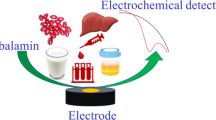
Abstract
The fast-growing healthcare demand for user-friendly and affordable analytical tools is driving the efforts to develop reliable platforms for the customization of therapy based on individual health conditions. In this overall scenario, we developed a paper-based electrochemical sensor for the quantification of iron ions in serum as a cost-effective sensing tool for the correct supplement administration. In detail, the working electrode of the screen-printed device has been modified with a nanocomposite constituted of carbon black and gold nanoparticles with a drop-casting procedure. Square wave voltammetry has been adopted as an electrochemical technique. This sensor was further modified with Nafion for iron quantification in serum after sample treatment with trifluoroacetic acid. Under optimized conditions, iron ions have been detected with a LOD down to 0.05 mg/L and a linearity up to 10 mg/L in standard solution. The obtained results have been compared with reference methods namely commercial colorimetric assay and atomic absorption spectroscopy, obtaining a good correlation within the experimental errors. These results demonstrated the suitability of the developed paper-based sensor for future applications in precision medicine of iron-deficiency diseases.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The healthcare system is moving toward a new era where decentralized and fast response analysis is a fundamental request. As reported by World Economic Forum, by 2030, the very nature of the disease will be further disrupted by technology. The fourth industrial revolution will ensure that humans live longer and healthier lives so that the hospitals of the future will become more like NASCAR pit stops than inescapable black holes. We will go to the hospital to be patched up and put back on track, revolutionizing healthcare from hospital to home-spital [1]. As a result, conventional clinical laboratory analyses are decreasing their dominant and global use on behalf of cost-effective laboratory analyses and decentralized point-of-care testing (POC). Switching from traditional testing to new-born sensing procedures is gaining high attraction. Indeed, the use of miniaturized, accessible, user-friendly, and equipment-free detection tools reduces drastically decision-making time for further testing or treatment. In addition, delays in the test results are no longer caused by specimen transport and preparation, being rapidly available at the point of care; thus, diagnosis can be performed directly in a medical facility or a primary care level, allowing for a prompt response and rapid treatment decision. Nevertheless, these tests should always offer high accuracy and sensitivity, maintaining a reliable and high-quality diagnosis process.
The World Health Organization (WHO) describes useful diagnostic tests with the acronym REASSURED [2] which means Real-time connectivity, Ease of specimen collection, Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment free or simple Environmentally friendly, Deliverable to end-users, being the required features for diagnostic devices.
Reliable and cost-effective sensors are promising candidates to fulfill these requirements as they are based on (i) minimal sample volume, (ii) miniaturized and portable devices, and (iii) user-friendly procedures. In addition, various detection techniques are used to enable the fabrication of highly sensitive and accurate sensing platforms, i.e., colorimetry, fluorimetry, and electrochemistry [3,4,5]. Among the others, the latter displays important features such as miniaturization, portability, environmental friendliness, and cost reduction, with the possibility to work in turbid and colorful matrices. In this regard, Green Analytical Chemistry aimed at eliminating plastic and testing cartridges currently used in analytical methods, as they cannot be recycled or disposed of properly [6, 7]. Paper is an obvious choice of substrate material, with many advantages over standard plastic materials. Indeed, paper-based electrochemical detection tools have been gaining high relevance in the past 10 years, thanks to numerous advantages, including cost-effectiveness, environmental friendliness, and simplicity of flow analyses in comparison with polyester or ceramic-based supports [8, 9].
Furthermore, the porosity of the cellulose allows for the fabrication of capillary-driven microfluidics and reagent-free devices [10, 11]. Paper has been used for the development of innovative electrochemical sensors with application in several fields, including environmental [12], biomedical [13], agrifood [14], and defense [15]. Noteworthy, our research group recently highlighted that the use of paper goes beyond the simple replacement of plastic or ceramic supports, but radically improves the features of electrochemical devices [16]. Indeed, paper-based electrochemical devices are capable to detect the target analytes in untreated samples [17], in the gas phase without any external sampling system [18], in solid surface [19], and in biological samples using paper as a mini-reactor for in situ nanomaterial-modified sensor copper [20]. Furthermore, the use of nanomaterials as sensor modifiers has been demonstrated to improve the electroanalytical properties of paper-based devices [21].
Iron is an essential component of the human body, as a relevant metal of several biological pathways and diverse cellular functions. Indeed, the body requires iron to form oxygen transport proteins, namely hemoglobin and myoglobin, and to synthesize other heme-based enzymes or non-heme compounds involved in electron transfer and oxidation–reduction processes [22, 23]. Iron is stored in the liver, spleen, and bone marrow complexed into ferritin and hemosiderin, ready to be delivered to tissues by circulating transferrin [24, 25]. Iron requirements depend on age and sex. Less than 1 mg/day is needed by newborns in their first years, while up to 2 mg/day during adolescence and 0.9–0.8 in adulthood. Noteworthy, pregnant women’s requirement is up to 6.3 mg/day during the second/third trimester [26].
Physiological serum iron levels are 65–175 µg/dL (0.65–1.75 mg/L) and 50–170 µg/dL (0.5–0.17 mg/L) for adult males and females, respectively [27].
Elevated iron serum levels are found in patients with aplastic anemia, acute liver injury, loading disorders such as hemochromatosis, or connected to metal poisoning or the use of hormonal contraceptives [27].
An iron deficiency occurs, instead, when iron stores are depleted, and iron metabolic demand does not meet the intake from the diet, because of a wrong diet or excessive blood loss [28, 29]. The most relevant consequence of iron deficiency is anemia, which is characterized by a low number of red blood cells or a low amount of hemoglobin [24], with severe functional damages affecting cognitive and learning ability [30], immunity mechanism [31], and neural capacity [32].
Iron deficiency is usually managed with tablets or supplements, but this treatment, if not strictly controlled, can produce more risks than relative benefits [33]. In this context, the development of a precision medicine approach represents an effective strategy to perform an accurate diagnosis for tailoring an individual therapy based on the patient’s health condition [34]. Implementing diagnosis with techniques that help to customize the treatment is highly requested to boost this approach.
Current methods for the detection of iron ions in blood samples are based on UV spectrophotometry and atomic absorption spectrometry analysis [35], but these analyses require a laboratory setup and a long analysis time.
In the last years, several alternative methods have been used for iron detection combined with customized sample treatment (Table S1). Although the potential use of sensing tools for routine analysis, they suffer from some drawbacks. Kawasaki et al. coupled electrochemical detection with a laboratory set-up-based HPLC for separation purposes [36]; Dorner et al. applied potentiostatic coulometry using a complex analyzer Ferrochem 3050 [37]; Kremplova et al. used a voltammetric automated bench analyzer with a mercury drop working electrode [38]; Hourani et al. employed an iodine-modified platinum working electrode in a bulk electrochemical cell comprising inlets/outlets for nitrogen purging [39].
Herein, we developed an electrochemical paper-based sensor for the detection of iron ions by exploiting nanomaterials, i.e., carbon black (CB) and commercially available gold nanoparticles (AuNPs) to deliver a highly sensitive analytical tool. CB is a well-known carbon-based nanomaterial widely used to enhance the electrochemical performances of the analytical platform thanks to its peculiar features including a high number of defect sites and nanodimension onion-like structure [40, 41]. Interestingly, CB has been applied in combination with metal nanoparticles (NPs) to ensure high electrochemical performances and homogeneous deposition of the NPs, as well. Indeed, the CB has been exploited in combination with copper nanoparticles for aminoacid detection [42] and with AuNPs for metal quantification [43, 44], demonstrating the effectiveness of the CB-NPs as nanocomposite for sensitive and accurate detection of the target analyte [45].
Applying this synergic combination for iron quantification, Compton’s group developed a gold ensemble-modified carbon nanocomposite electrodes (carbon black-polythene = CB/PE) (Au-MEE) for iron ion determination in water samples [46].
In this work, the AuNPs have been homogeneously deposited onto a CB-modified screen-printed electrode (SPE) with the final aim of fabricating a highly sensitive miniaturized electrochemical sensor for the detection of iron ions in serum samples.
Furthermore, the developed sensor is printed on an office paper substrate, delivering a cost-effective and highly sustainable detection tool. Indeed, the use of office paper allows for cost-reducing and the incineration of the sensor after the use.
Finally, the sensor was applied for iron determination in serum. After further modification with Nafion to remove fouling issues at the working electrode surface, the modified SPEs have been used for iron ions detection in serum samples, achieving comparable results with the standard methods, namely commercial colorimetric assay and AAS (Fig. 1).
Materials and methods
Reagents
Carbon black (CB) N220 was supplied by Cabot Corporation (Ravenna, Italy), hydrochloric acid, Iron standard, gold nanoparticles (5 nm diameter, OD 1, stabilized suspension in citrate buffer), Nafion 117 (5% in a mixture of lower aliphatic alcohols and water), trifluoroacetic acid (TFA), and trichloroacetic acid (TCA) were purchased from Sigma-Aldrich. IRON2 by Roche was used as a colorimetric assay.
Fabrication of the paper-based sensor
Paper-based SPEs were home-produced following a procedure previously optimized [47]. A wax pattern was drawn by Adobe illustrator and printed through a solid ink printer (Xerox ColorQube 8580) onto the office paper substrate (Copy 2, 80 g/m2, Fabriano, Italy). A first hydrophobic layer was wax printed to easily modify the working electrode surface, and then a second pattern was printed around the working electrode area to create a hydrophobic zone around. Subsequently, office paper was thermally treated at 100 °C for 2 min to allow the wax to permeate the paper network.
After, the three working electrode system was manually screen-printed using a squeegee and conductive inks. Firstly, silver/silver chloride (Electrodag 6038 SS, Loctite, Henkel) was used to print the pseudo-reference electrode, while graphite ink (Electrodag 423 SS, Loctite, Henkel) was used to print both the working and the counter electrodes. The screen-printed electrodes are characterized by a dimension of 25.5 mm × 6 mm. The working electrode has a diameter of 4 mm, resulting in a geometrical area of 12.56 mm2 (Fig. S1).
In order to investigate the repeatability of independently manufactured sensors (inter-assay repeatability, n = 3), we used a set of modified electrodes prepared on three different days for the detection of iron ions at a concentration of 4 mg/L in HCl 0.1 M. An RSD % equal to 7% was obtained, highlighting the satisfactory response from independently manufactured sensors.
Preparation of carbon black dispersion
CB powder was dispersed in a mixture of dimethylformamide/water in a ratio 1:1 (v/v) at a final concentration of 1 mg/mL. In detail, 10 mg of CB were first dipped in 5 mL of dimethylformamide, and then 5 mL of water was added. The dispersion was sonicated for 60 min at 59 kHz.
Preparation of the modified paper-based sensor
The surface of the working electrode was modified through three different modifiers. First, 2 µL of CB dispersion were drop-cast, followed by 20 µL of AuNPs dispersion. After each modification, the electrodes were dried for 30 min at RT. Finally, the sensors were modified with 2 µL of Nafion 1% v/v and dried at 25 °C for 30 min. Storage stability study was carried out using Nafion-AuNPs-CB after storing the sensors at 4 °C under vacuum for 30 days. We observed a decrease in the current intensity within 1 week and after the signal was stable within the experimental errors for at least 3 weeks.
In order to evaluate the repeatability of the developed electrodes, cyclic voltammetry measurements were performed in potassium ferro/ferricyanide 5 mM. In detail, by using the same paper-based SPEs (intra-assay repeatability, n = 3), we obtained an RSD% equal to 5.5%, while using different paper-based SPEs (inter-assay repeatability, n = 3), we obtained an RSD% equal to 7%, indicating good repeatability for the fabricated electrodes.
Electrochemical measurement of iron in standard solution
Square wave voltammetry measurements have been performed by a portable PalmSens3 potentiostat (PalmSens, Netherlands) connected to a laptop and controlled by PSTrace 5 software. Iron ions were detected by dropping 60 μL of the solution onto the three working electrode system obtaining an output stable signal in less than 30 s.
Electrochemical measurement of iron in serum
Serum samples were obtained from people enrolled for routine analysis at the San Giovanni Battista Hospital “Molinette” (Turin, Italy) and analyzed, at least, 3 weeks after the sampling.
Ten microliters of TFA were added to 500 µL of serum samples for a final concentration of 0.2 M. The resulting solution was centrifuged at 12,000 rpm for 10 min. Afterward, 60 µL of the supernatant was collected and cast on the SPE for the electrochemical measurement.
Iron measurement in serum using reference method
Colorimetric test was performed by IRON2 system (Roche) with an automatic Cobas system. The flame atomic absorption spectroscopy was carried out through contrAA 700 spectrometer from Analytik Jena corp following UNI EN ISO/IEC 17,025:2017 and the method of Rocks et al. [48].
Results and discussion
Nanomaterial-based electrochemical sensor
In order to obtain a highly sensitive detection platform, the surface of the working electrode has been modified with CB and AuNPs. Indeed, the combination of these two nanomaterials is able to detect iron ions with high sensitivity. The high surface-to-volume ratio of CB has been exploited to improve the area for the subsequent drop-cast of AuNPs, which enables high electrochemical performance, thanks to their electrocatalytic activity.
The modification procedure consists of two drop-cast modification steps, the first one applying CB dispersion and the second one by drop-casting AuNPs dispersion. With the aim of optimizing these two steps, evaluation studies have been carried out by detecting Fe (III) 5 mg/L in HCl 0.1 M, through square wave voltammetry measurements.
The first parameter under examination was the amount of CB dispersion to be drop-cast onto the surface of the working electrode. SWV measurements have been carried out by varying CB amounts, namely 2 µg, 4 µg, and 6 µg, maintaining a constant amount of AuNPs, i.e., 2 μL. As depicted in Fig. 2a when applying CB on the sensors almost a twofold signal enhancement was recorded, while the increase in CB amount did not significantly improve the electrochemical performances of the sensor. In order to employ fewer amounts of reagents and because of a similar signal and reproducibility of the measurements (RSD % equal to 4.5% and 2.7% for 2 µg and 4 µg, respectively), 2 µg was selected.
a Histogram bars obtained for optimization of working electrode surface modification with different amounts of CB and a fixed amount of 2 µL of AuNPs. b Histogram bars obtained for optimization of working electrode surface modification in the absence and in the presence of different volumes of AuNPs, with a fixed amount of 2 µg of CB. SWV detection of 3 mg/L of iron ions in HCl 0.1 M. SWV: Estep = 0.5 mV, frequency = 1 Hz, E amplitude = 0.25 mV, 60 µL of sample, n = 3
Afterward, the effect of AuNPs volume to be drop-cast on the CB-modified working electrode was evaluated. Figure 2b highlights the current signal improvement when combining CB with AuNPs; indeed, a low signal has been observed in the absence of AuNPs, while an improvement in the sensitivity of the sensor has been recorded with the increase of the AuNPs amount until to 20 µL and after a decrease in peak intensity was observed when modified with 30 µL. The decrease in the case of modification of the working electrode surface with 30 µL is probably ascribed to the increase in the thickness of the deposited AuNPs onto the surface of the working electrode, because the enhancement of the AuNPs layer thickness can decrease the conductivity. As an example, Yu et al. [49] investigated the resistance of an Au film, observing that the prepared Au nanoparticles film at 20 °C, at a thickness of 165 nm revealed a lower resistance than the one prepared at 175 nm.
In this work, an amount of 20 µL has been selected, giving the best performance in terms of current intensity and reproducibility equal to 0.9 µA with an RSD % of 3.3%.
Optimization of electrochemical parameters
The electrochemical conditions relative to the square wave voltammetry were successively examined. As shown in Fig. 3, amplitude, frequency, and step potential have been evaluated. All the optimizations have been carried out in the presence of iron ions 5 mg/L dissolved in HCl 0.1 M.
Histogram bars obtained for optimization of electrochemical technique parameters: a amplitude; b frequency; c step potential; d calibration curve obtained for the detection of iron ions in HCl 0.1 M by using the CB-AuNPs SPE and relative voltammograms in the inset (SWV parameters: Estep = 3 mV, frequency = 7 Hz, E amplitude = 50 mV, 60 µL of sample, n = 3); e interference study in the presence of zinc, lead, nickel, and copper ions; f calibration curve obtained for the detection of iron ions in HCl 0.1 M by using the Nafion-CB-AuNPs SPE and relative voltammograms in the inset
The effect of the amplitude of the voltammetric wave has been studied in the range between 10 and 100 mV. Figure 3a depicts the typical bell-shaped measurement trend, centered at 50 mV. Thus, this value has been selected for the higher current intensity (namely ca. 1.5 µA), having similar reproducibility (RSD % = 4.2%) with measurements performed at an amplitude of 25 mV (RSD % = 3.3%). After, the frequency was evaluated from 1 to 10 Hz. As reported in Fig. 3b, an increase in the current intensity has been recorded applying a frequency up to 10 Hz, joined by the decay of reproducibility. For this reason, a frequency equal to 7 Hz has been selected for the following measurements (current intensity = 5.5 µA, RSD % = 6.5%).
Finally, the step potential has been investigated (Fig. 3c), varying from 1 to 10 mV. A value of 3 mV has been selected as a compromise between peak intensity and reproducibility of the measurements (current intensity = 5.6 µA, RSD % = 2.5%).
Analytical performances in standard solution
Under the optimized conditions, AuNP-CB-SPEs have been tested using standard solutions of iron ions, as depicted in Fig. 3d. A linear range was obtained up to 10 mg/L (1000 µg/dL) described by the following equation y = − (2.7 ± 0.4) × − (0.4 ± 0.3), R2 = 0.997. The limit of detection, calculated as 3 σb/S (where σb represents the standard deviation of 10 blank measurements, and S the slope of the calibration curve) was equal to 0.035 mg/L.
Once the effectiveness of the AuNP-CB-SPE toward iron ions has been demonstrated, the selectivity of the sensor has been assessed considering the application in serum. Taking into account that the presence of other metals can affect the quantification, the selectivity of the platform by testing copper, lead, zinc, and nickel was evaluated. As depicted in Fig. 3e, the presence of these species has not shown any significant effect on the SPE response, demonstrating the good selectivity of the paper-based sensor developed. This is ascribed to the optimized fabrication of AuNP-CB-SPE as well as to the optimized working conditions, as previously reported by Compton’s group [46].
Analytical features of the Nafion-based modified sensor
With the final aim to use the sensor for analysis of serum samples, a further modification of the electrode surface with Nafion was necessary. Indeed, the presence of lipids and proteins in the serum matrix can affect the response of the working electrode surface due to the fouling issue. Thus, Nafion has been used as it is able to reduce the fouling of the working electrode surface owing to these compounds [50, 51].
By employing the Nafion-AuNP-CB modified electrode, a calibration curve has been performed in HCl 0.1 M standard solution, obtaining a linear correlation up to 10 mg/L described by the following equation y = − (3.3 ± 0.3) × − (1 ± 1), R2 = 0.976 (Fig. 3f). The limit of detection calculated as 3 σb/S was equal to 0.063 mg/L.
Detection in serum: sample treatment and iron quantification
Iron ions in the bloodstream are mainly complexed in the heme group of hemoglobin or myoglobin, to transport oxygen in the body, or bond to transferrin, to be delivered in the tissues or organs when requested. In order to obtain the release of iron ions from transferrin, an acidification treatment has been evaluated with three acid solutions, namely HCl 0.1 M, TCA 0.2 M, and TFA 0.2 M. In detail, 500 µL of serum samples spiked with 4 mg/L of iron ions were treated with 10 µL of acid solution. As depicted in Fig. 4a, the treatment with HCl and TCA was not enough to allow the quantification of iron, while TFA enables the detection in serum samples.
a Histogram bars obtained for optimization of serum treatment using HCL 0.1 M, TCA 0.2 M, and TFA 0.2 M for the detection in serum spiked with 4 mg/L of iron ions, by using the Nafion-CB-AuNPs SPE. b Calibration curve obtained for iron ions detection in TFA 0.2 M and relative voltammograms in the inset, by using the Nafion-CB-AuNPs SPE
Once TFA was selected as the preferred solution to further treat serum samples, a first evaluation was focused on testing the Nafion-AuNPs-CB SPE in this acid solution, by varying the concentration of iron ions. A linear correlation has been obtained in the iron range up to 10 mg/L, described by the following equation y = − (2.66 ± 0.08) × − (0.8 ± 0.3), R2 = 0.995 (Fig. 4b) with the limit of detection equal to 0.05 mg/L.
Finally, the effectiveness of the paper-based sensor has been assessed by the quantification of 10 serum samples, and the results have been compared with the reference methods, namely a colorimetric kit and AAS.
Serum samples were first treated with the TFA solution and then centrifuged at 12,000 rpm for 10 min. The supernatant was then collected, and electrochemical measurements were performed.
Iron ions were quantified through the interpolation method, by interpolating the current peak obtained after measuring different serum samples. Figure 5 shows the comparison of the results obtained by the developed sensor vs. colorimetric kit (Fig. 5a) and vs. AAS (Fig. 5b). In detail, a good agreement has been obtained with the colorimetric method and AAS, with a correlation factor equal to 0.976 and 0.980, respectively.
Conclusions
In this work, a paper-based electrochemical sensor for the detection of iron ions in serum samples has been developed. Several modifications have been carried out with the final aim of delivering a high-performance analytical tool. The nanomaterial modification by CB-AuNPs nanocomposite allowed for sensitive and selective detection of the target analyte, while Nafion was used to enable the detection of iron ions in the complex serum matrix.
Furthermore, the use of paper as a substrate for sensors provided a more sustainable material compared to polyester and ceramic, as the paper device can be easily incinerated, allowing for safety and sustainable waste management.
The suitability of the sensor has been demonstrated by measuring iron ions in serum, after ad hoc treatment of samples with TFA solution. We would like to highlight that in the sample treatment, the different amounts of TFA and the possible addition of other acids could be required depending on the lifetime of serum samples. By comparing the results with the ones obtained using atomic absorption spectroscopy and a commercial colorimetric kit as the reference methods, a good correlation within the experimental error has been obtained.
The results achieved in this study pointed out the possibility of using the developed sensor to realize POC devices, with the aim to perform the diagnosis for a customized administration of supplements and drugs in iron deficiency treatments. Indeed, further improvement with microfluidic systems for sample treatment can make easier the analytical procedures for sample treatment, allowing for its application as a POC diagnostic device for precision medicine in iron deficiency diseases.
References
Healthcare in 2030: goodbye hospital, hello home-spital. In: World Economic Forum.https://www.weforum.org/agenda/2016/11/healthcare-in-2030-goodbye-hospital-hello-home-spital/. Accessed 27 Sep 2022.
Land KJ, Boeras DI, Chen X-S, Ramsay AR, Peeling RW. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat Microbiol. 2019;4:46–54.
Vashist SK, Luppa PB, Yeo LY, Ozcan A, Luong JHT. Emerging technologies for next-generation point-of-care testing. Trend Biotechnol. 2015;33:692–705.
Yan T, Zhang G, Chai H, Qu L, Zhang X. Flexible biosensors based on colorimetry, fluorescence, and electrochemistry for point-of-care testing. Front Bioeng Biotechnol. 2021;9: 753692.
Yang J, Wang K, Xu H, Yan W, Jin Q, Cui D. Detection platforms for point-of-care testing based on colorimetric, luminescent and magnetic assays: a review. Talanta. 2019;202:96–110.
Gałuszka A, Migaszewski Z, Namiesnik J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC - Trends Anal Chem. 2013;50:78–84.
Nowak PM, Wietecha-Posłuszny R, Pawliszyn J. White analytical chemistry: an approach to reconcile the principles of green analytical chemistry and functionality. TrAC - Trends Anal Chem. 2021;138: 116223.
Noviana E, Henry CS. Simultaneous electrochemical detection in paper-based analytical devices. Curr Opin Electrochem. 2020;23:1–6.
Meredith NA, Quinn C, Cate DM, Reilly TH, Volckens J, Henry CS. Paper-based analytical devices for environmental analysis. Analyst. 2016;141:1874–87.
Cate DM, Adkins JA, Mettakoonpitak J, Henry CS. Recent developments in paper-based microfluidic devices. Anal Chem. 2015;87:19–41.
Martinez AW, Phillips ST, Whitesides GM, Carrilho E. Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal Chem. 2010;82:3–10.
Kung CT, Hou CY, Wang YN, Fu LM. Microfluidic paper-based analytical devices for environmental analysis of soil, air, ecology and river water. Sensor Actuat B-Chem. 2019;301: 126855.
Noviana E, Carrão DB, Pratiwi R, Henry CS. Emerging applications of paper-based analytical devices for drug analysis: a review. Anal Chim Acta. 2020;1116:70–90.
Arduini F, Micheli L, Scognamiglio V, Mazzaracchio V, Moscone D. Sustainable materials for the design of forefront printed (bio) sensors applied in agrifood sector. TrAC - Trends Anal Chem. 2020;128: 115909.
Arduini F. Nanomaterials and cross-cutting technologies for fostering smart electrochemical biosensors in the detection of chemical warfare agents. Appl Sci. 2021;2021(11):720.
Arduini F. Electrochemical paper-based devices: when the simple replacement of the support to print ecodesigned electrodes radically improves the features of the electrochemical devices. Curr Opin Electrochem. 2022;35: 101090.
Cinti S, Fiore L, Massoud R, Cortese C, Moscone D, Palleschi G, Arduini F. Low-cost and reagent-free paper-based device to detect chloride ions in serum and sweat. Talanta. 2018;179:186–92.
Maier D, Laubender E, Basavanna A, Schumann S, Güder F, Urban GA, Dincer C. Toward continuous monitoring of breath biochemistry: a paper-based wearable sensor for real-time hydrogen peroxide measurement in simulated breath. ACS sensors. 2019;4:2945–51.
Colozza N, Tazzioli S, Sassolini A, Agosta L, di Monte MG, Hermansson K, Arduini F. Multiparametric analysis by paper-assisted potentiometric sensors for diagnostic and monitoring of reinforced concrete structures. Sensor Actuat B-Chem. 2021;345: 130352.
Bagheri N, Mazzaracchio V, Cinti S, Colozza N, Di Natale C, Netti PA, Saraji M, Roggero S, Moscone D, Arduini F. Electroanalytical sensor based on gold-nanoparticle-decorated paper for sensitive detection of copper ions in sweat and serum. Anal Chem. 2021;93:5225–33.
Caratelli V, Meo ED, Colozza N, Fabiani L, Fiore L, Moscone D, Arduini F. Nanomaterials and paper-based electrochemical devices: merging strategies for fostering sustainable detection of biomarkers. J Mater Chem B. 2022;10:9021–39.
McDowell LR. Minerals in animal and human nutrition. 2nd ed. Amsterdam: Elsevier Science; 2003. p. 660.
Hurrell RF. Bioavailability of iron. Eur J Clin Nutr. 1997;51:S4-8.
Wood RJ, Ronnenberg A. Iron. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern nutrition in health and disease. 10th ed. Baltimore: Lippinco Williams & Wilkins; 2005. p. 248–70.
Nadadur SS, Srirama K, Mudipalli A. Iron transport and homeostasis mechanisms: their role in health and disease. Indian J Med Res. 2008;128:533–44.
Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19:164–74.
Burtis CA, Bruns DE, Sawyer BG, Tietz NW. Tietz fundamentals of clinical chemistry and molecular diagnostics. 7th ed. St. Louis, Missouri: Elsevier/Saunders; 2015.
WHO. Report of the WHO informal consultation on hookworm infection and anaemia in girls and women. Geneva: World Health Organization; 1995. p. 46.
Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:115–20.
Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58.
Failla ML. Trace elements and host defense: recent advances and continuing challenges. J Nutr. 2003;133:S1443–7.
Viteri FE, Torun B. Anemia and physical work capacity. In: Garby L, editor. Clinics in Hematology, vol. 3. London: WB Saunders; 1974. p. 609–26.
Georgieff MK, Krebs NF, Cusick SE. The benefits and risks of iron supplementation in pregnancy and childhood. Annu Rev Nutr. 2019;39:121–46.
Seymour CW, Gomez H, Chang C-CH, Clermont G, Kellum JA, Kennedy J, Yende S, Angus DC. Precision medicine for all? Challenges and opportunities for a precision medicine approach to critical illness. Crit Care. 2017;21:257.
Liang L, D’Haese PC, Lamberts LV, De Broe ME. Direct determination of iron in urine and serum using graphite furnace atomic absorption spectrometry. Analyst. 1989;114:143–7.
Kawasaki N, Tanimoto T, Tanaka A, Hayakawa T, Miyasaka N. Determination of non-protein-bound iron in human synovial fluid by high-performance liquid chromatography with electrochemical detection. J Chromatograph B. 1994;656:436–40.
Dorner K, Gustmann H, Sippell W. A new method for the determination of serum iron: potentiostatic coulometry with the Ferrochem 3050. J Chim Chem Clin Biochem. 1981;19:967–70.
Kremplova M, Krejcova L, Hynek D, Barath P, Majzlik P, Horak V, Adam V, Sochor J, Cernei N, Hubalek J, Vrba R, Kizek R. Automated electrochemical detection of iron ions in erythrocytes from MeLiM Minipigs suffering from melanoma. Int J Electrochem Sci. 2012;7:5893–909.
Hourani MK, Amayreh M, Hourani WH. A voltammetric sensor based on iodine-coated platinum electrode for determination of iron in blood serum. Anal Bioanal Electrochem. 2018;10:1620–8.
Vicentini FC, Ravanini AE, Figueiredo-Filho LCS, Iniesta J, Banks CE, Fatibello-Filho O. Imparting improvements in electrochemical sensors: evaluation of different carbon blacks that give rise to significant improvement in the performance of electroanalytical sensing platforms. Electrochim Acta. 2015;157:125–33.
Mazzaracchio V, Tomei MR, Cacciotti I, Chiodoni A, Novara C, Castellino M, Scordo G, Amine A, Moscone D, Arduini F. Inside the different types of carbon black as nanomodifiers for screen-printed electrodes. Electrochim Acta. 2019;317:673–83.
Carvalho RC, Mandil A, Prathish KP, Amine A, Brett CMA. Carbon nanotube, carbon black and copper nanoparticle modified screen printed electrodes for amino acid determination. Electroanal. 2013;25:903–13.
Cinti S, Politi S, Moscone D, Palleschi G, Arduini F. Stripping analysis of As(III) by means of screen-printed electrodes modified with gold nanoparticles and carbon Black Nanocomposite. Electroanal. 2014;26:931–9.
Cinti S, Santella F, Moscone D, Arduini F. Hg2+ detection using a disposable and miniaturized screen-printed electrode modified with nanocomposite carbon black and gold nanoparticles. Environ Sci Pollut Res. 2016;23:8192–9.
Arduini F, Zanardi C, Cinti S, Terzi F, Moscone D, Palleschi G, Seeber R. Effective electrochemical sensor based on screen-printed electrodes modified with a carbon black-Au nanoparticles composite. Sensor Actuat B-Chem. 2015;212:536–43.
Zakharova EA, Elesova EE, Noskova GN, Lu M, Compton RG. Direct voltammetric determination of total iron with a gold microelectrode ensemble. Electroanal. 2012;24:2061–9.
Caratelli V, Fillo S, D’Amore N, Rossetto O, Pirazzini M, Moccia M, Avitabile C, Moscone D, Lista F, Arduini F. Paper-based electrochemical peptide sensor for on-site detection of botulinum neurotoxin serotype A and C. Biosens Bioelectron. 2021;183: 113210.
Rocks FB, Sherwood RA, Turner Z. J, Riley C. Serum iron and total iron-binding capacity determination by flow injection analysis with atomic absorption detection Ann Clin Biochem. 1983;20:72–6.
Yu Q, Huang H, Peng X, Ye Z. Ultrathin free-standing close-packed gold nanoparticle films: conductivity and Raman scattering enhancement. Nanoscale. 2011;3:3868.
Hoyer B, Florence TM, Batley GE. Application of polymer-coated glassy carbon electrodes in anodic stripping voltammetry. Anal Chem. 1987;59:1608–14.
Torma F, Kádár M, Tóth K, Tatár E. Nafion®/2,2 bipyridyl-modified bismuth film electrode for anodic stripping voltammetry Anal Chim Acta. 2008;619:173.
Acknowledgements
We would like to thank Prof. Giulio Mengozzi of San Giovanni Battista Hospital “Molinette” for serum samples, and “Cardiovascular lab s.r.l (Milan, Italy)” for funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Source of biological material
Human serum samples were obtained from people enrolled for routine analysis at the San Giovanni Battista Hospital “Molinette” (Turin, Italy). The sampling of blood at the San Giovanni Battista Hospital “Molinette” (Turin, Italy) was carried out by medical doctors from patients and healthy subjects.
Consent to participate
Written informed consent was obtained from all the patients and healthy subjects.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection Electrochemical Biosensors – Driving Personalized Medicine with guest editors Susana Campuzano Ruiz and Maria Jesus Lobo-Castañón.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mazzaracchio, V., Bagheri, N., Chiara, F. et al. A smart paper-based electrochemical sensor for reliable detection of iron ions in serum. Anal Bioanal Chem 415, 1149–1157 (2023). https://doi.org/10.1007/s00216-023-04537-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04537-6