Abstract
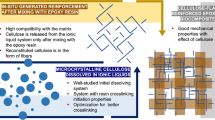
Development of high-performance bio-nanocomposite adhesives is of high interest due to their environmentally friendly nature and superior mechanical properties in outdoor environments. Nano-crystalline cellulose (NCC) and resilin are among the most promising bio-nanofillers, providing strength and elasticity, respectively. A novel bio-nanocomposite comprised of NCC and resilin fused to a cellulose binding domain (Res.-CBD) is presented. As a case study, commercial epoxy adhesive was chosen as a matrix for the bio-nanocomposite adhesive. Insertion of hydrophilic NCC into hydrophobic resins, such as epoxy, is typically performed using solvent exchange, chemical modification, emulsifier addition or mixing with water-borne resins, techniques which either limit the material’s application range or which are considered environmentally unfriendly. The unique approach presented here employed Res.-CBD as a surfactant-like agent supportive of the direct insertion of water-suspended NCC into an epoxy resin. The presented approach involves binding of Res.-CBD to NCC through its CBD domain and a chemical reaction between the resin epoxide groups and Res.-CBD amine moieties. The resulting bio-nano material shows a 50 % increase in the Young’s modulus and a 20 % decrease in the tan(δ), compared to pristine epoxy. This novel epoxy adhesive can be advantageous in applications where higher elasticity and Young’s modulus are required.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The search for high-performance materials has led to extensive development of polymeric nanocomposites. Nanoscale materials have a relatively large surface area for a given volume (Jyi-Jiin and Daniel 2003), leading to properties substantially different from materials of the same composition with larger filler dimensions. In the case of fillers such as particles and fibers, the surface area per unit volume is inversely proportional to the their diameter (Jyi-Jiin and Daniel 2003; Hussain et al. 2006). The potential of nanocomposites to enhance physical properties of materials has led to developments in various fields, such as electrical (Sanjines et al. 2011; Tanaka 2009), environmental (Xin et al. 2011), flame retardancy (Pereira 2009) and optical (Risbud 2004) applications. The enhanced physical properties of polymer nanocomposites are related to high surface to volume ratio of the reinforcing phase properties at small filler loadings, resulting in strong filler to matrix interaction. Factors such as local chemistry reactions, degree of thermoset cure, polymer chain mobility, polymer chain conformation, and degree of polymer crystallinity can vary significantly from the interface with the reinforcement into the bulk of the matrix, resulting in enhanced physical properties (Ajayan 2003).
Bio-nanocomposites exploit bio-based nanoparticles, which serve as building blocks and feature unique multifunctional and self-assembly properties (Jutz and Boker 2011). The development of bio-nanocomposites and innovative process technologies can reduce negative human impact and dependence on fossil fuels by utilizing natural in place of industrial nanofillers. The shift toward natural bio-based nanofillers that feature outstanding mechanical properties, will increase sustainability of the human environment, to the benefit of mankind (Rhim et al. 2013).
Nano-crystalline cellulose (NCC) and resilin, two bio-based nanofillers, were studied in this work. The elastomeric resilin is an insect-derived protein involved in many cyclic and dynamic movements in nature, such as the jumping mechanism of fleas and the efficient pivoting mechanism of insect wings (Donoughe et al. 2011). Resilin is the most elastic protein known, and can be stretched to over 300 % of its original length before failure (Elvin et al. 2005) with only 3 % of its stored energy is lost as heat. Recently, E. coli were genetically engineered to express recombinant resilin fused to a cellulose binding domain (Res.-CBD), which was shown to have high affinity to cellulose (Rivkin et al. 2013).
Cellulose is the most abundant bio-polymer on Earth (Monserrate et al. 2001). NCC extracted from heat and acid-treated cellulose powder (Rivkin et al. 2013), is among the most promising bio-based building blocks for improvement of mechanical properties (Eichhorn et al. 2010). NCC is considered an ideal load-bearing substance, due to its high aspect ratio, high bending strength (~10 GPa) and high Young’s modulus (~150 GPa) (Sturcova et al. 2005). Addition of 4 wt% NCC to a polyurethane matrix was reported to increase the Young’s modulus by 1,220 %, the tensile strength by 70 % and the elongation at break by 29 % (Cao et al. 2009; Reddy 2011). NCC has also been reported to enhance the mechanical properties of epoxy resins (Pan et al. 2012). For example, 4 wt% NCC added to an epoxy resin led to a 14 % increase in the storage modulus, at a temperature below the material’s T g; more dramatic mechanical reinforcement was observed above T g. The 4 wt% NCC epoxy nanocomposite exhibited a storage modulus of 355 MPa, compared to 15 MPa of the neat epoxy (Tang and Weder 2010). Such enhancement were attributed to the strong interactions between NCC and the epoxy network (Ruiz et al. 2000).
In general, dispersion of nano-scale particles in polymeric materials has proven difficult and frequently results in phase separation and agglomeration (Mackay et al. 2006). One of the greatest challenges in the incorporation of NCC into an epoxy matrix is the incompatibility between the hydrophilic NCC and the hydrophobic matrix (Ruiz et al. 2001). One means of overcoming this obstacle is the usage of waterborne epoxy. Through this procedure, addition of 15 wt% NCC to a waterborne epoxy was reported to increase its storage modulus by 100 % and the tensile strength from 40 to 60 MPa. Such enhanced mechanical properties suggest good adhesion between the NCC and the epoxy (Xu et al. 2013). However, the high water content in waterborne epoxy results in significant shrinkage, limiting its use to coating and thin film applications (Gao et al. 2011; Zhong-Hua et al. 2008). Another approach which may enable NCC insertion into non-polar media and usage of the final nanocomposite as a construction material, requires reaction of the NCC hydroxyl groups with appropriate reactants and covalently bonding of the desired function at the NCC surface (Tingaut et al. 2012). Alternatively, a sol/gel process through solvent exchange, involving periodic addition of acetone to an aqueous solution containing NCC, can ensure insertion of the hydrophilic NCC into the hydrophobic epoxy resin. This process leads to the formation of an NCC-acetone gel, which can be converted into an aerogel, through supercritical extraction, and then filled with a matrix polymer (Capadona et al. 2007) to form the desired nanocomposite. Alternatively, the NCC-acetone gel can be redispersed in DMF and mixed with an epoxy resin upon acetone evaporation (Tang and Weder 2010). However, such a solvent-exchange-based process demands large quantities of solvents and is considered environmentally unfriendly.
This work presents a case study of the insertion of NCC into a commercial epoxy resin using a novel, clean and environmentally friendly process involving Res.-CBD. This novel process empowers the fabrication of a novel bio-nanocomposite, which can be used as a construction and coating material, featuring both enhanced Young’s modulus and elastic properties compared to nonmodified epoxy.
Experimental
Materials
The nanocomposites studied in this work are based on a commercial epoxy matrix reinforced by bio-nano agents. EPON 828 and Epikure 3140 (Momentive Specialty Chemicals Inc.) were used as resin and hardener, respectively (Momentive Specialty Chemicals Inc. 2005, 2007).
NCC was extracted from Avicel® PH-200 microcrystalline cellulose (FMC Biopolymer Inc.) via H2SO4 acid hydrolysis (Siro and Plackett 2010; Habibi et al. 2010; Rivkin et al. 2013) and a sonication processes.
The recombinant protein 6 histidine tag-17 resilin–cellulose binding domain (6H-17Res.-CBD) is composed of D. Melnogaster resilin exon1 17 elastic repeats (Guokui et al.; Rivkin et al. 2013) C-terminal-fused to C. Cellulovorans CBD (Scheme 1) (Goldstein et al. 1993). The protein was produced by the authors according to the previously described procedures (Rivkin et al. 2013).
Methods
NCC imaging was performed using a FEI Tecnai F20 Transmission electron microscope (TEM).
Determination of NCC sulfate ester content was performed using conductmetric titration. Acid-form NCC in an aqueous suspension (0.05 g, ca. 2 wt%) was combined with NaCl (1 mM, 100 mL), and titrated against dilute NaOH (2 mM), which had been previously standardized against HCl. The equivalence point from titration is determined by the surface sulfate half-ester content as has been shown previously (Dong et al. 1998).
NCC insertion into epoxy was confirmed and analyzed using various techniques. Fourier transform infrared (FTIR) spectra were obtained using a Nicolet 6700 spectrometer. 32 scans at a resolution of 4 cm−1 were performed on each sample. The epoxy resin samples containing resilin were deposited as thin films on Ge windows and dried for 3 h before being analyzed. FTIR samples were prepared by periodically sonicating EPON 828 and resilin/water solutions to form an emulsion which was kept in a closed apparatus at a temperature of 80 °C. After 16 h at 80 °C, a droplet from the emulsion was deposited on the Ge window and the water from the emulsion was evaporated. The Ge window was then placed in the FTIR device and the chemical effect of resilin on the EPON 828 epoxydation reaction was investigated. To account for the path-length differences of the various films deposited on the Ge, the intensity of the 914 cm−1 band which corresponds to the epoxide rings (Eitan et al. 2003; Schiering et al. 1987) was normalized by the intensity of the 1,184 cm−1 band which was used as an internal reference for each of the measurements (Lin et al. 1979; Enns and Gillham 1983).
Weighing in the precision mass analysis was performed using an ALD114 balance (Scale-House Electronics). Sonication of the epoxy resin containing NCC and Res.-CBD was performed using a CL4 ultrasonic converter (Misonix Inc.).
Particle size measurements, based on dynamic light scattering (DLS) were performed using a Zetasizer Nano-ZS (Malvern Instruments). Epoxy resin containing 1.75 wt% NCC and 0.175 wt% Res.-CBD was diluted 1:10 in acetone before being introduced, at room temperature, into the device, in a quartz cuvette which was illuminated by a laser at a wave length of 633 nm.
Dynamic mechanical analysis (DMA) measurements of NCC-Res.-CBD epoxy nanocomposites were performed using a Q800 DMA (TA Instruments). The analysis was performed under a controlled strain mode, at a frequency of 0.005, 0.01, 1 and 10 Hz and a temperature of 25 °C. Sample dimensions were 2 × 0.3 cm2 and 0.8 mm thick. Stress strain measurements were also obtained using the DMA at a rate of 1 N/min to a maximum load of 18 N.
Preparation of nanocomposite samples
8.25 ml of an aqueous solution containing 2 wt% NCC or Res.-CBD–NCC (10 NCC/1 Res.-CBD wt/wt) were mixed and sonicated with 8 ml EPON 828 to form a modified resin containing 1.75 wt% bio-nano agent. Nanocomposite samples were generated from a mixture of the modified EPON 828 and Epikure 3140 at a ratio of 1 volume of modified EPON 828 to 0.75 volume of Epikure 3140 (Momentive Specialty Chemicals Inc. 2005). A detailed description of the insertion method is provided in “Results and discussion” section. The resin and the hardener were stirred at 65 °C for 3.5 min and then degassed for 15 s, under low vacuum. The adhesive was then cast into aluminum molds, preheated to 65 °C, using a 1 ml syringe. Curing was performed at a temperature of 58 °C for 17 h.
Results and discussion
Avicel® PH-200 microcrystalline cellulose hydrolysis via sulfuric acid and heat treatment resulted in water-dispersed NCC with a sulfur content of 0.34 mmol sulfate half-ester per 1 g of cellulose, as determined by the conductmetric titration assay. After sonication of the acid-treated Avicel® PH-200 microcrystalline cellulose, a rod-like nanoparticle with an aspect ratio of about 1:10 was received (Fig. 1a). The NCC dimensions where measured via image analysis, using ImageJ software (Fig. 1b). The NCC used in this work was 100–200 nm long, with a diameter of 10–15 nm.
Resilin protein purification was carried out using chromatography methods (Guokui et al.), which resulted in highly purified 17Res.-CBD repeats, with a molecular weight of 55 kDa (Fig. 2a, lane T). SDS-PAGE analysis of cellulose binding to purified 17Res.-CBD (Fig. 2a) and to purified 17Res. (Fig. 2b) was performed. Almost all of the 17Res.-CBD was found in the bound fraction, demonstrating its specific binding capacity to cellulose (Fig. 2a, lane B). The 17Res. variant, which served as a negative control, showed only minimal non-specific binding to cellulose (Fig. 2b, lane B).
The first challenge faced when attempting insertion of bio-nano agents such as NCC or Res.-CBD, into non-waterborne commercial epoxy resin, evolves from the differences in their chemical nature. While NCC (Tingaut et al. 2012; Moon et al. 2011) and Res.-CBD (Qin et al. 2011) are dispersed and dissolved in aqueous phases, epoxy resin can be dispersed in organic and hydrophobic phases (Momentive Specialty Chemicals Inc. 2005). In this work, NCC and Res.-CBD–NCC were mixed and sonicated with EPON 828 to form a modified resin. Sonication of the aqueous solutions containing NCC or Res.-CBD–NCC with EPON 828 resulted in the formation of a white emulsion (Fig. 3a).
The emulsion contained tiny aqueous droplets surrounded by the EPON 828 organic phase. When heat was applied, pure NCC droplets tended to coalescence; upon water evaporation, the NCC precipitated from the EPON 828 phase, resulting in the formation of an NCC film on the surface of the EPON 828 phase (Fig. 3b). However, when heat was applied on an emulsion containing Res.-CBD–NCC droplets, the Res.-CBD-modified NCC did not precipitate, but rather, dispersed in the EPON 828 hydrophobic phase (Fig. 3c). The proposed mechanism underlying this phenomenon is discussed in the next sections.
Curing of the EPON 828 epoxy resin requires amine-based hardeners that covalently react with the resin’s epoxide functional groups (Vleeshouwers et al. 1989; Momentive Specialty Chemicals Inc. 2005). Res.-CBD also contains primary and secondary amine groups (Qin et al. 2012); it was therefore assumed that incorporation of the Res.-CBD–NCC in the EPON 828 was achieved via a covalent reaction between amine groups of the Res.-CBD and the epoxide groups of the EPON 828 epoxy resin. In order to verify this assumption, the chemical composition of EPON 828 emulsified by increasing quantities of resilin, was investigated using FTIR spectroscopy (Fig. 4a). The epoxide rings at either side of the EPON 828 monomer (inset of Fig. 4a) correspond to an absorption band at a wave number of 914 cm−1 (Eitan et al. 2003; Schiering et al. 1987). The 1,184 cm−1 band, which was used as internal reference, was assigned to C–C stretching of the carbon atom bridging two p-phenylene groups (inset of Fig. 4a) (Lin et al. 1979; Enns and Gillham 1983). Since this carbon atom does not participate in the reaction, the 1,184 cm−1 band intensity is expected to remain constant and unaffected by the resilin. As the ratio between resilin to EPON 828 increased, the intensity of the normalized epoxide band decreased (Fig. 4b), indicative of a chemical reaction between the amine groups from the resilin and the epoxide groups of the EPON 828. Beside this chemical interaction, the Res.-CBD is also bound to the surface of the NCC via the CBD domain (Goldstein et al. 1993; Boraston et al. 2004). It is therefore suggested that Res.-CBD stabilizes the transfer of NCC from the aqueous phase into the organic epoxy resin phase by opening a small number of the epoxide rings via the protein amine groups, leading to EPON 828 monomer grafting onto the Res.-CBD–NCC structure. Thus, aside from its intended role as a nano-agent with enhances elastic properties, Res.-CBD also serves as a surfactant-like mediator between the hydrophilic NCC and the hydrophobic EPON 828 resin.
As shown in Fig. 3, insertion of the Res.-CBD–NCC into the EPON 828 involved evaporation of water from the emulsion. To further understand the effect of the chemical reaction that takes place at the interface between the aqueous Res.-CBD–NCC droplet phase and the organic epoxy resin phase on the water evaporation rate, a single aqueous 12 µL droplet containing 2.5 wt% of either Res.-CBD–NCC or NCC, which was used as a reference, was inserted into the EPON 828 epoxy resin and maintained in an open cuvette (see Fig. 5a, b). In this configuration, the droplets were contained within the EPON 828 epoxy resin under its upper surface. The system was then brought to a temperature of 70 °C and the droplets were periodically examined through a digital camera. After 3 h at a temperature of 70 °C, the size of the NCC/water droplet was much smaller than the Res.-CBD–NCC/water droplet (see Fig. 5c, d respectively), suggesting a difference in the water evaporation rate between the NCC and Res.-CBD–NCC droplets.
The differences in droplet sizes were quantified by performing precision mass analysis of the cuvettes and monitoring the water mass loss. Variation of water evaporation rates from NCC/water and Res.-CBD–NCC/water droplets solutions through the EPON 828 phase, at a temperature of 70 °C, led to a 50 % reduction in the water content of the Res.-CBD–NCC/water droplet within 0.6 h (Fig. 6a). The NCC/water droplet lost 60 % of its water content within the same time interval. This difference gradually decreased, and after 2.5 h of evaporation, both droplets had lost the same amount of water.
Water evaporated more readily from the NCC/water solution than from the Res.-CBD–NCC/water solution. This finding is somewhat surprising due to the strong hydrogen bonds between NCC hydroxyl groups and the water molecules (Ito et al. 2002). At the same time, the specific binding of Res.-CBD to NCC, as well as its chemical bonding with epoxide groups at the droplet interface, may be the base of the slower rate of evaporation. The chemical reaction between the Res.-CBD amine groups and epoxy resin epoxide groups at the Res.-CBD–NCC/water drop-resin interface forms a shell-like structure which retards the ability of the water to evaporate. This hypothesis was supported by a complementary water evaporation experiment, in which the water evaporation kinetics of pure NCC/water and Res.-CBD–NCC/water EPON 828-free solutions were analyzed (Fig. 6b) by periodically measuring the mass of the solutions. In the absence of epoxide groups, the evaporation kinetics mirrored those presented in Fig. 6a. Namely, water from the Res.-CBD–NCC/water solutions evaporated at a 25 % higher rate than water from the NCC/water solution. Thus, it can be concluded that Res.-CBD not only binds NCC and epoxide monomers but also retards water evaporation from the emulsion. These finding influence the process in which Res.-CBD–NCC is inserted into the epoxy resin, as will be demonstrated in the next section.
This effect was further demonstrated using a series of emulsions, where the Res.-CBD–NCC concentration in EPON 828 was increased from 0.3 to 1.2 wt% (Fig. 7). After proper sonication, the emulsions appeared white, mostly due to the presence of water. After 20 h of evaporation at 60 °C, only the 0.3 wt% Res.-CBD–NCC emulsion lost all of its water and became clear. The 0.6 wt% Res.-CBD–NCC emulsion required 40 h of evaporation to reach the same state. The 0.9 wt% emulsion lost all of its water after 48 h of heating at 70 °C, much like the 1.2 wt% emulsion, which required 52 h of heating. The constant heating accompanied by repetitive sonication was discontinued when the white emulsions turned into a clear light orange epoxy resin. However, it was unclear whether the sonications altered the original dimensions of NCC.
DLS particle size distribution of pristine EPON 828 is characterized by one major peak centered at 9.5 nm. This size distribution peak is attributed to the epoxy resin monomers (Morgan and Oneal 1977) (Fig. 8). The particle size distribution of EPON 828 containing Res.-CBD–NCC showed two major peaks, the first centered at 20 nm, attributed to both the epoxy resin monomers and to the diameter of the NCC, and the second, centered at 200 nm, attributed to the length of the NCC (as in Fig. 1). These DLS results confirm that the EPON 828 epoxy resin is enriched with NCC, which remains intact while maintaining its original dimensions.
NCC can therefore be inserted into an epoxy resin via the recombinant protein Res.-CBD. The insertion mechanism is comprised of the following stages. In the initial emulsion state, an aqueous droplets phase containing Res.-CBD bound to NCC is surrounded by an epoxy resin phase (Fig. 9a). As heat is applied, a chemical reaction takes place between the Res.-CBD amine groups and the epoxy resin epoxide groups positioned at the interface between the phases. As the water evaporates, the insertion process reaches its final stages. In the final product, Res.-CBD is bound to NCC through its CBD group (Boraston et al. 2004; Goldstein et al. 1993) and also covalently bonded to the epoxy resin monomers through the epoxide functional groups (Fig. 9b). In this state, Res.-CBD serves as a surfactant-like molecule, which enables the insertion of NCC into the epoxy resin. In addition, Res.-CBD enhances the epoxy’s mechanical properties.
Schematics of the chemical reaction taking place at the interface between the emulsion’s aqueous droplet phase and the epoxy resin organic continuous phase. Green and red dots indicate Res. and CBD groups respectively, attached to NCC (a). As water evaporates, the Res.-CBD–NCC chemically bonded to the epoxide functional groups is inserted into the epoxy resin organic phase (b). (Color figure online)
A frequency sweep performed using the DMA samples, demonstrated that as the frequency was increased, the tan(δ) values for both pristine epoxy and 1 wt% Res.-CBD–NCC epoxy nanocomposite decreased (Fig. 10a), as reported for other nanocomposite systems as well (Osman and Atallah 2005). This decrease can be attributed to the increase in the epoxy’s storage modulus and a decrease in its loss modulus (Heimann et al. 2009). However, the tan(δ) values of the nanocomposite were approximately 20 % lower compared to those of the pristine epoxy, indicative of the higher elasticity of the nanocomposite (Putz et al. 2007). The improved elasticity is attributed to the mechanical properties of Res.-CBD and to its position between the rigid NCC and the epoxy chains, enabling it to serve as a bearing. The dynamic elastic function of Res.-CBD in its solid state is dependent on the protein-bound water content, which allows β-turn transition (Guokui et al. 2010; Qin et al. 2011, 2012). The bound water molecules begin to evaporate at temperatures above 65 °C and are fully lost at temperatures above 150 °C (Qin et al. 2012). During the Res.-CBD–NCC insertion process for preparation of the DMA samples, the temperature was maintained below 65 °C during the water evaporation stage, thereby maintaining the Res.-CBD-bound water molecules and resilin functionality.
Stress–strain measurements (Fig. 10b) showed a 50 % increase in the nanocomposite’s Young’s modulus (977 MPa for 1 wt% Res.-CBD–NCC epoxy nanocomposite), when compared to the pristine epoxy (640 MPa). This increase is attributed to the mechanical properties of NCC, which has a Young’s modulus of 150 GPa (Iwamoto et al. 2009).
The presented characterization of a nanocomposite containing only 1 wt% of a novel bio-nano agent demonstrates the potential of Res.-CBD–NCC in enhancing the mechanical properties of polymeric matrixes and commercial adhesives.
Summary and conclusions
In this work, a novel and environmentally friendly means of inserting NCC into epoxy resins via the usage of the recombinant protein Res.-CBD, resulted in a novel bio-nanocomposite with enhanced mechanical properties. Res.-CBD is employed as a surfactant-like molecule, benefiting the final nanocomposite material through its unique elastic properties. The insertion mechanism relies on both Res.-CBD binding to the NCC nanorods via the CBD domain and on a chemical reaction between the protein amine groups and the EPON 828 epoxide groups. This novel insertion mechanism enables direct addition of the water-based NCC into the hydrophobic resin without requiring solvent exchange or any chemical modification of the NCC surface.
Mixing and sonication of the aqueous NCC-Res.-CBD phase with the epoxy resin organic phase, results in the formation of an emulsion comprised of tiny aqueous NCC-Res.-CBD-droplets surrounded by the epoxy resin. When heat is applied, two parallel processes occur. The first is a chemical reaction between Res.-CBD amine groups and EPON 828 resin epoxide groups through the interface of the two phases. The second, is the evaporation of water from the aqueous droplets, resulting in a clear epoxy resin which contains Res.-CBD–NCC.
This novel Res.-CBD–NCC insertion mechanism was verified through binding assays, FTIR analysis and evaporation kinetics measurements. Res.-CBD–NCC was also confirmed to reside within the epoxy resin in its original dimensions. When a commercial hardener was added to the Res.-CBD–NCC-containing resin, a new bio-nanocomposite was formed, with a dramatically higher Young’s modulus and enhanced elasticity.
References
Ajayan PM, Schadler LS, Braun PV (2003) Nanocomposite science and technology. Weinheim: WILEY-VCH Verlag GmbH Co. KGaA
Boraston AB, Bolam DN, Gilbert HJ, Davies GJ (2004) Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem J 382:769–781
Cao XD, Habibi Y, Lucia LA (2009) One-pot polymerization, surface grafting, and processing of waterborne polyurethane–cellulose nanocrystal nanocomposites. J Mater Chem 19:7137–7145
Capadona JR, Van Den Berg O, Capadona LA, Schroeter M, Rowan SJ, Tyler DJ, Weder C (2007) A versatile approach for the processing of polymer nanocomposites with self-assembled nanofibre templates. Nat Nanotechnol 2:765–769
Dong XM, Revol JF, Gray DG (1998) Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 5:19–32
Donoughe S, Crall JD, Merz RA, Combes SA (2011) Resilin in dragonfly and damselfly wings and its implications for wing flexibility. J Morphol 272:1409
Eichhorn SJ, Dufresne A, Aranguren M, Marcovich NE, Capadona JR, Rowan SJ, Weder C, Thielemans W, Roman M, Renneckar S, Gindl W, Veigel S, Keckes J, Yano H, Abe K, Nogi M, Nakagaito AN, Mangalam A, Simonsen J, Benight AS, Bismarck A, Berglund LA, Peijs T (2010) Review: current international research into cellulose nanofibres and nanocomposites. J Mater Sci 45:1
Eitan A, Jiang KY, Dukes D, Andrews R, Schadler LS (2003) Surface modification of multiwalled carbon nanotubes: toward the tailoring of the interface in polymer composites. Chem Mater 15:3198–3201
Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, Liyou NE, Wong DCC, Merritt DJ, Dixon NE (2005) Synthesis and properties of crosslinked recombinant pro-resilin. Nature 437:999–1002
Enns JB, Gillham JK (1983) Time temperature transformation (Ttt) cure diagram—modeling the cure behavior of thermosets. J Appl Polym Sci 28:2567–2591
Gao F-F, Jin Y, Chen Z-H, Zhang H, Peng L (2011) Preparation of waterborne epoxy interior coating for container. Electroplat Finish 30:61
Goldstein MA, Takagi M, Hashida S, Shoseyov O, Doi RH, Segel IH (1993) Characterization of the cellulose-binding domain of the Clostridium cellulovorans cellulose-binding protein A. J Bacteriol 175:5762–5768
Guokui Q, Lapidot S, Numata K, Xiao H, Meirovitch S, Dekel M, Podoler I, Shoseyov O, Kaplan DL (2010) 36th Annual northeast bioengineering conference. IEEE, Piscataway, NJ, USA, pp 1–2
Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev 110:3479–3500
Heimann M, Boehme B, Scheffler S, Wirts-Ruetters M, Wolter KJ (2009) Electronic components and technology conference. IEEE, Piscataway, NJ, USA
Hussain F, Hojjati M, Okamoto M, Gorga RE (2006) Review article: polymer-matrix nanocomposites, processing, manufacturing, and application: an overview. J Compos Mater 40:1511
Ito T, Hirata Y, Sawa F, Shirakawa N (2002) Hydrogen bond and crystal deformation of cellulose in sub/super-critical water. Jpn J Appl Phys Part 1 41:5809
Iwamoto S, Kai W, Isogai A, Iwata T (2009) Elastic modulus of single cellulose microfibrils from tunicate measured by atomic force microscopy. Biomacromolecules 10:2571–2576
Jutz G, Boker A (2011) Bionanoparticles as functional macromolecular building blocks—a new class of nanomaterials. Polymer 52:211
Jyi-Jiin L, Daniel IM (2003) Characterization and modeling of mechanical behavior of polymer/clay nanocomposites. Compos Sci Technol 63:1607
Lin SC, Bulkin BJ, Pearce EM (1979) Epoxy-resins. 3. Application of fourier-transform ir to degradation studies of epoxy systems. J Polym Sci Polym Chem 17:3121–3148
Mackay ME, Tuteja A, Duxbury PM, Hawker CJ, Van Horn B, Guan ZB, Chen GH, Krishnan RS (2006) General strategies for nanoparticle dispersion. Science 311:1740–1743
Momentive Specialty Chemicals Inc. (2005) EPON™ resin 828 technical data sheet. http://www.momentive.com/Products/TechnicalDataSheet.aspx?id=3942
Momentive Specialty Chemicals Inc. (2007) EPIKURE™ curing agent 3140 technical data sheet. http://www.momentive.com/Products/TechnicalDataSheet.aspx?id=2654
Monserrate E, Leschine SB, Canale-Parola E (2001) Clostridium hungatei sp nov., a mesophilic, N-2-fixing cellulolytic bacterium isolated from soil. Int J Syst Evol Microbiol 51:123–132
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–3994
Morgan RJ, Oneal JE (1977) Microscopic failure processes and their relation to structure of amine-cured bis-phenol-a-diglycidyl ether epoxies. J Mater Sci 12:1966–1980
Osman MA, Atallah A (2005) Interparticle and particle-matrix interactions in polyethylene reinforcement and viscoelasticity. Polymer 46:9476
Pan HF, Song L, Ma LY, Hu Y (2012) Transparent epoxy acrylate resin nanocomposites reinforced with cellulose nanocrystals. Ind Eng Chem Res 51:16326–16332
Pereira CMC (2009) Flame retardancy of thermoset polymers based on nanoparticles and carbon nanotubes. Diffus Defect Data Part B Solid State Phenom 151:79
Putz K, Krishnamoorti R, Green PF (2007) The role of interfacial interactions in the dynamic mechanical response of functionalized SWNT-PS nanocomposites. Polymer 48:3540–3545
Qin GK, Rivkin A, Lapidot S, Hu X, Preis I, Arinus SB, Dgany O, Shoseyov O, Kaplan DL (2011) Recombinant exon-encoded resilins for elastomeric biomaterials. Biomaterials 32:9231–9243
Qin GK, Hu X, Cebe P, Kaplan DL (2012) Mechanism of resilin elasticity. Nat Commun 3:1–9
Reddy B (2011) Advances in diverse industrial applications of nanocomposites. InTech, Rijeka
Rhim J-W, Park H-M, Ha C-S (2013) Bio-nanocomposites for food packaging applications. Prog Polym Sci 38:1629
Risbud SH (2004) Assembling quantum dots in glasses and polymers. SPIE - The International Society for Optical Engineering 5359:246
Rivkin A, Meirovitch S, Roth S, Arinos SB, Nevo Y, Dgany O, Preis I, Kaplan DL, Shoseyov O, Lapidot S (2013) Bio-Inspired elastic composites of nano crystalline cellulose and cellulose binding resilin. In: Madsen B, Lilholt H, Kusano Y, Feaster S, Ralph B (ed) 34th Riso international symposium on materials science: processing of fibre composites-challenge for maximum materials performance, Technical University of Denmark, Roskilde, pp 93–107
Ruiz MM, Cavaille JY, Dufresne A, Gerard JF, Graillat C (2000) Processing and characterization of new thermoset nanocomposites based on cellulose whiskers. Compos Interfaces 7:117–131
Ruiz MM, Cavaille JY, Dufresne A, Graillat C, Gerard JF (2001) New waterborne epoxy coatings based on cellulose nanofillers. Macromol Symp 169:211–222
Sanjines R, Abad MD, Vaju C, Smajda R, Mionic M, Magrez A (2011) Electrical properties and applications of carbon based nanocomposite materials: an overview. Surf Coat Technol 206:727
Schiering DW, Katon JE, Drzal LT, Gupta VB (1987) An infrared spectroscopic investigation of the curing reactions of the epon-828 meta-phenylenediamine system. J Appl Polym Sci 34:2367–2375
Siro I, Plackett D (2010) Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17:459–494
Sturcova A, Davies GR, Eichhorn SJ (2005) Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules 6:1055–1061
Tanaka T (2009) Polymer nanocomposite innovating on insulating materials. IEEJ Trans Electr Electron Eng 4:8
Tang LM, Weder C (2010) Cellulose whisker/epoxy resin nanocomposites (vol 2, p. 1073, 2010). ACS Appl Mater Interfaces 2:3396
Tingaut P, Zimmermann T, Sebe G (2012) Cellulose nanocrystals and microfibrillated cellulose as building blocks for the design of hierarchical functional materials. J Mater Chem 22:20105–20111
Vleeshouwers S, Jamieson AM, Simha R (1989) Effect of physical aging on tensile-stress relaxation and tensile creep of cured epon-828 epoxy adhesives in the linear viscoelastic region. Polym Eng Sci 29:662–670
Xin Z, Lu L, Bingcai P, Weiming Z, Shujuan Z, Quanxing Z (2011) Polymer-supported nanocomposites for environmental application: a review. Chem Eng J 170:381
Xu S, Girouard N, Schueneman G, Shofner ML, Meredith JC (2013) Mechanical and thermal properties of waterborne epoxy composites containing cellulose nanocrystals. Polymer (United Kingdom) 54:6589
Zhong-Hua C, Ying T, Fei Y, Jian-Hua C, Hai-Hong C (2008) Preparation of light color antistatic and anticorrosive waterborne epoxy coating for oil tanks. J Coat Technol Res 5:259
Author information
Authors and Affiliations
Corresponding author
Additional information
R. Verker and A. Rivkin have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Verker, R., Rivkin, A., Zilberman, G. et al. Insertion of nano-crystalline cellulose into epoxy resin via resilin to construct a novel elastic adhesive. Cellulose 21, 4369–4379 (2014). https://doi.org/10.1007/s10570-014-0460-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0460-7