Abstract
In this article, we reported a facile method for synthesizing poly (sodium acrylate) (PAANa)-modified TEMPO-oxidized cellulose nanofibril (TOCN) aerogel spheres. The water absorbent capacity of the spheres could be as high as 1030 g/g, which has never been reported before. Practically, the 1 wt% TOCN suspension was simply dropped into an HCl solution to prepare physically crosslinked TOCN hydrogel spheres. Afterwards, PAANa, an anionic monomer, was added into TOCN gel spheres by solution replacement, and the monomer was polymerized and grafted to the TOCN spheres in the presence of N,N-methylenebisacrylamide as a crosslinker through in situ free radical polymerization. The physical crosslinking (through hydrogen bonding) of the spheres was replaced by chemical crosslinking after washing the gel spheres to neutral, which resulted in high volume expansion (about 27 times v/v) of the washed sphere. The expansion rate and morphologies of the obtained hybrid gel spheres were strongly dependent on the dosage of the crosslinker and monomer. The structure and morphologies of the hybrid spheres were characterized by FTIR, TGA and SEM. The water-absorbing behaviors of the hybrid spheres were dependent on the pH, concentration of the salt solution and crosslinker content. The freeze-dried aerogel spheres were highly porous (approximately 99.88 %) with density as low as 1.5 mg/cm3. All results indicated that TOCN–PAANa hybrid aerogel spheres could serve as a new type of superabsorbent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Super water absorbents are 3D networks of hydrophilic polymers that can absorb a large amount of water (tens to thousands of times its own weight) in a short time (Weeaver et al. 1977). The superabsorbents have received considerable attention and are now applied to many fields, such as agriculture, hygienic products, wastewater treatment and controlled drug release (Dadhaniya et al. 2006).
Various types of superabsorbents including resin, hydrogel and aerogel have been investigated in recent years. The traditional superabsorbents from poly (sodium acrylate) often have some limitation with poor biodegradability (Zhang et al. 2006). Cellulose is the most abundant biopolymer on earth. Moreover, it is safe, stable, nontoxic and biodegradable in nature (Sim and Youn 2016). The composite absorbent based on cellulose and poly (sodium acrylate) has been widely investigated (Yang et al. 2014). However, the water-absorbing capacity of poly (sodium acrylate)/cellulose composite absorbents is limited (under 600 times its weight) because of their compact structure (high density, low pore fraction) and non-uniform pore distribution (Liu et al. 2009; Spagnol et al. 2012;). Aerogel appears to be a new promising class of high-capacity absorbents because of its light weight, high pore fraction and large surface area. As a new generation of materials, cellulose aerogel has received much attention in applications such as oil–water separation, waste water treatment, supercapacitors and drug-release agent (Cervin et al. 2012; Zhang et al. 2014b, 2015; García-González et al. 2011). With abundant hydroxyls and other functional hydrophilic groups, the superabsorption properties of cellulose aerogels have been widely reported (Jiang and Hsieh 2014). Basically, there are two different methods for preparing cellulose-based aerogel. The first method uses dissolved cellulose. It was reported that with this method, the cellulose dissolution and solvent exchange after the aerogel formed were very slow, taking several days (Sehaqui et al. 2014). Furthermore, the solvents that can dissolve cellulose are usually very expensive and toxic and have a low dissolution capability for high-molecular-weight cellulose (Egal et al. 2007). The second method for fabricating cellulose-based aerogels uses cellulose nanofibrils but not soluble cellulose. Supercritical or freeze-dried aqueous nanocellulose suspensions are usually adopted for the final aerogel formation step (Chen et al. 2007). Sphere-structured aerogel is a unique material that can be used as a template for drug delivery, a superabsorbent, energy storage device, etc. (García-González et al. 2015; Zhai et al. 2016; Liu et al. 2006). Although the sphere particles of the aerogel have advantages in some applications, such as drug delivery, cellulose nanofibril-based aerogel spheres have only been seldom reported.
TEMPO oxidation has been utilized for selective oxidization of carboxylate primary groups of cellulose to carboxylate groups. Furthermore, Saito et al. reported that cellulose nanofibrils with lengths of dozens of microns can be isolated from TEMPO oxidized pulp fibers by simply blending or ultrasonication (Saito et al. 2007). However, the gelation behavior of nanocellulose at a higher concentration or at a lower pH limits its application as an absorbent material. Using the CNF skeleton structure for synthesizing polymer-modified aerogel composites is a suitable method for obtaining a hybrid aerogel with uniform structure. Fang et al. reported a novel method to synthesize PNIPAm-modified CNF aerogel microspheres using crosslinked CNF aerogel microspheres as a microreactor (Zhang et al. 2016). However, if considering the polymerization of monomers and the synthesis of crosslinked cellulose nanofibril aerogel, the complete procedure was tedious, and it would take more than 1 week to obtain the final product.
In this study, a facile approach for preparation of ultralight poly (sodium acrylate)-modified TEMPO-oxidized cellulose nanofibril (TOCN) aerogel spheres by using TOCN gel spheres as a reactor is reported. As a result, this method for TOCN–PAANa synthesis completely avoided the formation of a non-uniform composite structure caused by the “one-step-blending” preparation process. Also, breakage of the hydrogen bond of the TOCN reactor resulted in high swelling of hybrid spheres (about 27 times the volume of TOCN gels). Moreover, the TOCN–PAANa sphere synthesizing method avoided the use of a toxic solvent, so no dissolution or purification process is required. After one-step freeze-drying, the obtained aerogel spheres are very light-weight (about 1.5 mg/cm3) (density is only one tenth of the silica aerogel) with high porosity and have an excellent water absorption capacity (about 1030 times its original weight). This water absorption value was higher than for pure PAANa resin (439 g/g) and most reported cellulose-PAANa composite absorbent materials. Due to the high water absorption capacity, the aerogel-poly (sodium acrylate) composites have great potential use as a drug-loaded material and superabsorbent because they are ultralight, highly porous and biodegradabile.
Experimental section
Materials
Commercial fully bleached softwood kraft pulp (Hao Sheng Inc, China) was used as the raw material. Acyclic acid (AA, A.R.), potassium persulfate (KPS, A.R.) and N,N-methylenebisacrylamide (BMA, A.R.) were purchased from Shanghai China. Paraffin liquid was purchased from Shanghai China. Span-85 was purchased from Sinopharm China. 2,2,6,6-Tetramethyl-1-piperidinyloxy (TEMPO, 98 wt%), sodium hydroxide, ethanol, sodium chlorite, sodium bromide, sodium hypochlorite solution, and other chemicals were of analytical grade and used without further purification.
Preparation of TOCN gel spheres
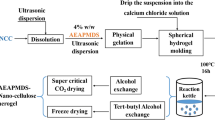
Commercial fully bleached softwood kraft pulp was used as the starting material for preparing TEMPO-oxidized cellulose nanofibrils. Briefly, 3 g cellulose fibers was suspended in 300 ml water containing TEMPO and sodium bromide (0.016 and 0.16 g per gram of cellulose, respectively) at room temperature (22 °C). The oxidation was started by adding the desired amount of NaClO (20 mmol NaClO per gram of cellulose). The pH was maintained at 10.5 by adding 2 mol/l NaOH solution. After 2 h, the obtained cellulose was washed with distilled water. Then, the 1 wt% oxidized cellulose suspension was subjected to the mechanical defibrillation process by using a blender at 10,000 rpm for 3 min and then sonicated for 3 min (output: 300 W, frequency: 20 kHz, pattern: continuous) using an FS-300 Ultrasonic processor to obtain TOCN. In order to obtain the physically crosslinked TOCN gel spheres, 1 wt% TOCN suspension at pH 7 was extruded dropwise through a syringe needle (syringe capacity: 2.5 ml, needle diameter: 0.6 mm) into a 0.1 mol/l HCl aqueous solution under gentle stirring.
Preparation of TOCN–PAANa aerogel spheres and pure PAANa resin
The TOCN–PAANa hybrid composites were prepared by free radical polymerization of sodium acrylate (AANa) inside the TOCN gel spheres in the presence of a crosslinker as described in Scheme 1. First, the monomer, crosslinking agent N,N’-methylenebisacrylamide (BMA) and initiator potassium persulfate (KPS) were dissolved in distilled water (22 °C) to make a uniform solution, and the concentration of AANa monomer was controlled at 5, 10, 15 and 20 % (w/v), respectively. The concentrations of BMA and KPS were controlled at 1 and 2 % based on the weight of the monomer. After soaking in the mixed solution for 3 h with gentle stirring, the TOCN gel spheres saturated with AANa monomer aqueous solution were filtered out and transferred to a three-neck bottle; paraffin oil and the emulsifier span-85 were added almost at the same time in order to prevent the crosslinking between spheres. The TOCN gels saturated with the mixed aqueous solution were dispersed in paraffin oil by vigorous stirring and heated to 80 °C under the protection of nitrogen. After 2 h polymerization, the obtained composite spheres were washed to neutral by excessive acetone and distilled water several times. Then, the purified samples were frozen by liquid nitrogen and further dried by a freeze-dryer. The samples with different monomer concentrations during the preparation process were named TOCN–PAANa-1, TOCN–PAANa-2, TOCN–PAANa-3 and TOCN–PAANa-4, respectively. The samples prepared by saturation with 5 % (w/v) monomer solution and different amounts of crosslinker (1, 2, 3, 4 and 5 % based on the weight of BMA) were named TOCN–PAANa-1-1, TOCN–PAANa-1-2, TOCN–PAANa-1-3, TOCN–PAANa-1-4 and TOCN–PAANa-1-5, respectively. In order to make a comparison, some TOCN–PAANa hydrogel spheres were directly dried at room temperature (22 °C) for 2 days followed by vacuum drying (60 °C) for 24 h. Pure PAANa hydrogel was prepared with 10 wt% monomer solution, 1 wt% crosslinker and KPS as described above. After 2 h polymerization, PAANa hydrogel was transferred to an oven and heated at 60 °C until it reached constant weight. Then, the dried sample was crushed by a pulverizer, and the resin was collected using a 200-mesh screen.
Characterization
Surface morphology of the superabsorbent aerogel spheres
Scanning electron microscopy (SEM, JSM-7600F) was used for morphological characterization. Samples were attached to the holders with conductive double-sided carbon tape and sputter coated with gold to avoid charging during the tests.
IR spectroscopy
TOCN–PAANa aerogel spheres were analyzed by Fourier transform infrared spectroscopy (FTIR). The spectra were recorded using Bruker Vertex 80 equipment with a detector at 4 cm−1 resolution from 500 to 4000 cm−1 and 32 scans per sample.
Thermogravimetric analysis
Thermal stability of the TOCN–PAANa was tested by thermogravimetric analysis (TGA) using a PerkinElmer STA 6000 thermal analyzer. About 10 mg of the sample was used for the test. The experiment was performed at a heating rate of 10 °C/min under a nitrogen flow rate of 20 ml/min. Temperature range was 30–600 °C.
Density
The TOCN–PAANa gel was cut to an approximately 5-mm-long section. After freeze-drying to aerogel, the mass and dimensions were measured using a digital caliper and balanced to 0.01 mm and 0.1 mg resolution in order to calculate the density of the aerogel.
Calculation of aerogel pore fraction
The density of the solid materials was calculated according to Eq. (1) based on the solid density of each component and their weight ratios used in the formulation (Zheng et al. 2014).
where W is the weight percentage of the different components, and ρ s is the solid density of the composite material. ρ TOCN and ρ s are the solid density of CNF and PAANa taken as 1.46 and 1.32 g/cm3, respectively (Zheng et al. 2014).
The pore fraction of aerogels was calculated according to Eq. (2).
where ρ was the density of the aerogel, and ρ s was the density of the solid materials.
Analysis of water absorbency properties
Water absorbency
To determine the water absorption capability (Q), a weighted quantity (w 1) of the superabsorbent composite was immersed in distilled water for 24 h at room temperature. Then, the swollen sample was taken out, and the excess water was removed by filter paper. The saturated aerogel was weighed (w 2). The water absorption percentage is calculated as Eq. (3) as follows.
Water absorbency at various pHs and saline solutions
The effects of pH and saline on water absorption were measured as follows: The pH adjustment was done using 0.1 mol/l HCl and 0.1 mol/l NaOH solutions. All the absorption capacities measured after absorbing NaCl solutions or HCl/NaOH solutions were calculated by the above method.
Results and discussion
Preparation and morphology
Softwood pulp fibers with diameters between 20 and 50 um were used as the starting material to prepare TOCN. After TEMPO oxidation, the carboxyl group content of the cellulose was 1.83 mmol/g, which was measured by the conductance titration method (Saito et al. 2007). Afterwards, the TEMPO-oxidized cellulose suspension was subject to a mechanical defibrillation process by using a blender and ultrasonic processor to obtain TOCNs with diameters mostly under 100 nm. It has been reported that aqueous TCON suspensions consist of anion charges at about pH 7 and thus are highly homogeneous. The homogeneous TOCN dispersion turned to gel spheres after dropwise extrusion of the TOCN suspension with a syringe needle into 0.1 mol/l HCl aqueous solution. This was because COO− is strongly affected by ionic strength. On one hand, the hydrogen-bonding interaction between COOH and OH was strengthened so that physical crosslinking was generated; on the other hand, the electrostatic repulsion among the carboxylate sodium group was restricted.
TOCN–PAANa hybrid spheres were synthesized inside the TOCN gel spheres by free radical polymerization of AANa monomer in the presence of the BMA crosslinking agent using KPS as an initiator. Because PAANa is water soluble but paraffin oil insoluble, the monomer aqueous solution was locked inside the TOCN spheres during the polymerization process along with constant stirring. As a result, the spheres were almost spherical in shape because of the physical crosslinking of the TOCN gels after polymerization. The crosslinking took place because of the free radical polymerization of BMA and PAANa. Some of PAANa could directly graft to the CNF surface by a free radical chain transfer reaction on the polysaccharide substrate. After washing the gel to neutral, some hydrogen bonds between COOH and OH disappeared, but the chemical crosslinking limited the possibility of unlimited expansion of the TOCN–PAANa gel spheres.
Table 1 gathers the physical properties of the TOCN–PAANa aerogel spheres in respect of the component ratio, aerogel density and pore fraction obtained from different preparation conditions previously described in the experimental section. The component ratio (PAANa/TOCN) was measured by the gravimetric method. Owing to the high volume expansion of the composite gel, the density of TOCN–PAANa-1 is even much lower than that of its pristine TOCN aerogel spheres. The ultra-low density of TOCN–PAANa-1 is approximately one tenth of that of the silica aerogel microspheres with high porosity (about 99.89 %). By comparing different samples, the hybrid aerogels presented densities varying from 1.5 to 15.2 mg cm−3. Although the density of TOCN–PAANa-4 was almost ten times that of TOCN–PAANa-1, the pore volume was only decreased from 99.88 to 98.83 %, confirming all aerogels were super lightweight and highly porous.
According to the observation described in Fig. 1a, very little shrinkage occurred in TOCN–PAANa aerogel spheres compared to their initial hydrogel dimensions. It has been previously reported that the freezing conditions have a large influence on the shrinkage of the aerogel. The rapid freezing by liquid nitrogen promotes the formation of small ice crystals and most likely also the formation of small-sized pores in the aerogels (Zhang et al. 2014a). There appears to be no significant change in size by comparing samples with different amounts of monomer during the copolymerization, as shown in Fig. 1b. However, by comparing the gel spheres with different crosslinker contents, as shown in Fig. 1c, it could be concluded that the dosage of BMA plays a dominate role in controlling size of spheres in the preparation of TOCN–PAANa gels. This was because the expansion rate was determined by the chemical crosslinking degree of the spheres. From b–d, it was clear that when the crosslinking degree increased, the size of the spheres became smaller. As the hydrogel sphere had a higher expansion rate, it would show a higher pore fraction and lighter weight. Moreover, it should be mentioned that if the BMA dosage was below 1 % based on the weight of the monomer, the gel would be no longer exist after washing.
Digital camera pictures of a a pristine TOCN gel sphere, TOCN–PAANa hydrogel sphere and TOCN–PAANa aerogel sphere after freeze-drying. b TOCN–PAANa hygrogel spheres prepared with different dosages of monomer during copolymerization. c TOCN–PAANa hydrogel spheres prepared with different amounts of crosslinker during copolymerization
The morphologies of poly (sodium acrylate)-modified TOCN aerogel spheres were evaluated through SEM pictures (Fig. 2). From (a), it was clear that the CNF aerogel microspheres are a highly porous homogeneous network. The higher magnification in (b) showed that the diameters of some nanofibril bundles were approximately dozens of nanometers. The pore sizes from (b) were typically in the range of nanometers to micrometers, which were significantly smaller than in (c–f). It was attributed to the fibrous-cellular structure of (a, b), whereas the overall surfaces of (c–f) were cellular, which can be seen in Fig. 1. Comparing the SEM images of (a–f), it was obvious that when the PAANa monomer concentration increased during the copolymerization process, the pore size became bigger and the spheres more compact. The fibrous-celluar structure from (a) and (b) was more convenient for the penetration of water into the polymeric network because of their high porous structure and specific surface area (Brodin and Theliander 2013). When the aerogel sphere was dipped into an aqueous medium, the medium easily diffused into the aerogel matrix through the micropores and was then locked in the small pores. Also, the fibrous-cellular structure of (a) and (b) could increase the number of hydrophilic groups based on their more highly porous structure and the specific surface area of the aerogel compared with the cellular structure, which makes the diffusion of water into the aerogel matrix easier and faster. The result from the observation is in good agreement with the absorption tests.
FTIR and TGA characterizations
Figure 3 shows the FTIR spectra of TOCN, PAANa and the synthesized TOCN–PAANa composites. For pristine TOCN, the presented bands at 3344, 2900 and 1646 cm−1 are assigned to stretching of –OH groups, C–H stretching and –OH bending of the absorbed water. Moreover, the band at 1720 cm−1 of TOCN confirms the presence of carboxylic acid groups, which is due to the selective TEMPO oxidation of the hydroxyl group at the C-6 position in the glucose ring (Silva et al. 2012). After the polymerization reaction, TOCN–PAANA had new absorption bands at 1721 (–COOH stretching), 1552, 1409 cm−1 (C=O asymmetric stretching in the carboxylate anion) and 1451 cm−1 (–CO–O– and OH coupling interactions of the carboxylic group) (Liu et al. 2009). Also, some of the carboxyl groups of TOCN may have been converted from –COOH to –COONa groups by ion exchange between TOCN and AANa during the polymerization process. The results indicated that the PAANa-modified TOCN composite had been successfully synthesized through radical copolymerization.
The thermogravimetric analysis (TGA) results of TOCN–PAANa aerogel spheres are shown in Fig. 4. The initial 5 % weight loss was below 105 °C, and it suggested the loss of bound water in the aerogel network. From approximately 172 to 321 °C, the composite had significant weight loss of nearly 19 %, which suggested complicated processes including the dehydration of TOCN chains and the breaking of the C–O–C glycosidic bond. From the DTG curve of TOCN–PAANa aerogel spheres, the maximum decomposition rate occurred at about 397 °C. This can be attributed to the elimination of the water molecule from the two neighboring carboxylic groups of the polymer chains because of the formation of anhydride, main-chain scission and destruction of crosslinked network structure (Huang et al. 2007). The 20 % weight loss from 427 to 527 °C was approximately due to the breakage of chains of PAANa.
Superabsorbent properties
TOCN-1 has excellent water absorption capacity (about 1035 g/g). This absorbency value is significantly higher than that of carboxymethyl cellulose/starch-co-poly (AA) composite polymers (300–680 g/g), which have been extensively studied as adsorbent materials because of their low cost and eco-friendly properties (Pourjava et al. 2006; Liu et al. 2009). TOCN-1 also has a much higher water absorption capacity than pure PAANa resin (439 g/g). The swelling properties of superabsorbent composites filled with nanocellulose were improved because of their high specific area and improved porous structure, which have also been reported previously (Dai and Kadla 2009). The hybrid aerogel densities vary from 1.5 to 15.2 mg cm−3, which can be seen in Table 1. However, the water absorption capacities of these absorbents varied widely from 80 to 1030 g/g. The water absorption of aerogel decreased while the monomer dosage increased. The phenomenon is presumably caused by the different pore structures of those aerogels. The pore structures of those composites became more compact when more amounts of AANa monomer were introduced into the pristine TOCN, which resulted in lower water storability. Based on the observed unchanged aerogel volume upon water saturation, water absorption capacities showed an inverse relationship with aerogel densities as shown in Table 1 and Fig. 5. A close study of the aerogel morphologies and physical properties of these TOCN–PAANa aerogels led to the conclusion that TOCN–PAANa-1 promotes a more effective dispersion of polymer chains into interconnected porous structures and thus favors water absorption. The water absorption capacities of TOCN–PAANa samples prepared by different drying conditions are shown in Fig. 5a, which does not show significant differences between freeze-dried and room-temperature dried samples with relatively high PAANa content. However, the absorption capacity of TOCN–PAANa-1 prepared by room-temperature drying could only absorb about 73 % of that by freeze-drying. This may be because the collapsed pores could not completely recover because of the strong hydrogen bond formation between TOCN chains during drying. From the swelling kinetics experiment, as can be found in Fig. 5b, the degree of swelling of the TOCN–PAANa-1 prepared by freeze-drying increased quickly within 1 h, reaching ca. 98 % of the equilibrium value. However, the equilibrium swelling degree of the room-temperature-dried sample reached more than 24 h. The swelling kinetics of a superabsorbent material were significantly influenced by various factors such as the composition of the superabsorbent, particle size and surface area (Elazzouzi-Hafraoui et al. 2008). From the structure and morphologies studied in this article, it could be concluded that the structure of TOCN–PAANa-1 aerogel spheres makes water absorption easier.
The effect of the amount of BMA, which was used as the crosslinker in the polymerization, is shown in Fig. 6a. Crosslinkers are indispensable for forming an absorbent in order to prevent dissolution of the hydrophilic polymer chains in aqueous solution (Lionetto et al. 2005). As the crosslinker content was increased, the aerogel’s water absorption capacity was decreased, and the highest absorbency could be as high as 1030 g/g when 5 wt% of PAANa monomers was used to make the hybrid aerogel. It should be pointed out that the hydrogel has very poor dimensional stability when the crosslinker concentration is lower than 1 wt%. According to Floy’s network theory, the increase of BMA concentration may cause the generation of more crosslinking points during radical polymerization and the increase of crosslinking density of polymer network, so the water absorption of the superabsorbent was decreased as the concentration of crosslinker increased (Pourjava et al. 2006). The conclusion is also in good agreement with the digital images shown in Fig. 1c. Moreover, if the BMA dosage was below 1 % based on the weight of the monomer, the gel would be no longer exist after washing.
The TOCN–PAANa aerogel contains carboxylate groups, which make up the majority of anionic type absorbents. The absorbency of the ionic superabsorbent could be strongly affected by the pH values of the solution. The absorbency results show that the network tends to shrink and the absorbency values decrease under an acidic environment. On the other hand, when the aqueous solution was under an alkaline environment, the absorbency of TOCN–PAANa aerogel spheres was limited because of the absence of anion-anion repulsion. A similar dependence of water absorption on pH has been reported for other anionic superabsorbents (Bao et al. 2011). Ionic strength can be influenced by the concentration of the saline solution as well (Zhao et al. 2014). The osmotic pressure between the polymeric network and external saline solution decreases with the increase of NaCl solution concentration. As a result, the water absorbency decreases as the concentration of external saline solutions increases. Figure 6b shows the effect of the NaCl concentration on the water absorbency of the superabsorbent. The water absorbency decreases as the concentration of the NaCl solution increases because of the well-known salting-out effect. The absorbency of TOCN–PAANa is larger than 158 g/g at 1.5 wt% NaCl concentration. Moreover, the penetration of salt ions into the polymeric network makes the screening effect on the anionic group of TOCN and PAANa (COO−) more evident, which decreases the water absorbency of the composite superabsorbent. In addition, the complexing ability of carboxylate groups on the composite superabsorbent network can induce the formation of intra- and intermolecular complexes with salt ions (Bao et al. 2011). PAANa-based superabsorbents show approximately the highest water absorption capacity among the most commonly used superabsorbent polymers; however, at a higher salt conditions, the water absorbency of PAANa is decreased remarkably. The introduction of a nonionic polymer such as polyacrylamide into the nanocellulose matrix should be one way to maintain the superabsorbent property of the cellulose nanofibril-based composite at higher salt concentrations.
Conclusion
In summary, we successfully fabricated PAANa-modified TOCN aerogel spheres by using physically crosslinked TEMPO-oxidized cellulose nanofibril gel spheres as the reactor. The skeleton structure of TOCN gel allowed the TOCN–PAANa aerogel spheres to have good homogeneity. The synthesized hybrid aerogel sphere with high PAANa content was even lighter and more porous than the pristine TOCN sphere. Moreover, the facile synthetic process for TOCN–PAANa hybrid aerogel spheres without using any solvent and purification methods showed promising potential for industrial applications. Based on this study, the structure of the aerogel spheres changed greatly by varying the crosslinker or monomer dosage during the preparation process. All the results demonstrated that TOCN–PAANa-1 is the best with highest absorbencies of 1,035 g/g in distilled water and 158 g/g in 1.5 wt% NaCl solution, respectively. The hybrid aerogel spheres turned out to be very sensitive to the pH environment in our study. The open-frame structure also leads to a rapid absorption process. In addition, this facile synthesis technique using physically crosslinked TOCN spheres as a reactor for preparing high-performance functional porous material could be extended to a variety of other systems.
References
Bao Y, Ma J, Li N (2011) Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly (AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr Polym 84:76–82
Brodin FW, Theliander HA (2013) Comparison of softwood and birch kraft pulp fibers as raw materials for production of TEMPO-oxidized pulp, MFC and superabsorbent foam. Cellulose 20:2825–2838
Cervin NT, Aulin C, Larsson PT (2012) Ultra porous nanocellulose aerogels as separation medium for mixtures of oil/water liquids. Cellulose 19:401–410
Chen W, Yu H, Li Q, Liu Y, Li J (2007) Ultralight and highly flexible aerogels with long cellulose I nanofibers. Soft Matter 7:10360–10368
Dadhaniya PV, Patel MP, Patel RG (2006) Swelling and dye adsorption study of novel superswelling [Acrylamide/N-vinylpyrrolidone/3(2-hydroxyethyl carbamoyl) acrylic acid] hydrogels. Polym Bull 57:21–31
Dai Q, Kadla JF (2009) Effect of nanofillers on carboxymethyl cellulose/hydroxyethyl cellulose hydrogels. J Appl Polym Sci 114:1664–1669
Egal M, Budtova T, Navard P (2007) Structure of aqueous solutions of microcrystalline cellulose/sodium hydroxide below 0 °C and the limit of cellulose dissolution. Biomacromolecules 8:2282–2287
Elazzouzi-Hafraoui S, Nishiyama Y, Putaux JL, Heux L, Dubreuil F, Rochas C (2008) The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 9:57–65
García-González CA, Alnaief M, Smirnova I (2011) Polysaccharide-based aerogels-promising biodegradable carriers for drug delivery systems. Carbohydr Polym 86:1425–1438
García-González CA, Jin M, Gerth J (2015) Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr Polym 117:797–806
Huang YH, Lu J, Xiao CB (2007) Thermal and mechanical properties of cationic guar gum/poly(acrylic acid) hydrogel membranes. Polym Degrad Stab 92:1072–1081
Jiang F, Hsieh YL (2014) Amphiphilic superabsorbent cellulose nanofibril aerogels. J Mater Chem A 2:6337–6342
Lionetto F, Sannino A, Maffezzoli A (2005) Ultrasonic monitoring of the network formation in superabsorbent cellulose based hydrogels. Polymer 46:1796–1803
Liu N, Fu RW, Wang JB (2006) Studies on the energy storage performance of carbon aerogel spheres. Acta Scientiarum Naturalium Universitatis Sunyatseni 45:59–63
Liu Z, Miao Y, Wang Z, Yin G (2009) Synthesis and characterization of a novel super-absorbent based on chemically modified pulverized wheat straw and acrylic acid. Carbohydr Polym 77:131–135
Pourjava A, Barzegar S, Mahdavinia GR (2006) MBA-crosslinked Na–Alg/CMC as a smart full-polysaccharide superabsorbent hydrogels. Carbohydr Polym 66:386–395
Saito T, Kimura S, Nishiyama Y, Isogai A (2007) Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 8:2485–2491
Sehaqui H, Zhou Q, Berglund LA (2014) High-porosity aerogels of high specific surface area prepared from nanofibrillated cellulose (NFC). Compos Sci Technol 71:1593–1599
Silva TCF, Habibi Y, Colodette JL, Elder T, Lucia LAA (2012) fundamental investigation of the microarchitecture and mechanical properties of tempo-oxidized nanofibrillated cellulose (NFC)-based aerogels. Cellulose 19:1945–1956
Sim K, Youn HJ (2016) Preparation of porous sheets with high mechanical strength by the addition of cellulose nanofibrils. Cellulose 2016:1–10
Spagnol C, Rodrigues FHA, Pereira AGB, Fajardo AR, Rubia AF, Muniz EC (2012) Superabsorbent hydrogel nanocomposites based on starch-g-poly (sodium acrylate) matrix filled with cellulose nanowhiskers. Cellulose 19:1225–1237
Weeaver MQ, Montgomery RR, Miller LD, Sohns VE, Fanta GF, Doane WM (1977) A practical process for the preparation of super slurper, a starch-based polymer with a large capacity to absorb water. Starch-starke 29:413–422
Yang J, Zhao JJ, Han CR (2014) Tough nanocomposite hydrogels from cellulose nanocrystals/poly (acrylamide) clusters: influence of the charge density, aspect ratio and surface coating with PEG. Cellulose 21:541–551
Zhai T, Zheng Q, Cai Z (2016) Synthesis of polyvinyl alcohol/cellulose nanofibril hybrid aerogel microspheres and their use as oil/solvent superabsorbents. Carbohydr Polym 148:300–308
Zhang J, Li A, Wang A (2006) Synthesis and characterization of multifunctional poly (acrylic acid-co-acrylamide)/sodium humate superabsorbent composite. React Funct Polym 66:747–756
Zhang W, Zhang Y, Lu C, Deng Y (2014a) Aerogels from crosslinked cellulose nano/micro-fibrils and their fast shape recovery property in water. J Mater Chem 22:11642–11650
Zhang X, Lin Z, Chen B, Zhang W, Sharma S, Gu W, Deng Y (2014b) Solid-state flexible polyaniline/silver cellulose nanofibrils aerogel supercapacitors. J Power Sources 246:283–289
Zhang F, Wu W, Sharma S, Tong G, Deng Y (2015) Synthesis of cyclodextrin-functionalized cellulose nanofibril aerogel as a highly effective adsorbent for phenol pollutant removal. BioResources 10:7555–7568
Zhang F, Wu W, Zhang X, Meng X, Tong G, Deng Y (2016) Temperature-sensitive poly-NIPAm modified cellulose nanofibril cryogel microspheres for controlled drug release. Cellulose 23:415–425
Zhao Y, Fang L, Tan T (2014) Optimization of the preparation of a poly (aspartic acid) superabsorbent resin with response surface methodology. J Appl Polym Sci 38:2616–2622
Zheng Q, Cai Z, Gong S (2014) Green synthesis of polyvinyl alcohol (PVA)-cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. J Mater Chem A 2:3110–3118
Acknowledgments
These works were supported by the Doctorate Fellowship Foundation of Nanjing Forestry University and National Natural Science Foundation of China (31470593), and we thank The Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, F., Ren, H., Tong, G. et al. Ultra-lightweight poly (sodium acrylate) modified TEMPO-oxidized cellulose nanofibril aerogel spheres and their superabsorbent properties. Cellulose 23, 3665–3676 (2016). https://doi.org/10.1007/s10570-016-1041-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-1041-8