Abstract
Thermo-compression process—compression of pure cellulose under high temperatures and pressures—is a recent method to produce biodegradable materials. For such processes, experimental measurements and study of properties and behavior of cellulose are difficult to carry out. To overcome these difficulties, a complete pressure–volume–temperature investigation is needed as carried out in this work. To develop a predictive thermodynamic PVT model of cellulose theoretically, the modified Sanchez and Lacombe equation of state together with the implementation of the Hoftyzer and van Krevelen group contribution method and the Boudouris modification to the Constantinou and Gani’s group contribution method were coupled to the Compressible Regular Solution theory. The developed method is a pure predictive model and to examine the accuracy of theoretically calculated PVT data by the model, some available PVT data of cellulose at temperatures from 25 to 180 °C and pressures from 19.6 to 196 MPa (219 data points) were collected from literature. The comparisons were made and the agreement between the calculations and the experimental data were acceptable with a Cumulative Absolute Relative Deviation of 0.04 %. Consequently, the model can be used for prediction of thermodynamic properties of cellulose and cellulose-containing mixtures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose, as one of the most abundant biomolecules on the earth, is currently being used for production of a wide variety of products, especially paperboards and papers (Jallabert et al. 2013). A new innovative way to produce biodegradable materials from cellulose is the compression of pure cellulose at high temperatures and pressures (Jallabert et al. 2013; Nilsson et al. 2010; Pintiaux et al. 2013; Vaca-Medina et al. 2013; Zhang et al. 2012). For such a process, experimental measurements and study of properties and behavior of cellulose are difficult to be done (Jallabert et al. 2013; Vaca-Medina et al. 2013). Therefore, theoretical modeling and investigation of such a process is required and interesting. To understand the behavior of cellulose over various operating conditions, a complete pressure–volume–temperature (PVT) investigation must be carried out as described in the following sections.
Modeling thermophysical properties of cellulose
Due to moisture of surrounding air and its sorption into cellulose, cellulose can be regarded as a mixture of water (moisture) and cellulose. Therefore, for a thermodynamic modeling of moist cellulose, mixture-related property calculations must be used. For the calculation of dry (as pure) cellulose, the modified Sanchez and Lacombe equation of state (SL-EOS) was used. By the extension of SL-EOS to mixtures, a large amount of numerical calculations and the availability of mixture experimental data are required to determine required adjustable parameters through appropriate correlations and fitting of experimental data. Therefore, here, to avoid such disadvantages, an alternative method for calculation of moist cellulose is developed.
Considering the moist cellulose as a final product of moisture (water) sorption in dry cellulose, alternatively the volume of moist cellulose can be determined from the fractional change in volume upon sorption (say mixing). It is viable to determine the fractional change in volume upon mixing (\({{\Delta _{mixing} V} \mathord{\left/ {\vphantom {{\Delta _{mixing} V} {V_{0} }}} \right. \kern-0pt} {V_{0} }}\)) theoretically using some chemical thermodynamic theories. The derivative of the Gibbs free energy (\(\Delta _{mix} g\)) with respect to pressure gives the ratio (\({{\Delta _{mixing} V} \mathord{\left/ {\vphantom {{\Delta _{mixing} V} {V_{0} }}} \right. \kern-0pt} {V_{0} }}\)) as given in Eq. 1, where V 0 is the total volume occupied by the dry cellulose at T and P (Ruzette and Mayes 2001).
To employ Eq. 1, a Gibbs free energy model must be used. In this work, a compressible regular solution free energy model (CRS) was used and given in Eq. 2 (Ruzette and Mayes 2001). The CRS model, due to the high and desirable accuracy, has found many applications for a wide range of systems (Keshavarz et al. 2015). In this theory, no interaction parameters have been defined and only pure component properties are needed for model calculations.
Here, \(\phi_{i}\) indicates the volume fraction of component i, \(\tilde{\rho }_{i}\) is the reduced density (hard-core density), N i is number of segments present in a hard-core volume of \(\nu_{i}\), \(\delta_{i,0}\) is the hard-core solubility parameter at reference temperature of 298 K (\(\delta_{i}^{2} \left( {298} \right)\)), k is the Boltzmann constant.
For calculation of \(N_{i} v_{i}\), one might use \(N_{i} v_{i} = {{Mw} \mathord{\left/ {\vphantom {{Mw} {\rho^{*} }}} \right. \kern-0pt} {\rho^{*} }}\) equality, where Mw is the molecular weight of components. δ i is the hard-core solubility parameter at temperature of system (\(\delta_{i} \left( T \right)\)) that can be calculated using \(\delta_{i}^{2} \left( T \right) = \delta_{i}^{2} \left( {298} \right)\left[ {{{\rho_{i} \left( T \right)} \mathord{\left/ {\vphantom {{\rho_{i} \left( T \right)} {\rho_{i}^{0} \left( T \right)}}} \right. \kern-0pt} {\rho_{i}^{0} \left( T \right)}}} \right]\). The hard-core density at system temperature can be calculated using \(\rho = \tilde{\rho }\rho^{*}\) where \(\tilde{\rho }\) is obtained from the modified SL-EOS (Boudouris et al. 1997). \(\delta_{i}^{2} \left( {298} \right)\) can be calculated from a group contribution method (van Krevelen and Nijenhuis 2008).
For calculation of reduced density, the modified version (Boudouris et al. 1997) of SL-EOS (Sanchez and Stone 2000; Sandler 1993) was used as presented in Eq. 3.
Here \(\tilde{P}\), \(\tilde{T}\), \(\tilde{\rho }\) and \(\tilde{v}\) are reduced pressure, temperature, density and volume which are defined as follows;
where (Eq. 4), \(P^{*}\), \(T^{*}\) and \(\rho^{*}\) are characteristic pressure, temperature and density that have been developed for SL-EoS (Boudouris et al. 1997). In the literature (Boudouris et al. 1997; Poling et al. 1987), a group contribution method has been established for calculation of these characteristic parameters (i.e. \(P^{*}\), \(T^{*}\) and \(\rho^{*}\)). Here, the modified version of the Constantinou and Gani group contribution method was used (Boudouris et al. 1997).
The fractional change in volume upon mixing, then, can be calculated as given by Eq. 5 (Ruzette and Mayes 2001) using Eqs. 1–2;
Here β i is the isothermal compressibility which can be obtained by evaluation of \(\beta_{i} = \left( {{1 \mathord{\left/ {\vphantom {1 {\tilde{\rho }_{i} }}} \right. \kern-0pt} {\tilde{\rho }_{i} }}} \right)\left. {\left( {{{\partial \tilde{\rho }_{i} } \mathord{\left/ {\vphantom {{\partial \tilde{\rho }_{i} } {\partial P}}} \right. \kern-0pt} {\partial P}}} \right)} \right|_{T,\varphi }\) where \(\tilde{\rho }_{i}\) can be obtained from the modified SL-EOS relationship (Eq. 3).
Therefore, this way, the necessity of the time-consuming calculations of adjustable parameters as required in the original SL-EOS model and also the essence of availability of experimental data would be resolved. In other words, now, the calculations need the pure component properties that are well-known and computationally accessible.
The moist cellulose volume, then, can be obtained using Eq. 6;
Collected experimental data
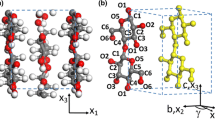
The experimental data for comparison of model findings were obtained (219 data points) from the literature (Jallabert et al. 2013). The chemical structure of cellulose is shown in Fig. 1.
Chemical structure of cellulose, created from the coordinates of the literature (Nishiyama et al. 2002)
Result and discussion
The calculated pure component properties that are required prior to the use of model are listed in Table 1, where parameters δ d , δ p , δ h , δ i,0 are given in \(\sqrt {{{\text{J}} \mathord{\left/ {\vphantom {{\text{J}} {{\text{m}}^{3} }}} \right. \kern-0pt} {{\text{m}}^{3} }}}\), ρ in \({{\text{g}} \mathord{\left/ {\vphantom {{\text{g}} {{\text{cm}}^{3} }}} \right. \kern-0pt} {{\text{cm}}^{3} }}\), α in 1/K and N i v i in \({{{\text{cm}}^{3} } \mathord{\left/ {\vphantom {{{\text{cm}}^{3} } {\text{mol}}}} \right. \kern-0pt} {\text{mol}}}\), α is the thermal expansion that was calculated using Eq. 7 (Keshavarz et al. 2015);
Using the developed model, the PVT data of Cellulose were predicted and satisfactory agreements were found as can be seen in Fig. 2 where the correlation results of predictive model for volume over all temperature and pressure ranges are plotted.
The correlation results of predictive model of cellulose PVT data; volume for all temperatures and pressures ranges; dashed line represent y = x line; solid line is the linear least-squares correlation equation (y = 1.0095x − 0.0024) with an R 2 = 0.9938 where x indicates the experimental V and y indicates the calculated V
In Figs. 3 and 4, the reliability of the developed model in prediction of PVT data is illustrated for P = 196.1 MPa and for P = 60 MPa, respectively. As it is expected, the errors in predictions are slightly increased as temperature increases. The error of model predictions for PVT data can be given in terms of Cumulative Absolute Relative Deviation (CARD %) [or Accumulative Absolute Relative Deviation (AARD %)] as defined in Eq. 8 where the summation is applied over all data points (NP = 219) for which the comparisons are made (all available experimental data). It must be noted that a CARD value closer to zero is desirable while a CARD value closer to unity is undesirable (0 < AARD < 1).
The model predictions reveal a CARD of 0.04 % which is satisfactory and desirable.
The visual presentation of correlation result of predictive model of Cellulose PVT for P = 196.1 MPa; the bold line is the model prediction and solid bullets are the experimental data obtained from the literature (Jallabert et al. 2013)
The visual presentation of correlation result of predictive model of Cellulose PVT for P = 60 MPa; the bold line is the model prediction and solid bullets are the experimental data obtained from the literature (Jallabert et al. 2013)
The application of model is simple and straightforward. The developed model was extensively analyzed and validated. Only using the structure of involved components, one would simply proceed to the prediction of PVT data of pure components or mixture under study. The presented model even can be used for prediction of sorption behavior of polymeric materials. The desirable accuracy of model, as demonstrated for the case of Cellulose, shows the potential of presented model for further studies.
Conclusions
Using the modified SL-EOS together with the Hoftyzer and van Krevelen group contribution method and the Boudouris modification to group contribution method of the Constantinou and Gani which were coupled to the CRS theory of Mayes, a predictive model was theoretically developed for thermodynamic calculation and prediction of cellulose PVT data. PVT data of cellulose has been collected at temperatures from 25 to 180 °C and pressures from 19.6 to 196 MPa from the literature (219 data points). The comparisons were made between model predicted data and the experimental data, and satisfactory agreements were found with a CARD of 0.04 %. The results confirmed that the proposed method is reliable, simple and accurate for thermophysical property calculations and can be extended to other systems.
References
Boudouris D, Constantinou L, Panayiotou C (1997) A group contribution estimation of the thermodynamic properties of polymers. Ind Eng Chem Res 36:3968–3973. doi:10.1021/ie970242g
Jallabert B, Vaca-Medina G, Cazalbou S, Rouilly A (2013) The pressure–volume–temperature relationship of cellulose. Cellulose 20:2279–2289. doi:10.1007/s10570-013-9986-3
Keshavarz L, Khansary MA, Shirazian S (2015) Phase diagram of ternary polymeric solutions containing nonsolvent/solvent/polymer: theoretical calculation and experimental validation. Polymer 73:1–8. doi:10.1016/j.polymer.2015.07.027
Nilsson H, Galland S, Larsson PT, Gamstedt EK, Nishino T, Berglund LA, Iversen T (2010) A non-solvent approach for high-stiffness all-cellulose biocomposites based on pure wood cellulose. Compos Sci Technol 70:1704–1712. doi:10.1016/j.compscitech.2010.06.016
Nishiyama Y, Langan P, Chanzy H (2002) Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 124:9074–9082. doi:10.1021/ja0257319
Pintiaux T, Viet D, Vandenbossche V, Rigal L, Rouilly A (2013) High pressure compression-molding of α-cellulose and effects of operating conditions. Materials 6:2240
Poling BE, Prausnitz JM, O’Connell JP (1987) Properties of gases and liquids, 4th edn. McGraw-Hill Professional, New York
Ruzette A-VG, Mayes AM (2001) A simple free energy model for weakly interacting polymer blends. Macromolecules 34:1894–1907. doi:10.1021/ma000712+
Sanchez I, Stone M (2000) Statistical thermodynamics of polymer solutions and blends volume 1: formulation. Polymer blends: formulation and performance. Wiley, New York
Sandler SI (1993) Models for thermodynamic and phase equilibria calculations chemical industries. CRC Press, Boca Raton
Vaca-Medina G, Jallabert B, Viet D, Peydecastaing J, Rouilly A (2013) Effect of temperature on high pressure cellulose compression. Cellulose 20:2311–2319. doi:10.1007/s10570-013-9999-y
van Krevelen DW, Nijenhuis KT (2008) Properties of polymers: their correlation with chemical structure; their numerical estimation and prediction from additive group contributions, chapter 7, 4th edn. Elsevier, Philadelphia, p 215
Zhang X, Wu X, Gao D, Xia K (2012) Bulk cellulose plastic materials from processing cellulose powder using back pressure-equal channel angular pressing. Carbohydr Polym 87:2470–2476. doi:10.1016/j.carbpol.2011.11.019
Acknowledgments
The authors gratefully acknowledge the important contribution and guidance provided by Al French (Editor-in-Chief in Cellulose) regarding the chemical structure of cellulose repeating unit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asgarpour Khansary, M., Shirazian, S. Theoretical modeling for thermophysical properties of cellulose: pressure/volume/temperature data. Cellulose 23, 1101–1105 (2016). https://doi.org/10.1007/s10570-016-0888-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-0888-z