while that of the chitosan-iodide combination increases with a rise in temperature reaching an optimum value of 99.72% at 60 °C. PDP results show that both chitosan and chitosan + KI behave as a mixed type inhibitor. A calculated synergism parameter confirms that the improved performance of chitosan is due to a synergistic effect. EDS results confirm the adsorption of inhibitor molecules on the metal surface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
St37 steel is known for its excellent strength and is preferred in ship and bridge construction, riveting, bolting, etc., where high mechanical strength is required (Bebon 2011). Mineral acids, particularly hydrochloric and sulfuric acids in the concentration range of 15–28%, are often used for industrial metal scale removal and cleaning as well as other practices such as acid descaling and oil well acidizing (Nataraja et al. 2011). These practices promote metal corrosion, and failure is inevitable if the corrosion process is not abated. The simplest and most cost effective approach toward metal corrosion control is the use of corrosion inhibitors (Umoren and Solomon 2014a). Inorganic (mostly chromates, nitrates, nitrites and phosphates) and organic (usually those having N, O, S, P and/or π-bonds in their structure) compounds have been utilized in this capacity for decades (Saji and Shibli 2002, 2005; Zhang et al. 2004; Sherif and Park 2006; Liu et al. 2014; Atta et al. 2011; Fekry and Ameer 2011). However, the growing awareness about environmental sustainability and the willingness to ensure ecosystem preservation have prompted a reduction in the volume of inorganic compounds used as corrosion inhibitors since their toxic level is unhealthy for the natural environment. The tedious synthesis route and the high prices of organic compounds are the limiting factors to their usage as corrosion inhibitors. At present, most researches are focused on polymers (both naturally occurring and synthetic) and plant part extracts (Solomon and Umoren 2015a; Umoren et al. 2014; Nasibi et al. 2013, 2014), which are readily available, cost effective and ecosystem friendly. Unfortunately, there is a setback on the use of polymeric materials as metal corrosion inhibitors as most of the materials studied are modest inhibitors (Umoren and Solomon 2014a) and tend to degrade at elevated temperature (Umoren and Solomon 2014a). Corrosion scientists have attributed this behavior to the partial solubility of polymers in the aqueous solution of usage because of the presence of a methylene group (–CH2–) in their structures and the fact that most of them exist as thermo-reversible gels at elevated temperature (Solomon et al. 2010; Umoren and Solomon 2014b). One of the many approaches devised to enhance the inhibition efficiency of polymers is the combination with substances capable of exerting a synergistic influence (Fares et al. 2013; Solomon and Umoren 2015b; Arukalam et al. 2014).
Synergism is a phenomenon whereby the combined effect of substances is greater than the sum of their individual effect. There are reports in the literature on the improvement of the inhibition efficiency of polymers by the addition of iodide ions. Arukalam et al. (2015) documented that addition of 500 mg/l KI to 2000 mg/l hydroxyethyl cellulose raised the inhibition efficiency of the polymer from 76.84 to 92.74% for mild steel in aerated sulfuric acid solution. Umoren et al. (2010) studied the influence of addition of 5 mM KI to 0.5 g carboxymethyl cellulose and reported that the inhibition efficiency of the polymer for mild steel in sulfuric acid medium increased from 65 to 89% on addition of iodide ions. A comprehensive review of the effect of halide ion addition on the inhibition efficiency of organic species including polymers can be found in Umoren and Solomon (2014b).
Chitosan (Fig. 1) is second to carboxymethyl cellulose as the most abundant naturally occurring polymer (Umoren et al. 2013) and has gained tremendous application in the medical and pharmaceutical fields owing to its biodegradability, biocompactibility and low toxicity (Yang et al. 2007). It has also found applications in the cosmetic, textile, paper and food industries (Jayakumar et al. 2005). It has been reported as an effective corrosion inhibitor for mild steel (Umoren et al. 2013) and copper (El-Haddad 2013) in HCl solution. Derivatives of chitosan, namely, acetyl thiourea chitosan (Fekry and Mohamed 2010), carboxymethyl chitosan (Cheng et al. 2007), chitosan-crontonaldehyde Schiff’s base (Mohamed and Fekry 2011) and amino thiourea-modified chitosan (Li et al. 2014) have all been reported as metal corrosion inhibitors in various corrosive environments. To the best of our knowledge, there is no documentation in the literature on the use of chitosan as an inhibitor for steel in H2SO4 solution.
This work was designed to examine the capability of unmodified chitosan to suppress the dissolution of St37 steel in 15% H2SO4 solution and the extent to which the inhibitive ability of the polymer can be boosted by the addition of a small amount of potassium iodide. The investigation was done using both weight loss (WL) and electrochemical (electrochemical impedance spectroscopy, potentiodynamic polarization and dynamic electrochemical impedance spectroscopy) techniques complemented with surface morphological examination using scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) at a temperature range of 25–60 °C.
Experimental
Materials
The specimens employed in this investigation were prepared from St37-2 (for convenience, St37 is used throughout the text) steel having a chemical composition of (wt%): C 0.17, Mn 1.40, P 0.05, Si 0.30, S 0.05 and the balance Fe (Hassan and Hadi 2015). The metal sheet was procured from Erdemir Steel Co., Turkey. Prior to the experiments, the metal samples were progressively ground with emery papers (grade 600, 800, 1500 and 2000), rinsed with distilled water several times, cleaned with ethanol and acetone, and then dried in warm air (Zou et al. 2014). The acid solution (15% H2SO4) was prepared by dilution of an analytical grade sulfuric acid with distilled water. Chitosan with the properties listed in Table 1 was used in the concentration range of 1–5 g per 1000 cm3 of acid solution, while the concentration of KI used was 5 mM. Chitosan and all the chemicals were procured from Sigma Aldrich and were used as purchased without further purification.
Electrochemical measurements
Electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PDP)
These measurements were performed using a computer-controlled Gamry instrument potentiostat/galvanostat/ZRA (Reference 600) embedded in a Gamry framework system composed of ESA410. The instrument applications include DC105 software for PDP measurements and EIS300 for EIS measurements. The prepared St37 (exposed area = 0.75 cm2) was used as the working electrode; the reference electrode was a saturated calomel electrode (SCE), while the counter electrode was a platinum plate. Before each experiment, the St37 electrode was immersed in test solutions for 2 h at 25 °C to attain a stable open circuit potential (OCP). All the electrochemical corrosion measurements were performed at least four times under the same conditions to obtain reproducible data.
The PDP studies were carried out at a constant sweep rate of 1 mV/s at −250 to +250 mV intervals with respect to the corrosion potential (E corr). Corrosion current density (i corr) values were obtained using the Tafel extrapolation technique (Zou et al. 2014) and by taking an extrapolation interval of 100 mV around the E corr value once stable. EIS measurements were performed at E corr using an amplitude signal of 10 mV peak to peak and using an AC signal at E corr in a frequency range of 10 mHz to 100 kHz. The analyses of EIS data were performed using the Echem6.32 program. The inhibition efficiency (%η) for the PDP and EIS experiments was generated from i corr and charge transfer resistance (R ct ) using Eqs. (1) and (2), respectively (Achary et al. 2008):
where \(i_{\text{corr}}^{0}\) and \(R_{ct}^{0}\) are the corrosion current density and charge transfer resistance in the absence of inhibitor; \(i_{\text{corr}}\) and \(R_{ct}\) are the corrosion current density and charge transfer resistance in the presence of inhibitor, respectively.
Dynamic electrochemical impedance spectroscopy (DEIS)
Dynamic electrochemical impedance spectroscopy (DEIS) studies were performed using a frequency response analyzer (FRA), which included a galvanostat model. The generation of the current perturbation was done with a National Instruments Ltd. PCI-4461 digital-analog card (Gerengi et al. 2016a). The same card was employed in the measurement of the current and voltage signals. The sampling frequency was 12.8 kHz, and the perturbation signal was a package composed of current sinusoids in the frequency range from 4.5 kHz to 700 MHz. The measurements were done for 15 h.
Weight loss experiments
In the weight loss measurements, the pre-cleaned St37 steel specimens (in triplicate) were suspended freely in glass reaction vessels containing 100 ml of different test solutions (15% H2SO4, 15% H2SO4 + chitosan and 15% H2SO4 + chitosan + KI) at 25, 40, 50 and 60 °C in a thermostated bath. The metal samples were retrieved after 10 h, washed thoroughly in 20% NaOH solution containing 200 g/l of zinc dust (Solomon et al. 2010), rinsed in running water, dried with warm air and then re-weighed. The weight loss (g) was taken as the difference in the weight of the St37 steel specimens before and after immersion in different test solutions, and the average weight loss was reported.
The corrosion rate (CR) (mpy) in the absence and presence of inhibitor was calculated using the following equation (Umoren et al. 2015):
where CR is the corrosion rate, W is the average weight loss (g), ρ is the density of the metal specimen (g cm−3), A is the surface area of the specimen (cm2), and T is the immersion duration (h). The inhibition efficiency (% η) of chitosan and chitosan + KI, respectively, was calculated from Eq. 4 (Verma et al. 2016):
where \(W_{0}\) and \(W_{e}\) are the weight losses of the coupons in the absence and presence of inhibitor, respectively, at the same temperature.
Surface morphological analysis
The surface morphologies of St37 steel specimens exposed to 15% H2SO4 solutions in the absence and presence of additives for 10 h were analyzed using a Quanta FEG 250 scanning electron microscope (FEI, The Netherlands). Elemental compositional analysis of the metal specimens before and after exposure to test solutions was done using electron dispersive X-ray spectroscopy.
Results and discussion
Potentiodynamic polarization (PDP)
The potentiodynamic polarization curves obtained for St37 steel in 15% H2SO4 solution (a) without and with various concentrations of chitosan and (b) in the absence and presence of 5 g chitosan, 5 mM KI and chitosan–iodide ion combination are presented in Fig. 2. The mechanism proposed previously by Bockris et al. (1961) has gained overwhelming acceptance as the anodic electro-dissolution pattern of iron in sulfate-containing solutions (Oguzie 2004; Umoren et al. 2015):
By this mechanism, the anodic oxidation reaction of Fe is dependent on the adsorbed intermediate, \(\left[ {\text{FeOH}} \right]_{\text{ads}}\). The corresponding cathodic reaction is the reduction of hydrogen ions according to the following equations (Umoren et al. 2015):
With inspection of the anodic branch of the Tafel curve for St37 steel in the acid solution devoid of inhibitor (Fig. 2), three distinctive regions designated as A–C can be identified. In the first region (A), the current density increases with a rise in the corrosion potential until a maximum is attained. This region may be ascribed to the initial rapid attack of the metal surface by hydroxyl ions according to Eq. 5. In the region labeled B, the current density decreases with increasing potential and attains a minimum value, which can be defined as the region of \(\left[ {\text{FeOH}} \right]_{\text{ads}}\) reduction to \(\left[ {\text{FeOH}} \right]^{ + }\) (Eq. 6). The current density is found to increase again with potential in region C, and this may correspond to the oxidation reaction in Eq. 7. Compared to the Tafel curves of the inhibited systems, the interference in the corrosion reactions can be seen; regions B and C are absent. This means that the additives inhibited the anodic corrosion process of St37 steel in 15% H2SO4 solution by aborting the reduction of \(\left[ {\text{FeOH}} \right]_{\text{ads}}\) to \(\left[ {\text{FeOH}} \right]^{ + }\) and the subsequent oxidation of \(\left[ {\text{FeOH}} \right]^{ + }\) to \({\text{Fe}}^{2 + }\). A comparison of the Tafel plots in Fig. 2a to that in 2b reveals that chitosan only slightly affected the anodic and cathodic densities with respect to that of the free acid solution, whereas KI and chitosan + KI combination significantly displaced the anodic and cathodic current densities toward lower values (Fig. 2b).This points to the low inhibition power of chitosan and the improvement brought about by iodide ion addition to the inhibition efficiency. Noteworthy in Fig. 2 is also the fact that the cathodic polarization curves of all the systems give rise to Tafel lines, indicating that the hydrogen evolution reaction is activation controlled (Li et al. 2015, 2016).
The potentiodynamic parameters, namely the corrosion potential (\(E_{\text{corr}}\)), corrosion current (\(I_{\text{corr}}\)), anodic and cathodic Tafel slopes (\(\beta_{a} \,{\text{and}}\,\beta_{c} )\), and corrosion rate (\(C_{R}\)), obtained from the extrapolation of the cathodic and anodic Tafel lines located next to the linear current regions (Sherif and Park 2006), are listed in Table 2. The percentage inhibition efficiency (\(\% \eta\)) also given in the table was computed using Eq. 1. It is obvious from the table that the \(E_{\text{corr}}\) values of the inhibited acid solutions are smaller than those of uninhibited but the difference between them is not up to the benchmark of \(\pm 85 {\text{mV}}\) given in the corrosion literature (Umoren et al. 2015) for categorizing an inhibitor as anodic or cathodic; thus, chitosan, KI and the chitosan + KI mixture can be classed as mixed type corrosion inhibitor in the studied system. Umoren et al. (2013) previously reported mixed type behavior for chitosan toward mild steel corrosion in HCl solution. As should be expected, the \(I_{\text{corr}}\) and \(C_{R}\) values for St37 steel in inhibited acid solutions are lower than those in acid solution without inhibitor. By increasing the chitosan concentration, \(I_{\text{corr}}\) and \(C_{R}\) values are found to further decrease, and as a consequence, the inhibition efficiency increases. For instance, the \(I_{\text{corr}}\) and \(C_{R}\) values of St37 steel in the free acid solution are 395.0 µAcm−2 and 74.13 mpy, respectively. Introduction of 1 g chitosan to the acid solution reduced both the \(I_{\text{corr}}\) and \(C_{R}\) values to 242.0 µAcm−2 and 45.43 mpy, respectively, corresponding to an inhibition efficiency of 38.73%. By increasing the concentration to 5 g, the \(I_{\text{corr}}\) and \(C_{R}\) values decreased to 185.0 µAcm−2 and 38.8 mpy, respectively, and the \(\% \eta\) increased to 53.16%. This decrease in the \(I_{\text{corr}}\) and \(C_{R}\) values in the presence of chitosan is indicative of the reduction in the rate of corrosive ion attack on the St37 steel surface, which probably arises from the adsorption of chitosan molecules on the metal surface. However, it can be seen that by combining 5 mM KI and 5 g chitosan, the \(I_{\text{corr}}\) and \(C_{R}\) values are substantially decreased compared to individual addition leading to a marked increase in the inhibition efficiency from 53.16% for 5 g chitosan and 79.37% for 5 mM KI to 97.65%. This seems to reflect a synergistic effect (Farag and Ali 2015). Table 2 also shows that both \(\beta_{a} \,{\text{and}}\,\beta_{c}\) values do not display any specific pattern with increasing inhibitor concentration, implying that the mechanisms of the anodic St37 steel oxidation and cathodic hydrogen ion reduction reactions were not modified by the presence of the additives in the corrosive solution (Li et al. 2016).
Electrochemical impedance spectroscopy (EIS) measurements
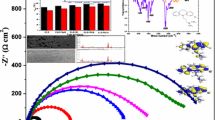
Figure 3 shows the electrochemical impedance results obtained for St37 steel in 15% H2SO4 solution in the absence and presence of various additives as exemplified in the (a, b) Nyquist and (c, d) bode modulus and phase angle representations. The Nyquist diagram for St37 steel in the free acid solution (Fig. 3a) consists of a large capacitive loop at high frequencies and a small inductive loop at low frequencies. Compared with those for the inhibited systems, the diameter of the capacitive loop increases, while the inductive loop gradually disappears and is almost completely absent in the Nyquist impedance curve for 5 g chitosan + 5 mM KI (Fig. 3b). Zheng et al. (2014) reported a similar observation for mild steel in H2SO4 solution without and with 1-butyl-3-methyl-1 H-benzimidazolium iodide, which was interpreted to indicate a ‘degradation’ phenomenon of impedance spectroscopy. It could also be associated with the delay in the charge transfer process caused by the growing up of the inhibiting film on the metal surface (Fuchs-Godec and Pavlovic 2012). The capacitive loop at high frequencies is associated with the time constant of the charge transfer and double-layer capacitance (Palomar-Pardavé et al. 2012; Farag and Hegazy 2013), while the inductive loop at low frequencies is assigned to the relaxation process of adsorption species such as \(\left( {{\text{SO}}_{4}^{2 - } } \right)_{\text{ads}}\) and \(\left( {{\text{H}}^{ + } } \right)_{\text{ads}}\) on the St37 steel surface (Palomar-Pardavé et al. 2012; Farag and Hegazy 2013) or the adsorption-desorption process of inhibitive molecules on the metal surface (Zheng et al. 2015; Ansari et al. 2015). However, some authors (Zheng et al. 2015; Amin and Ibrahim 2011) believed that, in sulfuric acid solution, \(\left( {{\text{SO}}_{4}^{2 - } } \right)_{\text{ads}}\) is the favored adsorption specie since the steel surface acquires positive net charge, meaning that the observed inductive loop in the impedance curve of the corrodent is due to the relaxation of adsorbed \(\left( {{\text{SO}}_{4}^{2 - } } \right)_{\text{ads}}\) species on the metal surface. By inspecting Fig. 3b, it is seen that the capacitive loop for the chitosan-iodide combination is remarkably larger than that of iodide ions and chitosan alone, emphasizing the better protection accorded the St37 steel surface by the mixture. Also noteworthy in the Nyquist diagrams is the imperfection of the semicircles, which describes the frequency dispersion caused by the roughness and non-uniformity of the St37 working electrode surface (Yilmaz et al. 2016; Ansari et al. 2015). In the Bode modulus and phase angle plots (Fig. 3c, d), the influence of additives can also clearly be seen. The introduction of chitosan in the acid solution causes an increase in the impedance modulus at low frequencies and also in the phase angle. This demonstrates that the presence of chitosan in the corrosive environment improves the corrosion resistance of St37 steel. It is however seen that addition of iodide ions to chitosan caused a further increase in the impedance modulus, and the phase angle approached 80° from medium to low frequencies, suggesting the formation of a highly stable and compact protective film (Wang et al. 2016).
For the analysis of the Nyquist impedance data, the equivalent circuit shown in Fig. 4 was used. The circuit was recently used by Choe et al. (2015) in their work on the evaluation of the electrochemical impedance properties of anti-corrosion films by the arc thermal metal spraying method. It allows the solution resistance (R s ), charge transfer resistance (\(R_{ct}\)) and film resistance (R f ) to be derived. The \(Q\) parameter denotes the constant phase element (CPE) and was used to compensate for the roughness and the non-uniformity of the St37 steel electrode. \(Q_{f}\) and \(Q_{dl}\) represent the capacitance for the surface film and double layer. The parameter \(Q\) is related to the impedance representation as (Solomon and Umoren 2016):
where \(Y_{0}\) is the CPE constant, and \(n\) is the CPE exponent; \(j = \left( { - 1} \right)^{1/2}\), which is an imaginary number, and ω is the angular frequency in rad/s. The double-layer capacitance (\(C_{dl}\)) values were calculated from the expression (Solomon and Umoren 2015a):
where ω is the angular frequency (ω = 2πf max); f max is the frequency at which the imaginary component of the impedance is maximum, and n is the phase shift (\(- 1 \le n \le 1\)); when \(n = 0\), the CPE represents the pure resistor; if \(n = - 1\), the CPE stands for the inductor, and if \(n = + 1\), the CPE represents the pure capacitor (Obot and Madhankumar 2016; Solomon and Umoren 2016). The goodness of the equivalent circuit fitting and the validity of the derived parameters are adjudged by the quite small chi-square values (i.e., χ2 ranged between \(1.439 \times 10^{ - 4}\) and \(5.264 \times 10^{ - 4}\)). All the derived parameters are listed in Table 3. The %η also given in the table was computed using Eq. 2. The results in the table show that the \(R_{ct}\) and R f values of the inhibited solutions are higher compared to those of the uninhibited and increased with increasing inhibitor concentration, corresponding to the increase in the diameter of the Nyquist diagrams (Fig. 3a, b), impedance modulus and phase angle (Fig. 3c, d). The reverse is observed for \(C_{dl}\) values, that is, the \(C_{dl}\) values for additives containing solutions are smaller than those without additives and decrease with increasing concentration. The higher values of \(R_{ct}\) and R f indicate the corrosion inhibition by chitosan alone and in combination with iodide ions. The inhibition process by chitosan is found to be a function of concentration. The decrease in the \(C_{dl}\) value with increasing inhibitor concentration is in accordance with the Helmholtz equation given as: \(C_{dl} = \frac{{\varepsilon_{o} \varepsilon A}}{d}\) (Chidiebere et al. 2014) where \(\varepsilon_{o}\) is the permittivity of air, \(\varepsilon\) is the local dielectric constant, \(d\) is the thickness of the adsorbed film, and \(A\) is the surface area of the electrode. By this equation, it follows that the decrease in \(C_{dl}\) could arise from a decrease in the local dielectric constant and/or an increase in the thickness of the electrical double layer. It is arguable to say that the decrease in the present case was due to adsorption of inhibitor molecules on the St37 steel surface. Further inspection of Table 3 reveals that the highest inhibition efficiency afforded by the highest studied chitosan concentration is 46.98 and 80.70% for 5 mM KI. By mixing the two, the inhibition efficiency of chitosan increased by 50.67%, that is, from 46.98 to 92.72%, which is in good agreement with the PDP results. The \(n\) values are found to be very close to unity, signifying that the interface behavior is nearly capacitive (Zheng et al. 2014, 2015).
Dynamic electrochemical impedance spectroscopy (DEIS) measurements
The uniqueness of DEIS lies in the fact that corrosion measurement can be performed over a prolonged immersion time in a non-stationary system. The weakness of EIS has been the assumption that the corrosion system is a stationary one as this investigated system should remain constant throughout the time of impedance measurement (Nagarajan and Rajendran 2009). To gain insight into the corrosion and corrosion inhibition process of St37 steel in 15% H2SO4 solution without and with chitosan alone and in combination with KI as time elapses, DEIS experiments were undertaken at 25 °C for 15 h. Figure 5 shows the DEIS spectra obtained for St37 steel in (1) 15% H2SO4 solution, (2) acid solution containing 5 g chitosan and (3) acid solution containing 5 g chitosan + 5 mM KI after 15 h of corrosion measurement. The spectra are flat and depressed semicircles agreeing with the EIS results that the metal corrosion process is controlled by charge transfer. However, the effect of the chitosan and chitosan-iodide mixture can clearly be seen in Fig. 5b and c, respectively. At 1 h after the inhibitor has been introduced, there is a disconnection, and the diameter of the semicircle becomes larger, indicating greater resistance posed by the additives on the charge transfer process. Greater effect is observed in Fig. 5c compared to Fig. 5b, again showing the improved inhibition by the chitosan-iodide mixture. Also noteworthy in the chitosan-iodide spectrum (Fig. 5c) is the monotonous increase in the size of the semicircle with immersion time. This suggests that the adsorbed protective film is more stable at longer immersion time (Gerengi et al. 2013). To give quantitative information on the DEIS study, the spectra were analyzed using the same R(QR)(QR) equivalent circuit used for the analysis of EIS results, and the results are presented in Table 4. Inspection of the table revealed that \(R_{ct}\) and \(R_{f}\) vary in the same manner as those obtained from the EIS method with respect to the values for the blank, and the \(n\) parameter also points toward capacitive behavior. Nevertheless, it is observed that the %\(\eta\) for the chitosan and chitosan-iodide combination are higher than those from the EIS method. For instance, the inhibition efficiency for 5 g chitosan alone and in combination with KI from the EIS technique is 46.98 and 92.72%, respectively, but 63.48 and 94.19% for the DEIS method. This could be due to the adsorption of more inhibitor molecules and the stability of the adsorbed film as time elapsed.
Weight loss (WL) measurements
The corrosion-inhibiting ability of chitosan alone and in combination with 5 mM KI for St37 steel in 15% H2SO4 solution was also studied by the weight loss (WL) technique for 10 h. The parameters such as weight loss (g), corrosion rate (mpy), surface coverage (θ) and %η obtained from the WL method are listed in Table 5. As the table shows, the weight loss and corrosion rate of the metal are reduced in acid solution containing various concentrations of chitosan and 5 mM KI and significantly in chitosan + KI containing acid solution. For instance, the weight loss of 0.4212 g and the corrosion rate of 2003.34 mpy of the St37 steel in free acid solution is reduced to 0.0081 g and 38.53 mpy, respectively, by introduction of the chitosan-iodide ion mixture into the acid solution. This shows that the mixture effectively protected the metal surface against corrosive attack in the acid solution. Again, the effectiveness of the chitosan-iodide ion mixture is affirmed by the larger surface coverage value (0.98) and higher inhibition efficiency of 98.08% as against the 59.86 and 70.09% afforded by chitosan and 5 mM KI, respectively. This suggests a synergistic effect between chitosan and iodide ions (Obot and Madhankumar 2016). The WL results are in good agreement with the results from electrochemical techniques (Table 2, 3).
Mechanism of adsorption of chitosan in the presence of iodide ions
The optimum average percentage inhibition efficiency from the PDP, EIS, DEIS and WL methods afforded by 5 g chitosan (the highest studied concentration) is 53.57%, and this portrays chitosan as a moderate inhibitor for St37 steel in 15% H2SO4 solution. Inhibition of metal corrosion by an organic inhibitor is brought about by the adsorption and stability of inhibitor molecules on the metal surface. However, certain factors, such as the metal surface charge, form in which the inhibitor molecules exist in the corrosive solution; the concentration of the electrolytes, temperature and immersion duration affect the adsorption and stability of the adsorbed inhibitor film as well as the inhibition efficiency. It has been shown (Chidiebere et al. 2014; Farag and Ali 2015) that the steel surface in the sulfuric acid solution acquired a net positive charge and is hydrated with anions such as sulfate ions. The molecular structure of chitosan given in Fig. 1 reveals that the polymer is characterized by hydroxyl (–OH) and amine (–NH2) functional groups. The N and O heteroatoms would undoubtedly serve as the adsorption center for chitosan molecules. Nevertheless, in the strong acid solution, these groups would be protonated such that the polymer exists as polycation (Umoren et al. 2013). Direct interaction between the positively charged steel surface and the polycations would be difficult because of the electrostatic repulsion. The chitosan protonated species are therefore adsorbed on the sulfate layer, but our results suggest that the \({\text{SO}}_{4}^{2 - }\) ions did not effectively replenish the St37 surface to allow a sufficient amount of the inhibitor species to be adsorbed. In the presence of iodide ions, a competitive adsorption between the \({\text{SO}}_{4}^{2 - }\) and \({\text{I}}^{ - }\) ions is inevitable on the charged metal surface. Mourya et al. (2016) recently demonstrated through the use of X-ray photoelectron spectroscopy that, in a sulfuric acid solution containing iodide ions, \({\text{I}}_{3}^{ - }\) and \({\text{I}}^{ - }\) ions are the species adsorbed on the steel surface. According to the authors, iodide ions are oxidized by dissolved oxygen in \({\text{I}}_{3}^{ - }\) and exist in equilibrium with \({\text{I}}^{ - }\). It could therefore be said that chitosan polycations are attracted to the St37 steel surface whereby the \({\text{I}}_{3}^{ - }\) and \({\text{I}}^{ - }\) ions are adsorbed through coulombic attraction. The co-adsorption leads to an increase in the thickness of the protective film, and greater resistance is posed to the charge transfer as evident in the larger capacitive loop in Fig. 3b and the enhanced inhibition efficiency. It is interesting to note that the inhibition efficiency obtained in the present investigation for the chitosan + KI combination is significantly higher than the inhibition efficiency achieved by Umoren et al. (2013) in HCl solution even though a higher acid concentration was used in the present study. This clearly shows the influence of the larger ionic radius, high hydrophobicity and ease of polarizability of iodide ions compared to chloride ions on the recharging metal surface.
Adsorption consideration
Organic inhibitors are believed to inhibit metal corrosion by an adsorption mechanism whereby the inhibitor molecules gradually substitute the adsorbed water molecules on the metal surface (Liu et al. 2014; Mourya et al. 2016; Solomon and Umoren 2016). Two types of adsorption are defined in the literature: physisorption and chemisorptions (Tao et al. 2012; Solomon et al. 2010). Physisorption has to do with the electrostatic interaction between charged inhibitor molecules and a charged metal, while chemisorption involves charge sharing or transfer from the inhibitor components to a metal surface to form a coordinate type of bond (Solomon et al. 2010). Interestingly, these two types of adsorption can occur on the same surface. Certain factors such as the chemical composition of the inhibitor molecule, concentration of both the inhibitor and corrosive solution, electronic properties of the inhibitor molecule and the temperature of the aggressive medium (Tao et al. 2012) have an influence on the type of adsorption that prevails over the other in a metal surface. Theoretically, low temperatures favor physical adsorption because of the low heat of adsorption, while elevated temperatures favor chemical adsorption owing to its stronger adsorption energy compared to physisorption (Tao et al. 2012; Liu et al. 2015). Basic information regarding the predominant adsorption mechanism can be obtained through the use of a suitable adsorption isotherm (Gerengi et al. 2016b; Zheng et al. 2015; Tao et al. 2012). To explain the nature of the interaction between the chitosan molecules and St37 steel surface, the surface coverage values (Table 5) were fitted to various adsorption isotherms including the Langmuir, El-Awady et al. kinetic/thermodynamic, Temkin, Freundlich and Frumkin adsorption isotherms. Normally, the linear regression coefficient (\(R^{2}\)) value of the isotherm graph is used to adjudge the isotherm model that best fits the experimental data. In our case, the Langmuir adsorption isotherm [Fig. S1(a)] gave the best fit with the \(R^{2}\) value ranging between 0.9931 and 0.9998 (Table 6). However, the slope (Table 6) of the Langmuir plot deviated remarkably from the unity required by an ideal Langmuir isotherm equation. The deviation is likely to be due to interaction of adsorbed chitosan molecules on the St37 steel surface (Gerengi et al. 2016b; Solomon et al. 2010), a factor that was omitted during the derivation of the Langmuir isotherm equation (Gerengi et al. 2016b; Solomon et al. 2010). For this reason, the θ values were fitted into the El-Awady kinetic/thermodynamic adsorption model, which is a modification of the Langmuir isotherm and makes a provision for the omitted interaction parameter by the Langmuir isotherm. The El-Awady et al. kinetic/thermodynamic isotherm model has the following form (Solomon and Umoren 2015c):
where C is the inhibitor’s concentration; \(K_{\text{ads}} = K^{1/y}\) is the equilibrium constant of the adsorption process. \(1/y\) stands for the number of active sites occupied by an inhibitor molecule or the number of water molecules replaced by an inhibitor molecule (Solomon and Umoren 2015c). The straight line graphs obtained by plotting \(\log (\theta /1 - \theta )\) against \(\log C\) are given in Fig. S1(b) of the supplementary information. The free energy in the adsorption process (\(\Delta G_{\text{ads}}^{0}\)) can be deduced from the \(K_{\text{ads}}\) value using the following equation (Tao et al. 2012):
where \(R\) is the universal gas constant and \(T\) is the absolute temperature. The adsorption parameters derived from the El-Awady et al. isotherm plot are listed in Table 6. As can be seen from the table, the \(K_{\text{ads}}\) value decreases with increasing temperature, indicating a shift in the adsorption-desorption equilibrium toward desorption at elevated temperatures (Zheng et al. 2015; Xia et al. 2015). This behavior is typical of the physical adsorption mechanism (Zheng et al. 2015; Solomon et al. 2010). Further inspection of Table 6 reveals that the value of 1/y in all cases is significantly higher than unity, which is a clear indication that each molecule of chitosan became attached to more than one active center at the St37 steel surface. In the corrosion literature (Gerengi et al. 2016b; Tao et al. 2012; Xia et al. 2015), the \(\Delta G_{\text{ads}}^{0}\) value up to −20 kJ/mol is interpreted to indicate the physisorption mechanism, while that more negative than −40 kJ/mol is linked to the chemical adsorption mechanism (Zou et al. 2014). Our \(\Delta G_{\text{ads}}^{0}\) value is between 9.55 and 9.98 kJ/mol; as such, we conclude that the physical adsorption mechanism was the predominant mechanism in the adsorption process of chitosan molecules onto the St37 steel surface.
Synergism consideration
It is observed from all the applied methods that the chitosan-iodide ion combination exhibits higher inhibition efficiency than either chitosan or KI independently. As explained earlier, this could arise because of the co-adsorption of the iodide anions and the chitosan cations on the metal surface. However, co-adsorption could be competitive or cooperative (Farag and Ali 2015). In competitive co-adsorption, the anions and cations adsorb on different sites on the metal surface, whereas cooperative co-adsorption has to do with the adsorption of the cations on the anion layer (Mourya et al. 2016). Nevertheless, these two kinds of co-adsorption can take place simultaneously on the same metal surface. The synergism parameter (\(S_{\theta }\)) is usually used to establish the kind of co-adsorption prevailing over the other on a metal surface. Generally, \(S_{\theta } < 1\) is indicative of competitive co-adsorption and the existence of antagonistic behavior, while \(S_{\theta } > 1\) signifies the synergistic effect (Farag and Ali 2015; Mourya et al. 2016). To ascertain the kind of co-adsorption existing between chitosan polycations and iodide ions on the St37 steel surface in 15% H2SO4 solution, \(S_{\theta }\) was calculated using the formula given in Eq. 15 (Solomon et al. 2016), and the values obtained are given in Table 7. It is obvious that the enhanced corrosion inhibition of chitosan by iodide ions was due to a synergistic effect as the \(S_{\theta }\) values from all the techniques are greater than unity.
where \(\theta_{1}\) is the degree of surface coverage of iodide ions, \(\theta_{2}\) is the degree of surface coverage of chitosan, and \(\theta_{1 + 2}^{\imath }\) is the degree of surface coverage of chitosan + KI.
Effect of temperature on the inhibition efficiency and corrosion kinetic analysis
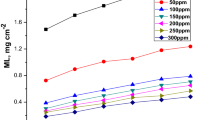
The influence of temperature on the corrosion and corrosion inhibition of the St37 steel in 15% H2SO4 solution without and with additives has been studied at temperatures ranging from 25 to 60 °C for 10 h. Figure 6 illustrates the variation of (1) the corrosion rate (mpy) and (2) the percentage inhibition efficiency (\(\eta\)) with a selected concentration of the chitosan and chitosan-iodide ion combination at different temperatures. The figure clearly shows that both the corrosion rate and inhibition efficiency are dependent on the concentration and temperature. While the corrosion rate decreases with increasing concentration, the inhibition efficiency increases. The figure also shows that the corrosion rate increases with an increase in the system temperature because of the intensified molecular thermal motion, while the inhibition efficiency of chitosan declines as the system temperature rises. This decrease in the inhibition efficiency of chitosan with increasing temperature is suggestive of a shift in the adsorption-desorption equilibrium toward desorption (Solomon et al. 2010). It also suggests the physical adsorption of chitosan molecules onto the St37 steel surface (Thirumoolan et al. 2014). By closely observing the bar charts for the inhibition efficiency of the chitosan-iodide mixture as it varies with temperature, it is noticed that \(\eta\) exhibits near constancy particularly at 40, 50 and 60 °C. For instance, \(\eta\) for the mixture at 40 °C is 99.07%. At 50 and 60 °C, the inhibition efficiency only slightly increased to 99.68 and 99.72%, respectively. A similar observation was reported in the literature and associated with the formation of dimeric protective film on the metal surface (Solomon et al. 2010; Norr 2005). In such a case, an increase in the system temperature only causes a gradual desorption of the outer layer such that the underlying layer still offers satisfactory protection to the metal surface.
The corrosion kinetic parameters for the studied system, namely the activation energy (\(E_{a}\)), activation enthalpy (\(\Delta H^{*}\)) and entropy of activation (\(\Delta S^{*}\)) were obtained from the Arrhenius and transition state equations, respectively (Solomon et al. 2016):
where \(C_{R}\) is the corrosion rate, \(R\) is the molar gas constant, \(T\) is the absolute temperature, \(A\) is the frequency factor, \(h\) is Planck’s constant, and \(N\) is Avogadro’s number. Represented in Figures S2 and S3 of the supplementary information are the plot of log \(C_{R}\) against 1/T and \(\log \left( {C_{R} /T} \right)\) versus (1/T) for St37 steel in 15% H2SO4 solution without and with the additives. From the slopes of Fig. S2 and S3, \(E_{a}\) and \(\Delta H^{*}\) values were computed, while the \(\Delta S^{*}\) values were calculated from the intercepts of the linear graphs in Fig. S3. The derived kinetic parameters are given in Table 8. The \(E_{a}\) values of chitosan-inhibited acid solutions are bigger than those of the blank solution and increase with increasing concentration, while those of the chitosan-iodide ion-inhibited system are smaller compared to the \(E_{a}\) value of the uninhibited ones. In the literature (Thirumoolan et al. 2014; Solomon et al. 2010; Mobin and Khan 2013), a bigger \(E_{a}\) value of the inhibited solution relative to the uninhibited one is interpreted to indicate the physical adsorption, while the reverse is indicative of the chemisorption mechanism. The variation of the \(E_{a}\) values of the acid solution with those of the free solution conforms with the variation of %η with temperature; we submit that chitosan polycations were adsorbed on the St37 steel surface via electrostatic interaction, while strong chemisorption takes place in the case of chitosan-iodide mixture adsorption. Table 8 shows that the \(\Delta H^{*}\) values vary in a similar manner as \(E_{a}\) and are almost the same. Zheng et al. (2014) had reported similar results. This allows the verification of the well-known thermodynamic relationship between \(E_{a}\) and \(\Delta H^{*}\), i.e., \(E_{a} - \Delta H^{*} = RT\). The \(\Delta S^{*}\) values are large and negative in all cases, indicating that the activated complex in the rate-determining step represents an association rather than dissociation step, meaning that a decrease in the degree of disorderliness of the system takes place on moving from reactants to the activated complex (Thirumoolan et al. 2014; Solomon et al. 2010).
Surface morphological assessment
Figure 7 shows the SEM images and EDS spectra obtained for St37 steel in the (a, b) abraded state, (c, d) exposed to 15% H2SO4 solution, (e, f) exposed to 15% H2SO4 solution containing 5 g chitosan and (g, h) exposed to 15% H2SO4 solution containing 5 g chitosan in combination with 5 mM KI after 10 h of immersion at 25 °C. The percentage weight (wt%) elemental composition of the metal specimen in different test environments is given in Table 9. The St37 steel surface in the abraded state is relatively smooth (Fig. 7a) and shows an intense Fe peak in the EDS spectrum (Fig. 7b) corresponding to 95.63 wt% (Table 9). Exposure to the acid solution caused serious damage to the metal surface as evident in the scaly image in Fig. 7c, and this resulted in the loss of some component elements of the metal. For instance, the Mn, Mg, Si and Cr peaks are absent in Fig. 7d and in Table 9. It is obvious from Fig. 7d that the St37 steel surface was hydrated with sulfate ions; the presence of an intense peak with 17.43 wt% (Table 9) supports this claim. By comparing Fig. 7e with Fig. 7c, it could be seen that the surface in Fig. 7e is smoother. An interesting observation is made on comparing the EDS spectrum in Fig. 7d and f. Beside the fact that the C peak is more intense in Fig. 7f while the S peak is less intense and a new peak (N) appears, the metal retained its component elements. The Mn, Cr and Mg peaks, which are absent in the spectrum in Fig. 7d, could be seen in Fig. 7f. Again, the wt% of Fe, which reduced from 95.63 to 41.22% on exposure to the corrosive environment, increased in the chitosan-inhibited solution to 78.21%, indicating the protection of the metal offered by chitosan. However, the smoother surface in Fig. 7g compared to the image in Fig. 7d and the higher wt% of Fe (86.17%) in the acid solution containing the chitosan-iodide ion combination confirm the experimental results that the mixture effectively inhibited St37 steel corrosion in 15% H2SO4 solution compared to chitosan alone. The appearance of a N peak in Fig. 7f and I and N peaks in Fig. 7h confirms the adsorption of the additives on the St37 steel surface.
Conclusions
In this work, the corrosion inhibition of St37 steel corrosion in 15% H2SO4 solution by chitosan and the effect of addition of a small amount of KI on the inhibition efficiency have been studied using both chemical and electrochemical methods. Chitosan moderately inhibits the dissolution of St37 steel in the acid solution. Addition of 5 mM KI to chitosan has a significant effect on the inhibition efficiency. An increase in the immersion time and temperature leads to an increase in the inhibition efficiency of chitosan-iodide mixture. Judging from the variation of the inhibition efficiency with temperature and the value of the activation energy, it is concluded that physical adsorption prevails during the adsorption of chitosan molecules on the St37 steel surface, while the chitosan-iodide ions adsorb via chemisorption mechanism. According to the PDP results, both chitosan and chitosan-iodide combination functioned in the studied system as a mixed-type corrosion inhibitor.
References
Achary G, Naik YA, Kumar SV, Venkatesha T, Sherigara BS (2008) An electroactive co-polymer as corrosion inhibitor for steel in sulphuric acid medium. Appl Surf Sci 254:5569–5573
Amin MA, Ibrahim MM (2011) Corrosion and corrosion control of mild steel in concentrated H2SO4 solutions by a newly synthesized glycine derivative. Corros Sci 53:873–885
Ansari KR, Quraishi MA, Singh A (2015) Isatin derivatives as a non-toxic corrosion inhibitor for mild steelin 20% H2SO4. Corros Sci 95:62–70
Arukalam IO, Madufor IC, Ogbobe O, Oguzie E (2014) Experimental and theoretical studies of hydroxyethyl cellulose as inhibitor for acid corrosion inhibition of mild steel and aluminium. Open Corros J 6:1–10
Arukalam IO, Madufor IC, Ogbobe O, Oguzie EE (2015) Inhibition of mild steel corrosion in sulfuric acid medium by hydroxyethyl cellulose. Chem Eng Commun 202(1):112–122
Atta NF, Fekry AM, Hassaneen HM (2011) Corrosion inhibition, hydrogen evolution and antibacterial properties of newly synthesized organic inhibitors on 316L stainless steel alloy in acid medium. Int J Hydrogen Energy 36:6462–6471
Bebon, H (2011). St37 steel plate. www.steel-plate-sheet.com/Steel-plate/D/N/St372.html. Accessed 2nd July 2016
Bockris JOM, Drazic D, Despic AR (1961).The electrode kinetics of the deposition and dissolution of iron. Electrochim Acta 4(2–4):325–361
Cheng S, Chen S, Liu T, Chang X, Yin Y (2007) Carboxymethyl chitosan + Cu2+ mixture as an inhibitor used for mild steel in 1.0 M HCl. Electrochim Acta 52:5932–5938
Chidiebere MA, Oguzie EE, Liu L, Li Y, Wang F (2014) Corrosion inhibition of Q235 mild steel in 0.5 M H2SO4 solution byphytic acid and synergistic iodide additives. Ind Eng Chem Res 53:7670–7679
Choe HB, Lee HS, Ismail MA, Hussin MW (2015) Evaluation of electrochemical ımpedance properties of anticorrosionfilms by arc thermal metal spraying method. Int J Electrochem Sci 10:9775–9789
El-Haddad MN (2013) Chitosan as a green inhibitor for copper corrosion in acidic medium. Int J Biol Macromol 55:142–149
Farag AA, Ali TA (2015) The enhancing of 2-pyrazinecarboxamide inhibition effect on the acid corrosion of carbon steel in presence of iodide ions. J Ind Eng Chem 21:627–634
Farag AA, Hegazy MA (2013) Synergistic inhibition effect of potassium iodide and novel Schiff bases on X65 steel corrosion in 0.5 M H2SO4. Corros Sci 74:168–177
Fares MM, Maayta AK, Al-Mustafa JA (2013) Synergistic corrosion inhibition of aluminum by polyethylene glycol and ciprofloxacin in acidic media. J Adhes Sci Technol 27(23):2495–2506
Fekry AM, Ameer MA (2011) Electrochemical investigation on the corrosion and hydrogen evolution rate of mild steel in sulphuric acid solution. Int J Hydrogen Energy 36:11207–11215
Fekry AM, Mohamed RR (2010) Acetyl thiourea chitosan as an eco-friendly inhibitor for mild steel in sulphuric acid medium. Electrochim Acta 55:1933–1939
Fuchs-Godec R, Pavlovic MG (2012) Synergistic effect between non-ionic surfactant and halide ions in the forms ofinorganic or organic salts for the corrosion inhibition of stainless-steel X4Cr13in sulphuric acid. Corros Sci 58:192–201
Gerengi H, Slepski P, Bereket G (2013) Dynamic electrochemical impedance spectroscopy andpolarization studies to evaluate the inhibition effect ofbenzotriazole on copper-manganese-aluminium alloyin artificial seawater. Mater Corros 64(11):1024–1031
Gerengi H, Bereket G, Kurtay M (2016a) A morphological and electrochemical comparison of the corrosion process of aluminum alloys under simulated acid rain conditions. J Taiwan Inst Chem Eng 58:509–516
Gerengi H, Mielniczek M, Gece G, Solomon MM (2016b) Experimental and quantum chemical evaluation of 8-hydroxyquinoline as a corrosion inhibitor for copper in 0.1 M HCl. Ind Eng Chem Res. doi:10.1021/acs.iecr.6b02414
Hassan SA, Hadi AK (2015) Sudan III as corrosion ınhıbıtor for carbon steel St37-2 in H2SO4 solutıons. Int J Recent Sci Res 6(7):5445–5453
Jayakumar R, Prabaharan M, Reis RL, Mano JF (2005) Graft copolymerized chitosan—present status and applications. Carbohydr Polym 62:142–158
Li M, Xu J, Li R, Wang D, Li T, Yuan M, Wang J (2014) Simple preparation of aminothiourea-modified chitosan as corrosion inhibitor and heavy metal ion adsorbent. J Colloid Interface Sci 417:131–136
Li XH, Xie XG, Deng SD, Du GB (2015) Inhibition effect of two mercaptopyrimidine derivatives on cold rolled steel in HCl solution. Corros Sci 92:136–147
Li X, Deng S, Xie X, Du G (2016) Synergistic inhibition effect of 5-aminotetrazole and 4,6- dihydroxypyrimidine on the corrosion of cold rolled steel in H3PO4 solution. Mater Chem Phys 181:33–46
Liu W, Singh A, Lin Y, Ebenso EE, Zhou L, Huang B (2014) 8-Hydroxyquinoline as an effective corrosion inhibitor for 7075 aluminium alloy in 3.5% NaCl solution. Int J Electrochem Sci 9:5574–5584
Liu Y, Zou C, Yan X, Xiao R, Wang T, Li M (2015) β-Cyclodextrin modified natural chitosan as a green inhibitor for carbon steel in acid solutions. Ind Eng Chem Res 54(21):5664–5672
Mobin M, Khan MA (2013) Synergistic influence of polyvınyl alcohol and surfactants on the corrosion inhibition of mıld steel ın 0.1 M H H2SO4. Chem Eng Commun 200(9):1149–1169
Mohamed RR, Fekry AM (2011) Antimicrobial and anticorrosive activity of adsorbents based on chitosan Schiff’s base. Int J Electrochem Sci 6:2488–2508
Mourya P, Singh P, Rastogi RB, Singh MM (2016) Inhibition of mild steel corrosion by1,4,6-trimethyl-2-oxo-1,2-dihydropyridine-3-carbonitrile and synergistic effect of halide ion in 0.5 M H2SO4. Appl Surf Sci 380:141–150
Nagarajan S, Rajendran N (2009) Crevice corrosion behaviour of superaustenitic stainless steels: dynamic electrochemical impedance spectroscopy and atomic force microscopy studies. Corros Sci 51:217–224
Nasibi M, Mohammady M, Ghasemi E, Ashrafi A, Zaarei D, Rashed G (2013) Corrosion inhibition of mild steel by Nettle (Urticadioica L.) extract: polarization, EIS, AFM, SEM and EDS studies. J Adhes Sci Technol 27(17):1873–1885
Nasibi M, Mohammady M, Ashrafi A, Akbar A, Khalaji D, Moshrefifar M, Rafiee E (2014) Nanosized scale roughness and corrosion protection of mild steel in hydrochloric acid solution and in the presence of Turmeric (Curcuma Longa) Extract as a green corrosion inhibitor: FTIR, polarization, EIS, SEM, EDS, AFM studies, and neural network modeling. J Adhes Sci Technol 28(20):2001–2015
Nataraja SE, Venkatesha TV, Manjunat K, Poojary B, Pavithra MK, Tandon HC (2011) Inhibition of the corrosion of steel in hydrochloric acid solution by some organic molecules containing the methylthiophenyl moiety. Corros Sci 53:2651–2659
Norr EA (2005) The inhibition of mild steel corrosion in phosphoric acid by some N-heterocyclic compounds in the salt form. Corros Sci 47(1):33–55
Obot IB, Madhankumar A (2016) Synergistic effect of iodide ion addition on the inhibition of mild steel corrosion in 1 M HCl by 3-amino-2 methylbenzylalcohol. Mater Chem Phys 177:266–275
Oguzie EE (2004) Influence of halide ions on the inhibitive effect of congo red dye on the corrosion of mild steel in sulphuric acid solution. Mater Chem Phys 87(1):212–217
Palomar-Pardavé M, Romero-Rom M, Herrera-Hernández H, Abreu-Quijano MA, Likhanova MA, Uruchurtu J, Juárez-García JM (2012) Influence of the alkyl chain length of 2 amino 5 alkyl 1,3,4 thiadiazole compounds on the corrosion inhibition of steel immersed in sulfuric acid solutions. Corros Sci 54:231–243
Saji VS, Shibli SMA (2002) Synergistic inhibition of carbon steel corrosion by sodium tungstate and sodium silicate in neutral aqueous media. Anti-Corros Methods Mater 49:433–443
Saji VS, Shibli SMA (2005) Co-inhibition characteristics of sodium tungstate with potassium iodate on mild steel corrosion. Corros Sci 47:2213–2224
Sherif EM, Park SM (2006) Inhibition of copper corrosion in acidic pickling solutions by N-phenyl-1,4-phenylenediamine. Electrochim Acta 51:4665–4673
Solomon MM, Umoren SA (2015a) Performance evaluation of poly (methacrylic acid) as corrosion inhibitor in the presence of iodide ions for mild steel in H2SO4 solution. J Adhes Sci Technol 29(11):1060–1080
Solomon MM, Umoren SA (2015b) Enhanced corrosion inhibition of mild steel by polypropylene glycol in the presence of iodide ions in acid solutions. J Environ Chem Eng 3:1812–1826
Solomon MM, Umoren SA (2015c) Electrochemical and gravimetric measurements of inhibition of aluminium corrosion by poly (methacrylic acid) in H2SO4 solution and synergistic effect of iodide ions. Measurement 76:104–116
Solomon MM, Umoren SA (2016) In-situ preparation, characterization and anticorrosion property ofpolypropylene glycol/silver nanoparticles composite for mild steelcorrosion in acid solution. J Colloid Interface Sci 462:29–41
Solomon MM, Umoren SA, Udousoro II, Udoh AP (2010) Inhibitive and adsorption behavior of carboxymethyl cellulose on mild steel corrosion in sulphuric acid solution. Corros Sci 52:1317–1325
Solomon MM, Umoren SA, Israel AU, Etim IG (2016) Synergistic inhibition of aluminium corrosion in H2SO4 solution by polypropylene glycol in the presence of iodide ions. Pigm Resin Technol 45(4):280–293
Tao Z, He W, Wang S, Zhang S, Zhou G (2012) A study of differential polarization curves and thermodynamic propertiesfor mild steel in acidic solution with nitrophenyltriazole derivative. Corros Sci 60:205–213
Thirumoolan D, Katkar VA, Gunasekaran G, Kanai T, Basha KA (2014) Hyperbranched poly(cyanurateamine): a new corrosion inhibitor for mild steel in hydrochloric acid medium. Prog Org Coat 77:1253–1263
Umoren SA, Solomon MM (2014a) Recent developments on the use of polymers as corrosion inhibitors—a review. Open Mater Sci J 8:30–45
Umoren SA, Solomon MM (2014b) Effect of halide ions on the corrosion inhibition efficiency of different organic species—a review. J Ind Eng Chem 21:81–100
Umoren SA, Solomon MM, Udousoro II, Udoh AP (2010) Synergistic and antagonistic effects between halide ions and carboxymethyl cellulose for the corrosion inhibition of mild steel in sulphuric acid solution. Cellulose 17:635–648
Umoren SA, Banera MJ, Alonso-Garcia T, Gervasi CA, Mirı´fico MV (2013) Inhibition of mild steel corrosion in HCl solution using chitosan. Cellulose 20:2529–2545
Umoren SA, Solomon MM, Eduok UB, Obot IB, Israel AU (2014) Inhibition of mild steel corrosion in H2SO4 solution by coconut coir dust extract obtained from different solvent systems and synergistic effect of iodide ions: Ethanol and acetone extracts. J Environ Chem Eng 2:1040–1060
Umoren SA, Solomon MM, Israel AU, Eduok UM, Jonah AE (2015) Comparative study of the corrosion inhibition efficacy of polypropylene glycol and poly (methacrylic acid) for mild steel in acid solution. J Dispersion Sci Technol 36(12):1721–1735
Verma C, Quraishi MA, Ebenso EE, Obot IB, El Assyry A (2016) 3-Amino alkylated indoles as corrosion inhibitors for mild steel in 1 M HCl: experimental and theoretical studies. J Mol Liq 219:647–660
Wang ZB, Hu HX, Zheng YG, Ke W, Qiao YX (2016) Comparison of the corrosion behavior of pure titanium and its alloys in fluoride-containing sulfuric acid. Corros Sci 103:50–65
Xia G, Jiang X, Zhou L, Liao Y, Duan M M, Wang H H, Pu Q, Zhou J (2015) Synergic effect of methyl acrylate and N-cetylpyridinium bromide in N-cetyl-3-(2-methoxycarbonylvinyl) pyridinium bromide molecule for X70 steel protection. Corros Sci 94:224–236
Yang KK, Wang XL, Wang YZ (2007) Progress in nanocomposite of biodegrable polymer. J Ind Eng Chem 13:485–500
Yilmaz N, Fitoz A, Ergun Ü, Emregül KC (2016) A combined electrochemical and theoretical study into the effect of 2-((thiazole-2-ylimino) methyl) phenol as a corrosion inhibitor for mild steel in a highly acidic environment. Corros Sci 111:110–120
Zhang D, Gao L, Zhou G (2004) Inhibition of copper corrosion by bis-(1-benzotriazolymethylene)-(2,5-thiadiazoly)-disulfide in chloride media. Appl Surf Sci 225:287–293
Zheng X, Zhang S, Li W, Yin L, He J, Wu J (2014) Investigation of 1-butyl-3-methyl-1H-benzimidazolium iodideas inhibitor for mild steel in sulfuric acid solution. Corros Sci 80:383–392
Zheng X, Zhang S, Li W, Gong M, Yin L (2015) Experimental and theoretical studies of two imidazolium-based ionic liquids as inhibitors for mild steel in sulfuric acid solution. Corros Sci 95:168–179
Zou C, Yan X, Qin Y, Wang M, Liu Y (2014) Inhibiting evaluation of β-Cyclodextrin-modified acrylamide polymer on alloy steel in sulfuric solution. Corros Sci 85:445–454
Acknowledgements
Moses M. Solomon acknowledges the financial support by The Scientific and Technological Research Council of Turkey (TÜBITAK) under the TÜBITAK 2216-Postdoctoral Research Fellowship (TUBITAK 21514107-115.02-56312) and Duzce Unıversity, Turkey, for providing the facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Solomon, M.M., Gerengi, H., Kaya, T. et al. Synergistic inhibition of St37 steel corrosion in 15% H2SO4 solution by chitosan and iodide ion additives. Cellulose 24, 931–950 (2017). https://doi.org/10.1007/s10570-016-1128-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-1128-2