Abstract
A whole paper electro-biosensor detection platform for detecting glucose is reported. Glucose oxidase was immobilized on paper-based screen-printed carbon electrodes. The developed biosensor has an excellent electrochemical characteristic and high heterogeneous electron transfer rate constant (ks = 4.23 × 10−4). This new detection platform exhibited good amperometric responses toward glucose, with a wide linear range up to 10 mM with R2 is 0.9973 and the sensitivity is 2.07 μA mM−1. The parallel measurements of glucose by different biosensors had a low relative standard deviation of 4.26 % and demonstrated it had good reproducibility. In the interference studies, there was no significant difference with ascorbic acid or uric acid. For stability, it retained 93.9 % of its initial response after a storage period of 31 days in 4 °C. Besides, the Parkes error grid analysis was indicated that paper-based electrochemical biosensor has a good accuracy on the glucose detection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is one of the most common chronic diseases in nearly all countries, and has been a huge threat to human health. In 2013, the estimates of International Diabetes Federation (IDF) indicated that approximately 382 million people have diabetes, and the number of people with the disease is set to rise beyond 592 million in less than 25 years (IDF 2013). Therefore, fast and accurate detection of glucose has great significance in further reducing the incidence of diabetic complications (Clark 2008). Most diabetics blood-glucose monitoring were used a disposable test strip (Yoo and Lee 2010). According to the American Diabetes Association (ADA) guidelines for self-monitoring blood glucose, diabetic people might frequently perform 4 or more daily tests, and then produced the 558 billion medical test strips annual. Nevertheless, the disposable test strips usually print on ceramic substrates or non-degradable plastic materials, such as PC, PVC and PET which are facing the challenge in medical waste pollution or recycling problems. Therefore, a completely biodegradable test strips for detecting glucose has great significance in the future.
Cellulose, the chain of glucose residues which form the principal component of the plant cell wall, is the most abundant and widespread biopolymer. Every year, plants make more than 1011 tons of cellulose (Read and Bacic 2002). Besides of its abundance, biodegradability, biocompatibility, renewable behavior and green recycling, cellulose has unique material properties: high Young’s modulus, dimensional stability and low thermal expansion coefficient. For a fascinating application of cellulose, paper-based flexible and disposable analytical devices have been suggested (Kim et al. 2014).
In recently, paper-based analytical devices mainly were used in colorimetric assays (Martinez et al. 2010). Colorimetric changes in diagnostic assays can be visualized by the naked eye to yield a yes/no answer. And imaging analysis by a cell phone is used to report quantitative readouts (Martinez et al. 2008). However, both qualitative and quantitative strategies suffer from low sensitivity and poor accuracy. Additionally, the color of sample, inconsistencies in lighting, or the presence of particulate contaminants may confuse the colorimetric result. For example, the colorimetric assays of blood glucose are interference seriously by hemoglobin.
Electrochemical analysis is a useful method for replacing colorimetric analysis for diagnostic test, because it measured the change of current or voltage via an electrochemical device. Moreover, it can be qualitative and quantitative more easily and insensitive to color contamination. According to recently research, Chee Shiong Kuek Lawrence et al. used a cellulose paper as a matrix for immobilizing enzymes placed on top of a plastic-based screen printed carbon electrode. This simple physical adsorption immobilization technique has been extremely low cost, and the cellulose matrix provided an effective stable microenvironment for GOx. After a period of 4 months, it was found that there was no significant difference in the current (less than 2 % difference in the signal) (Lawrence et al. 2014). However, the biosensor’s substrates were still using plastic materials and not completely resolved after the extensive use of biosensors in medical waste pollution.
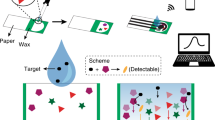
Herein, we report a whole paper electro-biosensor (PEB) platform for glucose detection which has completely biodegradable and it can be solve the medical waste pollution. The electrochemical characteristic of the PEB has been investigated by cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and chronoamperometry (CA). The glucose oxidase (GOx) immobilized on the surface of biosensor by physical adsorption has been further used for detecting glucose. The results showed that the developed PEB has high sensitivity and good accuracy for glucose detection. The selectivity, reproducibility and stability of the paper-based electrochemical biosensor have also been studied in detail.
Experimental methods
Chemicals and reagents
Conductive carbon ink (Electrodag 423SS) were purchased from Acheson Colloids Company (Ontrario, CA, USA). Cellulose paper, A4 size, gram weight of 160 g cm−2 and an average thickness of about 200 μm (Guangzhou, China). Glucose oxidase (GOx, from Aspergillus niger, Type X-S, lyophilized powder, 118 000 units/g solid), purchased from Sigma-Aldrich. Potassium ferri(III)cyanide (K3[Fe(CN)6]), D-(+)-glucose, carboxymethylcellulose sodium (CMC) was purchased from Tianjin Fuchen Chemical Reagents Factory. Hydrochloric acid, sodium hydroxide, acetone and ethanol were supplied from Beijing Chemical Reagent Company. All the chemicals used in this work were of analytical grade and were used as received without further purification. All solutions were prepared with deionized water of resistivity no less than 18.2 MΩ cm.
Fabrication of paper electro-biosensor
Firstly, carbon electrodes were printed using a semiautomatic screen-printing machine (MARABU, Dongguan, China), equipped with a 250 threads per inch polyester screen, a polyurethane squeegee, and stainless-steel flood blade. Paper-based carbon electrodes were printed onto an untreated white A4 paper of 200 μm thickness, and left to dry 30 min at 71 and 121 °C, respectively. Then, individual carbon electrodes were cut from the paper, and their working area (0.5 × 1 cm2) was defined with insulation tape.
After individual carbon electrodes fabrication, a disposable PEB was fabricated by physical adsorption GOx on the surface of the carbon electrodes. In detail, 30 μL of potassium ferri(III)cyanide solution was dropped upon working area of the electrodes then heated to cure at 30 °C for 30 min. Then, 20 μL of GOx solution which contain 0.05 g mL−1 carboxymethylcellulose sodium (CMC) was immobilized on the surface of the electrodes by drop coating and dry at 30 °C for 30 min, and the new PEB test strips were ready for use.
Surface characterization
Surface characteristics of the bare carbon electrodes and PEB were measured with a field emission scanning electron microscope (SEM, Hitachi S-4800, Japan) at 10.0 kV. ATR-FTIR spectra of modified electrode were recorded on a Bruker Vertex 70/80 FTIR spectrometer operated at a resolution of 4 cm−1.
Electrochemical characterization
The electrochemical properties of the PEB were characterized by cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and chronoamperometry (CA). CV and CA experiments were performed with CHI 400 electrochemical analyzer (CH Instruments Inc., USA). The experiments were conducted to investigate the effects of scan rate for CV technique on a characteristic of induced current. The experiments at different scan rates between 40 and 200 mV s−1 were performed with bare carbon electrodes at 50 mM potassium ferri(III)cyanide (K3[Fe(CN)6]) in 0.05 M PBS. EIS analyses were performed at the open-circuit potential with Autolab PGSTAT302N electrochemical workstation (Metrohm, Switzerland) controlled by Nova 1.10 software, and the impedance spectra were recorded in the frequency range 0.1 Hz–100 kHz by using a sinusoidal excitation signal (single sine) with an excitation amplitude of 10 mV. The impedance spectra were expressed with the Nyquist plot. CA experiments of PEB were tested in 0.05 M PBS at the fixed applied voltage of 0.45 V for 60 s. All experiments were performed at room temperature on biosensor and each PEB test strip was used in a single assay. Data points were plotted using ORIGIN (Northampton, MA, USA).
Effect of applied potential and pH
To determine the optimal applied potential and pH for glucose detection using chronoamperograms. Chronoamperograms were recorded using the PEB on 5 mM glucose at room temperature. A potential step was then applied, and the resulting current was monitored. The applied potential was varied from 0.1 to 0.9 V (in increments of 0.1 V) and current from the resulting chronoamperograms was sampled at 60 s. To optimize the working pH of phosphate buffer, the experiments employing CA technique are performed with PEB at 5 mM glucose solution at the optimal applied potential by varying pH of the phosphate buffer solution from 4.0 to 10.0.
Paper electro-biosensor response characteristics
The stock solution of D-(+)-glucose was prepared in a potassium phosphate buffer (pH 7.0) and allowed to equilibrate overnight before use. The PEB was tested using chronoamperometric detection technique to obtain the relationship between induced current and glucose concentration and real-time detection performance. The experiment was tested in PBS at pH of 7.0 at the fixed applied voltage of 0.45 V. In this measurement, the 20 μL droplet of different concentrations of glucose solution is added on the working area of PEB for 60 s. With this simple procedure, the relationship between the induced current and glucose concentration can be constructed.
Interference and thermal stability studies
The effect of potential interference on the response of the paper-based electrochemical biosensor was evaluated in 0.05 M PBS (pH 7.0) containing 5 mM glucose. After addition of 0.1 and 0.3 mM ascorbic acid or 0.05 and 0.15 mM uric acid, the effects of interferences were performed. The stability of the PEB was investigated at 4 °C, room temperature and 45 °C for a month.
Parkes error grid analysis based on ISO 15197:2013
The Parkes error grid analysis (PEG) was conducted to evaluate the accuracy of the paper electro-biosensor (Andreas et al. 2013). The PEG requires over 95 % of the glucose measurements to fall within zone A and 100 % to fall within zone A + B. PEG contains 5 zones (A, B, C, D, and E). Zone A represents bias within 20 % of reference values or the hypoglycemic region. Zone B denotes bias outside of 20 % of the reference values but no/benign treating effect. Values in zones C, D, and E may cause unnecessary corrective treatments, failure to administer treatment, and incorrect treatment, respectively. Notably, results within zones A or B are clinically acceptable, whereas those within zones C, D, or E are completely intolerable. In this investigation, the agreement between glucose values obtained from paper-based electrochemical biosensor was further assessed via regression analysis.
Results and discussion
Surface characterization
The electrode surfaces shows a configuration with randomly oriented flake-like graphitic layer, homogeneous ensemble into an insulator matrix as shown in Fig. 1a. The SEM image reveal a relatively rough surface structures and reduced polymeric binders indicating that the electrodes with the large electro active sites exhibit faster electron transfer rates (Fanjul-Bolado et al. 2008; Kadara et al. 2009). After modified ferricyanide and GOx (Fig. 1b, c), there are larger K3[Fe(CN)6] crystal particles attached on the surface of the carbon electrodes, particles size between 20 and 200 μm, and uneven distribution. Figure 1d shows the ATR-FTIR spectra of modified electrodes, there was no main characteristic peak on the bare carbon electrodes and the electrodes modified Ferri. After modified the GOx, the apparent peaks lying at 1640 and 1568 cm−1 were associated with the C=O stretching vibration of peptide, and the N–H bending and C–N stretching of protein backbone, respectivelyc (Altun et al. 2015). Furthermore, the appearance of new peaks in the 1200–800 cm−1 region was attributed to the characteristic peaks of GOx, which indicated that the enzyme was adsorbed on the surface of the electrodes (Chen et al. 2011).
Electrochemical characterization
Cyclic voltammograms (CV) is a valuable and convenient tool to monitor the behavior changes during the electrode modified process. In this work, CV was used to determine the glucose detection platform characteristics with potassium ferri(III)cyanide (Fig. 2a). Observation of voltammetric peak height, plotted as peak current (Ip) against square root of scan rate (v1/2) was found to be linear-ship, suggesting a diffusional process occurring at the electrode surface. The heterogeneous electron transfer rate constant (ks) was calculated to be 1.72 × 10−3, by Nicholson method (Nicholso 1965). This ks was higher than those previous reported on polyester SPE (7.26 × 10−4) (Metters et al. 2013), ultra-inH (3.9 × 10−4) (Morrin et al. 2003) and commercial zensor TE100 (2.5 × 10−4) (Kadara et al. 2009). The large ks value showed paper electro-biosensor platform had excellent electrochemical properties.
CVs of PEB modified Ferri and GOx exhibited clearly anodic and cathodic redox peaks with an average peak-to-peak separation (ΔEp) was about 1230 mV, much larger fourfolds than bare carbon electrodes (ΔEp = 278 mV). The results indicated the GOx affect the electron transfer between the surface of the electrode and the solution, caused a higher peak-to-peak separation. This result could be confirmed by electrochemical impedance spectroscopy (EIS). Figure 2c showed that the Nyquist plot of carbon electrodes modified Ferri includes a semicircle portion and a linear portion. The electron transfer resistance (can be quantified using the diameter of the semicircle portion, Rct = 12.8 kΩ) of the electrodes modified Ferri and GOx was much larger than that of the electrodes modified Ferri (Rct = 5.07 kΩ), suggesting that GOx resists the electron exchange between the carbon electrode surface and the redox probes in the solution (Kang et al. 2009). Figure 2b showed the influence of the scan rate of PEB modified Ferri and GOx, the results indicated that it is also a diffusional process. The ks was calculated to be about 4.23 × 10−4, which was also larger than ultra-inH (3.9 × 10−4) (Morrin et al. 2003) and commercial zensor TE100 (2.5 × 10−4) (Kang et al. 2009). To prove the precision and practicability of the proposed platform, the reproducibility was examined by CA (Fig. 2d). The relative standard deviation (RSD) of the platform was 4.26 % (n = 5) in 0.05 M PBS. Those results demonstrated the PEB platform could replace the plastic-based biosensor.
Effect of applied potential
Figure 3a shows the effect of applied potential on the response of the PEB platform. The results showed that the current increased with rising potential from 0.00 to 0.45 V and decreased during the potential range from 0.45 to 0.8 V. Thus, the potential of 0.45 V was selected for the subsequent experiments so as to achieve the greatest current response.
Effect of pH
The impact of the pH buffer is vital to the sensitivity of the PEB as the pH influences the bioactivity of the GOx (Tian and Zhu 2002). The effect of phosphate buffer solution (containing 5 mM glucose) pH on the PEB was investigated in the range of 4.0–10.0. As can be seen in Fig. 3b, the PEB exhibited an optimum response at pH 7.0. This is because strong acidic or alkaline environments causing enzymes denatured so that the electrochemical response of the PEB was weak. The results obtained in this experiment were consistent with previous studies (Qiu et al. 2009).
Paper electro-biosensor response characteristics
By employing the optimum conditions investigated, the chronoamperometric measurements of the proposed PEB for different glucose solution was obtained as shown in Fig. 4a. The current response time was less than 40 s, which indicated a fast response for detecting glucose. Figure 4b showed the calibration curve of PEB for glucose detection. The results showed good linearity in the concentration range of 0–10 mM, and gradually leveled off beyond 10 mM glucose due to the responses become saturated towards high concentrations. The linear regression equation was y = 2.07x + 7.91, with R2 value was found to be 0.9973 and the higher sensitivity is 2.07 μA mM−1. PEB has high sensitivity due to the highly porous microstructure of cellulose paper, which could absorb more GOx molecules and stored in the fiber matrix (Koga et al. 2012; Li et al. 2012).
Interference and thermal stability studies
The effect of potential interference on the response of the PEB was evaluated in 0.05 M PBS containing 5 mM glucose. The current ratio of interference (Ii) and without interference (Ig) in 5 mM glucose was used to evaluate the interference effect. Table 1 shows that the possible electro active species such as ascorbic acid and uric acid could cause no significant effect on the glucose detection, and PEB has a good selectivity due to the specificity of GOx.
For the thermal stability studies, the effect of storage time and temperature on the relative response of the new PEB was shown in Fig. 5. After a month, it retained 93.9, 90.9 and 85.4 % of its initial response when stored at 4 °C, room temperature and 45 °C, respectively. Thermal stability of the proposed new PEB was better than the ceramic-based biosensor (68 % for 3 weeks) (Tian and Zhu 2002) and plastic-based biosensor (~70 % after 31 days) (Weng et al. 2014). It indicated that the whole PEB detection platform has an excellent stability, because the cellulose substrates provide a biocompatible microenvironment for enzyme molecules, and largely protect the biological activity of the GOx enzyme (Sekar et al. 2014).
Parkes error grid analysis based on ISO 15197:2013
The Parkes error grid analysis (PEG) was conducted to evaluate the accuracy of the proposed PEB. The accurate measurements obtained by PEB, assessed using the glucose concentration as a yardstick. As shown in Fig. 6, all the measurements performed by proposed biosensor fell within zone A (no effect on clinical action) of the PEG, indicating that PEB has a good accuracy on the glucose detection.
The equation of regression analysis was y = 0.98x − 0.02 with R2 value of 0.94, indicated a good agreement. The total coefficient of variation (CV) calculated using all the measured test values was about 11.4 %, lower than ISO 15197:2013 standard (less than 15 %), indicated the PEB fit the requirements of practical application in point-of-care diagnostic devices.
Hence, the analytical performance of the whole PEB has been compared with previously plastic-based biosensor for the glucose detection (Table 2). It is evident the PEB exhibits wider linear range than other polymer-based biosensors and relatively high sensitivity and good stability.
Finally, in terms of cost, commercially available electro-chemical glucose test strips are typically made on a plastic substrate and their price in the US, $0.5 to 1.0 per strip (Heller and Feldman 2008), is impractically high for applications in the developing world. In contrast, paper electro-glucose test strips that would be produced at lower cost (each test strip costs about $0.01–0.02). Furthermore, paper is high abundance, biodegradability and green recycling compared to plastic substrates. Moreover the fabricated PEB test strip is disposable one and can be degraded by microorganisms within several months. Most importantly, the proposed paper electro-biosensor has high sensitivity and good accuracy, and that would assay glucose and replace the commercially available electrochemical glucose test strips. These advantages of our proposed PEB detection platform will gain much further attention and can be used for real time sensing of blood-glucose (normal blood glucose concentration is 4.4–6.1 mM).
Conclusion
In summary, we have successfully fabricated a whole paper electro-biosensor detection platform by screen printing technique for rapid detecting glucose. This platform has an excellent electrochemical properties (ks = 1.72 × 10−3), good sensitivity (2.07 μA mM−1), well stability and good accuracy. We believe that PEB platform could apply in point-of-care diagnostic devices and easy transfer or replace the current commercial plastic-based electrodes.
References
Altun S, Çakıroğlu B, Özacar M, Özacar M (2015) A facile and effective immobilization of glucose oxidase on tannic acid modified CoFe2O4 magnetic nanoparticles. Colloids Surf B 136:963–970
Andreas P, David CK, Scott P, Joan LP (2013) Technical aspects of the Parkes error grid. J Diabetes Sci Technol 7:1275–1281
Chen M, Zhang W, Jiang R, Diao G (2011) Development of glucose amperometric biosensor based on a novel attractive enzyme immobilization matrix: amino derivative of thiacalix[4]arene. Anal Chim Acta 687:177–183
Clark M (2008) Diabetes self-management education: a review of published studies. Primary Care Diabetes 2:113–120
Crouch E, Cowell DC, Hoskins S, Pittson RW, Hart JP (2005) Amperometric, screen-printed, glucose biosensor for analysis of human plasma samples using a biocomposite water-based carbon ink incorporating glucose oxidase. Anal Biochem 347:17–23
Fanjul-Bolado P, Hernández-Santos D, Lamas-Ardisana PJ, Martín-Pernía A, Costa-García A (2008) Electrochemical characterization of screen-printed and conventional carbon paste electrodes. Electrochim Acta 53:3635–3642
Heller A, Feldman B (2008) Electrochemical glucose sensors and their applications in diabetes management. Chem Rev 108:2482–2505
International Diabetes Federation (2013) Diabetes atlas, 6th edn
Kadara RO, Jenkinson N, Banks CE (2009) Characterisation of commercially available electrochemical sensing platforms. Sens Actuators B Chem 138:556–562
Kang X, Wang J, Wu H, Aksay IA, Liu J, Lin Y (2009) Glucose oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens Bioelectron 25:901–905
Kim J-H, Mun S, Ko H-U, Yun G-Y, Kim J (2014) Disposable chemical sensors and biosensors made on cellulose paper. Nanotechnology 25:092001
Koga H, Kitaoka T, Isogai A (2012) Paper-immobilized enzyme as a green microstructured catalyst. J Mater Chem 22:11591–11597
Laschi S, Bulukin E, Palchetti I, Cristea C, Mascini M (2008) Disposable electrodes modified with multi-wall carbon nanotubes for biosensor applications. IRBM 29:202–207
Lawrence CSK, Tan SN, Floresca CZ (2014) A “green” cellulose paper based glucose amperometric biosensor. Sens Actuators B Chem 193:536–541
Li X, Ballerini DR, Shen W (2012) A perspective on paper-based microfluidics: current status and future trends. Biomicrofluidics 6:011301
Martinez AW, Phillips ST, Carrilho E, Thomas SW, Sindi H, Whitesides GM (2008) Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal Chem 80:3699–3707
Martinez AW, Phillips ST, Whitesides GM, Carrilho E (2010) Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal Chem 82:3–10
Metters JP, Houssein SM, Kampouris DK, Banks CE (2013) Paper-based electroanalytical sensing platforms. Anal Methods 5:103–110
Morrin A, Killard AJ, Smyth MR (2003) Electrochemical characterization of commercial and home-made screen-printed carbon electrodes. Anal Lett 36:2021–2039
Nicholso RS (1965) Theory and Application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal Chem 37:1351–1355
Pchelintsev NA, Vakurov A, Millner PA (2009) Simultaneous deposition of Prussian Blue and creation of an electrostatic surface for rapid biosensor construction. Sens Actuators B Chem 138:461–466
Piano M, Serban S, Biddle N, Pittson R, Drago GA, Hart JP (2010) A flow injection system, comprising a biosensor based on a screen-printed carbon electrode containing Meldola’s Blue-Reinecke salt coated with glucose dehydrogenase, for the measurement of glucose. Anal Biochem 396:269–274
Qiu J-D, Zhou W-M, Guo J, Wang R, Liang R-P (2009) Amperometric sensor based on ferrocene-modified multiwalled carbon nanotube nanocomposites as electron mediator for the determination of glucose. Anal Biochem 385:264–269
Read SM, Bacic T (2002) Prime time for cellulose. Science 295:59–60
Sato N, Okuma H (2008) Development of single-wall carbon nanotubes modified screen-printed electrode using a ferrocene-modified cationic surfactant for amperometric glucose biosensor applications. Sens Actuators B Chem 129:188–194
Sekar NC, Shaegh SAM, Ng SH, Ge L, Tan SN (2014) A paper-based amperometric glucose biosensor developed with Prussian Blue-modified screen-printed electrodes. Sens Actuators B Chem 204:414–420
Tian F, Zhu G (2002) Bienzymatic amperometric biosensor for glucose based on polypyrrole/ceramic carbon as electrode material. Anal Chim Acta 451:251–258
Weng B, Morrin A, Shepherd R, Crowley K, Killard AJ, Innis PC, Wallace GG (2014) Wholly printed polypyrrole nanoparticle-based biosensors on flexible substrate. J Mater Chem B 2:793–799
Yoo EH, Lee SY (2010) Glucose biosensors: an overview of use in clinical practice. Sensors 10:4558–4576
Zuo S, Teng Y, Yuan H, Lan M (2008) Direct electrochemistry of glucose oxidase on screen-printed electrodes through one-step enzyme immobilization process with silica sol–gel/polyvinyl alcohol hybrid film. Sens Actuators B Chem 133:555–560
Acknowledgments
This work was supported by the State Key Project of Fundamental Research (Grant 2014CB931900), UCAS Young teacher research fund (Grant Y55103NY00, Y55103EY00 and Y25102TN00).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tao, C., Yen, CS., Liu, JT. et al. Analytical performance of paper electro-biosensor detection platform for point-of-care diagnosis. Cellulose 23, 3799–3808 (2016). https://doi.org/10.1007/s10570-016-1046-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-1046-3