Abstract
Epithelial-mesenchymal transition (EMT) is a plastic transition in tumor progression during which cancer cells undergo dramatic changes acquiring highly invasive properties. Transforming growth factor-β (TGF-β) is an inducer of EMT in epithelial cells and is obligatory for acquiring invasive phenotype in carcinoma. TGF-β plays a vital role in metastasis and tumorigenesis in prostate cancer, and mutations in the components of Wnt signaling pathways are associated with various kinds of cancers including prostate cancer. The purpose of this study was to identify alterations in Wnt signaling pathway components involved during prostate cancer progression and to determine the effect of quercetin on TGF-β-induced EMT in prostate cancer (PC-3) cell line. The expression of epithelial and mesenchymal markers and the components of Wnt signaling pathway were evaluated by real-time polymerase chain reaction. It was observed that quercetin prevented TGF-β-induced expression of vimentin and N-cadherin and increased the expression of E-cadherin in PC-3 cells, thus preventing TGF-β-induced EMT. Furthermore, the relative expression of Twist, Snail, and Slug showed that quercetin significantly decreased TGF-β-induced expression of Twist, Snail, and Slug. In the present study, the expression of epithelial markers were found to be upregulated in naive state and downregulated in induced state whereas the mesenchymal markers were found to be downregulated in naive state and upregulated in induced state. Thus, our study concludes that quercetin may prevent prostate cancer metastasis by regulating the components of Wnt pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) remains one of the major medical burdens in males. The estimated number of new cases and deaths from PCa in USA in 2015 are 220,800 and 27,540, respectively [1]. The mortality rate of metastatic prostate cancer is even higher despite the chemotherapies. Thus, there is an urgent need for therapies for the malignant forms of cancer.
Cancer cells undergo dynamic changes to acquire invasive phenotype, and epithelial-mesenchymal transition (EMT) plays an important role in the metastasis of tumors of epithelial origin [2]. It is a developmental program where there is downregulation of epithelial characteristics and upregulation of mesenchymal phenotypes. Loss of E-cadherin is associated with PCa progression and Gleason grade [3], indicating EMT to be a potent player of PCa metastasis. EMT is involved in the formation of many tissues and organs during development [4]. Transforming growth factor-β (TGF-β) is an inducer of EMT in epithelial cells and is obligatory for acquiring invasive phenotype in carcinoma [5, 6]. TGF-β acts as a potent inhibitor of epithelial cell proliferation, whereas in contexts with cancer progression, TGF-β promotes tumor cell survival through autocrine/paracrine interactions within tumor microenvironment. TGF-β also enhances tumor cell proliferation and simultaneously inhibits tumoricidal activity of the immune system, thus accelerating tumor progression [7]. Several transcriptional factors are involved in the downregulation of EMT such as two members of the Snail superfamily of zinc finger proteins namely Snail and Slug. Smad interacting protein-1 (SIP1) is another zinc finger protein that represses E-cadherin transcription via promoter binding. Another transcriptional factor that triggers EMT is Twist which is essential for transcribing N-cadherin expression. Twist is believed to be involved in E-cadherin to N-cadherin transition in EMT.
Increasing evidences reveal that various other molecular signaling pathways, such as Notch, Hedgehog, and specifically Wnt signaling pathway, induce EMT. Differential expression of Wnt is often correlated with the aggressiveness associated with prostate cancer [8], and the high rates of Wnt signaling pathway mutations in many different cancers and their association with initiation and progression of various kinds of tumors reveal their importance to carcinogenesis [9]. Thus, studying the molecular mechanisms underlying the association of Wnt activation and prostate cancer aggressiveness may prove to be an important step toward the cure for prostate cancer metastasis [10] .
Cancer chemoprevention, by use of natural or dietary agents, has become an appealing strategy to combat increasing cases of cancers. Several epidemiological and preclinical studies have emphasized on the potential benefits on flavonoids for cancer prevention and one such flavonoid widely reported is quercetin [11–13]. Quercetin shows a variety of anticancer effects, such as cell growth, kinase activity inhibition, apoptosis induction, differentiation, and suppression of the secretion of matrix metalloproteinases (MMPs). It has also been reported to show a reduction in tumor cell adhesion, invasive behavior, metastasis, and angiogenesis [14, 15]. Thus, it inspired us to evaluate the impact of quercetin on events such as tumor progression and invasion.
In the present study, we used PC-3 cells and EMT was induced by TGF-β. Subsequently, the effect of quercetin on the Wnt signaling molecules and EMT markers were studied at the molecular as well as at the protein level. To further validate our results at the molecular level, we have also studied the effect of quercetin on transcriptional repressors that regulate EMT. It has been reported that quercetin is a known inhibitor of Wnt in colon cancer [16]. Thus, in our present study, we have investigated the effect of quercetin on the process of EMT in PC-3 cells.
Materials and methods
Cell culture
PC-3 cell line was obtained from National Center for Cell Science (NCCS), Pune, India. The cells were of epithelial prostate grade IV, adenocarcinoma origin, and are adherent in nature. The cells were maintained in Rosewell Park Memorial Institute medium (RPMI 1640) with 10 % fetal bovine serum, 2 mM l-glutamine, and 100 U/ml penicillin–streptomycin solution (Invitrogen) under 5 % CO2 and humidified condition at 37 °C. When the cells were confluent, they were trypsinized and passaged. Cells were exposed to 10ng/mL of Human recombinant TGF-β to induce EMT.
3-4,5-Dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide (MTT) assay
To determine the proliferative activity, the PC-3 cells were seeded in three 96-well plates at a density (1 × 104 cells/well) based on the doubling time, with 200 μl of growth media (10 % fetal bovine serum (FBS)) and incubated for 24 h in an incubator with 5 % CO2 concentrated at 37 °C. The flavonoid, quercetin, was diluted into 14 different concentrations in the growth media. Simultaneously, the cells were treated with the solvent control (DMSO) to see its effect on cells. After 24 h, the old media was replaced with fresh growth media containing different concentrations of quercetin and was incubated for 24 and 48 h at 37 °C. To detect the cell viability, MTT working solution was prepared from the stock solution (stock 1 mg/ml PBS, pH 7.2) in growth medium with FBS. After 24 h of quercetin treatment, 50 μl of MTT working solution was added to each well and incubated for 4 h in a CO2 incubator. After incubation, the formazan precipitate obtained was dissolved in 150 μl of extraction fluid (0.1 N HCl and isopropanol). The colorimetric estimation of the formazan product was done at 570 nm using a microplate reader. The data was plotted against quercetin concentration and percent proliferation was calculated using the following formula: % proliferation = absorbance of treated cells / absorbance of control cells × 100.
Cell migration assay (scratch assay)
PC-3 cells were seeded (2 × 104 cells/well) in 24-well plates and incubated to form a confluent monolayer at 37 °C. After incubation for 24 h, the old media was removed and the monolayer of the cell was scraped with a sterile 200-μL tip to form a “scratch.” All the wells were washed with plain media to remove the debris. The cells were washed with 1 ml of growth media and then replaced with media containing additives (such as TGF-β and quercetin + TGF-β). Pictures were taken using an inverted microscope at different time points (0, 24, 48 h).
Colony forming assay (soft agar assay)
Cancer stem cells (CSCs) and normal stem cells have an important property of forming spheres and forming colonies in serum-free medium or in soft agar medium. To detect the transformation of cells, cells were harvested and suspended (5 × 10 cells/well) in serum-free medium supplemented with different concentrations of quercetin. One percent of base agar layer and 0.7 % top agar solution were used.
Cell cycle analysis
Propidium iodide (PI) staining was done to perform cell cycle analysis by FACS [17]. PC-3 cells were serum starved for 4 h followed by treatment with 20 and 25 μM concentration of quercetin. Cells were harvested and florescence-activated cell sorting analysis was performed using FACS Calibur (BD Science), and the percentage of cells in the G0-G1 and G2-M phases were observed.
Apoptosis detection
Caspase 3 assay kit (Sigma Aldrich) was used to analyze the activity of caspase 3. Cells were trypsinized and harvested in the concentration of 2 × 106 cells/ml. The cell lysate was prepared using the 1× lysis buffer provided with the kit. The lysates were transferred to 96-well plate. A peptide with a caspase 3 target motif DEVD bound to the chromophore p-nitroaniline (pNA) was added and incubated for 2 h at 37 °C. The absorbance was measured at 405 nm using a microplate reader.
Annexin V staining
Annexin V staining assay kit (Invitrogen) was used to differentiate between apoptotic and necrotic cells. Cells were treated with 20 and 25 μM quercetin for 24 and 48 h followed by harvesting in the concentration of 1 × 106 cells/ml. The treated and non-treated cells were stained with FITC-conjugated Annexin V and PI, according to the manufacturer’s instructions. Stained cells were analyzed by flow cytometry and data were analyzed by use of CellQuestPro (Becton-Dickinson, Sunnyvale, Calif).
Isolation of total cellular RNA and real-time PCR
Total cellular RNA was extracted from PC-3 cells using TRI reagent (Sigma) following the manufacturer’s instructions. Final preparation of RNA was estimated using a NanoDrop UV spectrophotometer. The isolated RNA was found to be pure and without any proteins and oligopeptide contamination. cDNA was synthesized from 2 μg of total cellular RNA by reverse transcription (High-Capacity cDNA Reverse Transcription kit ) (Himedia). Real-time PCR was carried out in Applied Biosystems Stepone Plus. RT-PCR was performed using SYBR green dye and the endogenous control used was β-actin. The data were normalized by comparing the threshold cycle ratios between the target genes and housekeeping gene β-actin. The data were analyzed by the comparative CT method [18]. The primers used were as follows: E-Cadherin, N-cadherin, cyclin D1, Twist, Snail, Slug, vimentin, Zeb1, MMP-9, β-catenin, survivin, and HIF-1α (Supplementary Table 1).
Western blot analysis
The cells were harvested after treatment with TGF-β (10 ng/ml), quercetin (20 μM), and quercetin (20 μM) + TGF-β (10 ng/ml) for 24 and 48 h followed by washing of the cell lysate with PBS. Radioimmunoprecipitation assay (RIPA) buffer and 1× protease inhibitor cocktail was added three times the dry weight of the cell pellet and was subsequently centrifuged at 10,000 rpm for 15 min to remove the cell debris, and supernatant was stored at −80 °C. The protein samples were estimated using Bradford assay. The membranes were then incubated with primary antibodies against cyclin D1 (1:500 dilution) and β-catenin (1:200 dilution) in PBS at 4 °C overnight. The membranes were then washed and incubated with horseradish peroxidase (HRP)-conjugated secondary anti-mouse IgG antibody (1:5000 dilution) or HRP-conjugated secondary anti-rabbit IgG antibody (1:5000 dilution) for 1 h at room temperature. The blots were visualized by enhanced chemiluminescence by using gel doc (Diversity 4 by syngene).
Statistical analysis
Data were expressed as mean ± S.E.M. Statistical analysis were performed using Student’s t test for comparing treatment values and control values using GraphPad Prism. P < 0.05 was considered to be statistically significant. The significance level was defined as *P < 0.05, **P < 0.01, and *** P < 0.001.
Results
Cell viability assay
Cell viability assay was performed to optimize the concentration of MTT and the cell number for the PC-3 cell line (Fig. 1a). The MTT stock concentration of 1 mg/ml and cell count of 1 × 104 were found to be optimum values.
The effect of quercetin on the viability and proliferation of PC-3 cells. a Proliferation curve: the effect of quercetin on the cell viability of PC-3 cells by MTT assay at 24-, 48-, and 72-h duration. b Inhibitory effect of quercetin on the proliferation of PC-3 cells after 24 and 48 h of treatment
IC50 value
The inhibitory effect of quercetin on the proliferation of the PC-3 cells was determined as described earlier in the “Materials and methods” section. The results showed that cell proliferation was inhibited both in a dose- and time-dependent manner. The IC50 was found to be 20 μ M (Fig. 1b).
Effect of quercetin on cell cycle
The effect of quercetin on cell cycle was analyzed by flow cytometry, and it was seen that in comparison to untreated cells, treatment with quercetin showed increase in the G2-M and S phase population and decrease in the G0-G1 phase. Also, the percentage of apoptotic cells increased remarkably upon quercetin treatment. Thus, quercetin affects all the stages of cell cycle, arresting cell progression and proliferation and ultimately increasing the rate of apoptosis in the cell population (Fig. 2a).
In vitro assessments of apoptosis in PC-3 cells by flow cytometry. a Analysis of the subpopulation of PC-3 cells in cell cycle phases G1, S, and G2/M and apoptotic cells. b Quantitation of cleaved caspase 3 in PC-3 cells treated with 20 and 25 μM quercetin for 24 and 48 h. c Flow cytometry plots of Annexin V-FITC and PI. Annexin V-FITC staining on the x-axis and PI on the y-axis. Cells in the lower right quadrant indicate Annexin-positive, early-apoptotic cells. Cells in the upper right quadrant indicate Annexin-positive/PI-positive, necrotic cells
Induction of apoptosis by quercetin
Caspase-3 activity, the biomarker of apoptosis, was determined in PC-3 cells, [19] posttreatment with 20 and 25 μM of quercetin at 24 and 48 h. Quercetin at both 20 and 25 μM concentrations significantly increased caspase 3 activity after 24 h of treatment, indicating apoptosis induction by quercetin (Fig. 2b); however, the increase was statistically significant at 20 μM.
Annexin V staining
The exposure of phosphatidylserine to the surface was studied by using fluorescein isothiocyanate-conjugated Annexin V (FITC-conjugated Annexin V) followed by flow cytometry analysis [20]. To differentiate between apoptotic and necrotic cells, cells were simultaneously stained with propidium iodide (PI). After 24 h, control cells showed negligible levels of Annexin V and PI staining (1.16 %) and the major percentage of cells was viable (Fig. 2c). Cells treated with 20 μM quercetin for 24 h showed an elevated level of apoptotic cells with 5.50 % of cells in the lower right quadrant (FITC+/PI−) (Fig. 2c). After treatment with 25 μM quercetin for 24 h, a slightly lower percentage of cells were in the lower right quadrant (4.88 %) (Fig. 2c). Similarly, after 48 h of treatment with 20 μM quercetin, 7.04 % of the cell population was in the upper right quadrant (FITC+/PI+) and 8.67 % of the cell population was FITC+/PI− (Fig. 2c). After treatment with 25 μM quercetin for 48 h, 8.16 % of the cell population was in the upper right quadrant (FITC+/PI+) and 7.04 % of the cell population was FITC+/PI+ (Fig. 2c). Among control cells after 48 h, 4.67 % of the cell population was FITC+/PI+ and a very negligible level (0.77 %) of the cell population was FITC+/PI− (Fig. 2c). This result indicates that there is a significant increase in the relative number of apoptotic cells (FITC+/PI−), but not of necrotic cells (FITC+/PI+) with increase in time and dose of quercetin (Fig. 2c).
Effect of quercetin on TGF-β-induced cell migration
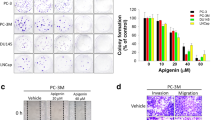
Migration of PC-3 cells was studied by wound healing (scratch assay) assay, which showed that cancer cell migration induced by TGF-β was significantly decreased upon quercetin treatment. It was observed that the gap was completely sealed after 48 h of TGF-β treatment. However, treatment of quercetin prevented the migration of cells and thus sealing of gap was not observed (Fig. 3a).
Effect of quercetin on the colony-forming ability of PC-3 cells
Quercetin at concentration of 20 μM significantly inhibited the colony-forming ability of PC-3 cells compared to the untreated control cultures (Fig. 3b). Lower concentrations of quercetin (<20 μM) did not have any significant effect on the colony-forming ability of PC-3 cells.
Effect of TGF-β and quercetin on transcriptional repressors
To understand the underlying mechanism by which quercetin regulates EMT, the effect of quercetin and TGF-β on transcriptional repressors Twist, Snail, and Slug were studied by real-time PCR (Fig. 4a). Treatment with TGF-β significantly increased Twist, Snail, and Slug expressions in PC-3 cells, whereas quercetin significantly decreased the expression of all these markers.
The effect of quercetin on the mRNA expression of a transcriptional repressors: real-time PCR data for Snail, Slug, and Twist were analyzed by the comparative CT method, and b EMT markers: the mRNA expression of E-cadherin, N-cadherin, and vimentin by real-time PCR. The real-time PCR data were analyzed by comparative CT method
Effect of quercetin on TGF-β-induced EMT
To determine the regulatory potential of quercetin on TGF-β-induced EMT, the expression levels of the characteristic markers of EMT viz. N-cadherin, E-cadherin, and vimentin were studied by real-time PCR. The expression of E-cadherin showed significant decrease post-TGF-β treatment, whereas treatment with quercetin reestablished its expression. On the contrary to E-cadherin expression level, the mesenchymal phenotype markers viz. N-cadherin and vimentin revealed significant increase post-TGF-β treatment, whereas quercetin treatment reduced N-cadherin and vimentin expression (Fig. 4b).
Effect of TGF-β and quercetin on Wnt signaling components
β-catenin and cyclin D1 are two components of Wnt signaling pathway that increases significantly and accumulates in the nucleus with activation of the Wnt signaling pathway. To determine the effect of TGF-β and quercetin on Wnt signaling components, real-time PCR was performed to analyze the expression of β-catenin and cyclin D1. The expression of β-catenin and cyclin D1 increased significantly upon TGF-β treatment, while quercetin impaired the expression level of both these genes (Fig. 5a). The protein expression of Wnt signaling pathways viz., β-catenin and cyclin D1 were downregulated upon quercetin treatment, which further validates real-time PCR results (Fig. 5b).
The effect of quercetin on the mRNA expression of a Wnt proteins: real-time PCR data for β-catenin and cyclin D1 were analyzed by the comparative CT method. b Western blot data of β-catenin and Cyclin D1 proteins in control PC3 cells (L1), TGF-β (10ng/mL) treated PC3 cells (L2), Quercetin (20μM) treated PC3 cells (L3) and Quercetin (20μM) and TGF-β (10ng/mL) treated PC3 cells (L4). c The effect of quercetin on the mRNA expression of MMP-9 by real-time PCR. The real-time PCR data were analyzed by comparative CT method
Effect of TGF-β and quercetin on ECM proteins
EMT in adult tissues is regulated by the composition and structure of the extra cellular matrix (ECM) components and ECM-remodeling matrix metalloproteinases (MMPs) [21, 22]. To determine the effect of TGF-β and quercetin on matrix metalloproteinases such as MMP-9, real-time PCR was performed. The expression of MMP-9 significantly increased with TGF-β treatment, whereas exposure to quercetin decreased the expression of MMP-9 (Fig. 5c).
Effect of TGF-β and quercetin on the IAP family
Survivin, encoded by the BIRC5 gene in humans, functions as inhibitor of apoptosis and regulates transformed cells by increasing their resistance to apoptosis [23]. To determine the effect of TGF-β and quercetin on survivin, real-time PCR was performed. It was observed that the expression of survivin significantly increased with TGF-β treatment, whereas exposure to quercetin decreased the expression of survivin (Fig. 6a).
Effect of quercetin on HIF-1α
Hypoxia-inducible factor 1-alpha (HIF-1α), encoded by the HIF1A gene, is the subunit of a heterodimeric transcription factor HIF-1. The overexpression of HIF-1α is associated with various kinds of tumors including prostate cancer, and it correlates with abnormal accumulation of the p53 gene and proliferation of cells. [24]. Also, to correlate the tumor-forming ability of PC-3 cells with HIF-1α expression, mediating hypoxic adaptation of PC-3 cells, we screened the expression of HIF-1α both in control and quercetin-treated samples. The effect of quercetin on the expression of HIF-1α was studied using real-time PCR (Fig. 6b). Treatment with quercetin significantly decreased the expression of HIF-1α in a dose- and time-dependent manner.
Discussion
EMT is one of the important fundamental processes involved in embryogenesis [2] and has a potent role in the developmental stages [4]. EMT reorganizes cellular as well as ECM architecture that overall compromises the integrity of cell–cell and cell–matrix environment and, consequently, the physiological integrity of the tissue and thereby mediates mesenchymal phenotypes to cancerous cells leading to metastasis [25–27].
TGF-β is a potent inducer of EMT through Smad signaling [28, 29]. TGF-β induces expressions of Snail and Slug via activating Smad signaling or via PI3K and ERK pathways, thereby inhibiting the expression of E-cadherin and regulating EMT during development and migration of both normal and cancerous cells [30, 31]. In addition TGF-β induces expression of Zeb1 and SIP1. SIP1 forms complex with pSmads and represses E-cadherin expression [32]. Smad complexes also induce expressions of mesenchymal markers viz., N-cadherin and vimentin and a SMA [25]. TGF-β is considered to be the prototypical cytokine for the induction of EMT because different isoforms mediate various aspects of EMT in many diverse cellular contexts, whereas the effects of other EMT inducers are often context dependent and variable [33].
Various plant flavonoids have been reported to have roles in inhibiting EMT and impairing metastasis in cancer [34, 35]. Quercetin, a plant flavonoid, acts both as an antioxidant and prooxidant. Flavonoid antioxidants limit the negative effects of free radicals through the quick transfer of hydrogen atoms to the radicals [36]. The anticancer activity of quercetin on prostate cancer cells (PC-3) was studied by performing MTT assay and flow cytometry. The inhibitory concentration 50 (IC50 ) value of quercetin was found to be 20 μM for PC-3 cells. Analysis of the effect of quercetin revealed that cells treated with quercetin showed increase in the G2-M phase and S phase population and decline in the G0-G1 phase as well as increase in apoptotic cells as compared to control untreated cells. Thus, quercetin affects all the stages of cell cycle, arresting cell progression, and proliferation and ultimately increasing the rate of apoptosis in the cell population. In addition, the effect of quercetin on the caspase 3 activity of PC-3 cells was quantified to determine its effect on apoptosis. Quercetin significantly increased caspase 3 activity in PC-3 cells at 20 μM concentration after 24 h of treatment. Further, to differentiate between viable, apoptotic, and necrotic cell population, Annexin V-FITC/PI double-staining was performed. After 24 h of treatment with 20 and 25 μM quercetin, the percentage of apoptotic cells increased from 0.13 to 5.50 % and 4.88 %, respectively. Similarly, after 48 h of treatment with 20 and 25 μM quercetin, the percentage of apoptotic cells increased from 0.77 to 8.67 % and 7.04 %, respectively. Thus, the above results show that quercetin at 20 μM induced the highest percentage of apoptosis in PC-3 cells after 48 h of treatment.
Moreover, to identify the effect of quercetin on the morphological transformation of PC-3 cell colonies, soft agar colony forming assay was performed for 192 h and the colonies were photographed. The results showed that quercetin has the potential to affect certain phenotypic properties of PC-3 cells such as loss of contact inhibition and anchorage independence in the 20 μM concentration. Also, to correlate the tumor-forming ability of PC-3 cells with HIF-1α expression, mediating hypoxic adaptation of PC-3 cells, we screened the expression of HIF-1α both in control and quercetin-treated samples. To understand the affect of quercetin on mRNA expression of HIF-1α, real-time PCR was performed. HIF-1α mediates hypoxic responses and regulates genes responsible for tumor angiogenesis. Treatment with quercetin significantly downregulated the mRNA expression of HIF-1α in a time- and dose-dependent manner, which further strengthens our results.
The overexpression of Slug is associated with tumor metastasis in prostate cancer cells [37]. In our study, we found that increased expression of Slug correlated with the migration of PC-3 cells. In contrast, reduced expression of Slug impaired migration in PC-3 cells. Thus, it can be concluded that quercetin affects migration in PC-3 cells by regulation of Slug. In addition, in pancreatic cancer cells, a higher expression of Slug correlates with a higher expression of MMP-9 [38]. In our study, we also found that Slug promotes migration in PC-3 cells with the upregulation of MMP-9 and downregulation of E-cadherin.
Further, to understand the molecular basis of how quercetin reverses TGF-β-induced EMT, the effect of quercetin on transcriptional repressors viz., Snail, Twist, and Slug, was studied by RT-PCR. Recent data suggested that Snail and Slug plays a potent role in the process of invasiveness and tumorigenicity, by regulating the expression of E-cadherin [39]. Twist suppresses the transcription of E-cadherin by binding to E-boxes on the E-cadherin promoter and also induces the expression of mesenchymal markers, such as fibronectin and N-cadherin, during EMT [40]. RT-PCR data suggested that there is decline in the expression of Snail, Twist, and Slug upon quercetin treatment which indicated that quercetin has the potential to reverse TGF-β-induced EMT and restrain the expression of E-cadherin, thereby inducing MET.
The key EMT markers viz., E-cadherin, N-cadherin, and vimentin, were studied by RT-PCR. The mechanism of EMT is associated with the common feature of decreased expression of E-cadherin with increased expression of N-cadherin and vimentin. N-cadherin promotes motility and vimentin is an important regulator of cell motility. Thus, the downregulation of E-cadherin with the upregulation of N-cadherin and vimentin is a prerequisite for EMT induction. In our work, quercetin upregulated E-cadherin and downregulated N-cadherin and vimentin thereby working as a preventer of TGF-β-induced EMT in PC-3 cells.
The functional loss of E-cadherin: the hallmark of EMT is associated with β-catenin, a transcriptional signaling pathway regulating EMT. The loss of E-cadherin from the adherens junction is followed by accumulation of β-catenin in the cytosol, and finally, it transfers to the nucleus where it transcribes Slug and Twist, mediated by the activation of LEF/TCF, and thereby inducing EMT [2]. Two components of the Wnt signaling pathway, cyclin D1 and β-catenin, were found to be upregulated with TGF-β treatment, whereas quercetin treatment restored the same. Also, the protein expression of these two components revealed significant decrease in level by treatment with quercetin.
Our findings of quercetin as an inducer of MET were further supported and strengthened by the observed upregulated expression of ECM proteins such as MMP-9 and the inhibitor of apoptosis (IAP) family such as survivin.
In a similar study, the authors have shown the potential of quercetin to inhibit epidermal growth factor (EGF)-induced EMT in PC-3 cells by regulating the EGFR/PI3K/Akt pathway [41]. Also, quercetin is a potent inhibitor of Wnt signaling components in breast cancer, B-cell lymphomas [42, 43], and leukemia [44]; however, the role of quercetin in regulating Wnt signaling components in prostate cancer has not been addressed to the best of our knowledge.
To conclude, the present study illustrates the potency of quercetin in preventing TGF-β-induced EMT process by suppressing the expression of mesenchymal markers viz., N-cadherin and vimentin, and increasing expression of epithelial marker E-cadherin in PC-3 cells. These findings are supported by the downregulation of expression of ECM proteins such as MMP-9 and Twist: a known master regulator of EMT. Reduced E-cadherin expression has been found in high-grade prostate cancers and is associated with poor prognosis, reflecting its critical role in tumor progression [45]. Our study has revealed that quercetin enhances E-cadherin expression; thus, quercetin appears to be a potential agent which can be used to target and prevent EMT in prostate cancer.
References
Siegel R, Miller K, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65(1):29.
Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–29.
Grant CM, Kyprianou N. Epithelial mesenchymal transition (EMT) in prostate growth and tumor progression. Transl Androl Urol. 2013;2(4):202–11.
Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90.
Moustakas A, Heldin P. Biochimica et Biophysica Acta TGF β and matrix-regulated epithelial to mesenchymal transition ☆. BBA - Gen Subj. 2014;1840(8):2621–2634.
Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J, et al. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109(12):1551–9.
Zavadil J, Böttinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764–74.
Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. Nature Publishing Group2012;13(1):11–26.
Valkenburg KC, Graveel CR, Zylstra-Diegel CR, Zhong Z, Williams BO. Wnt/β-catenin signaling in normal and cancer stem cells. Cancers (Basel). 2011;3(2):2050–79.
Baruah MM, Sharma N. Effect of flavonoid on reversal of TGF-β induced epithelial to mesenchymal transition in prostate cancer (PC-3) cell line. Ind J Clin Biochem. 2015;30(1):S83.
Chirumbolo S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm Allergy Drug Targets. 2010;9(4):263–85.
Papiez MA, Cierniak A, Krzysciak W, Bzowska M, Taha HM, Jozkowicz A, et al. The changes of antioxidant defense system caused by quercetin administration do not lead to DNA damage and apoptosis in the spleen and bone marrow cells of rats. Food Chem Toxicol. 2008;46(9):3053–8.
Baruah MM, Sharma N, Khandwekar AP, Lavale G-, Author C. Flavonoids and Prostate Cancer. AIJRFANS. 2016;15(1):01–07.
Kim GT, Lee SH, Kim II J, Kim YM. Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner. Int J Mol Med. 2014;33(4):863–9.
Chan S-T, Yang N-C, Huang C-S, Liao J-W, Yeh S-L. Quercetin enhances the antitumor activity of trichostatin a through upregulation of p53 protein expression in vitro and in vivo. PLoS One 2013;8(1):e54255.
Shan B-E, Wang M-X, Li R. Quercetin inhibit human SW480 colon cancer growth in association with inhibition of cyclin D1 and survivin expression through Wnt/beta-catenin signaling pathway. Cancer Investig. 2009;27(6):604–12.
Pozarowski P, Darzynkiewicz Z. Analysis of cell cycle by flow cytometry. Methods Mol Biol. 2004;281:301–11.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–8.
BD Biosciences. Caspase-3 Activation: an indicator of apoptosis in image-based assays. BD Biosciences. 2012. https://www.bdbiosciences.com/documents/Bioimaging_AppNote_Apoptosis.pdf. Accessed 10 Nov 2015.
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184(1):39–51.
Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118(19):4325–6.
Nistico P, Bissell MJ, Radisky DC. Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb Perspect Biol. 2012;4(2):a011908.
Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–21.
Zhong H, Marzo AM De, Laughner E, Lim M, Hilton D A, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1α in common human cancers and their metastases 1. New York. 2000;(22):5830–5.
Son H, Moon A. Epithelial-mesenchymal transition and cell invasion. Toxicol Res. 2010;26(4):245–52.
Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–69.
López-Nouoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1(6–7):303–14.
Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor-β employs HMGA2 to elicit epithelial-mesenchymal transition. J Cell Biol. 2006;174(2):175–83.
Nawshad A, LaGamba D, Polad A, Hay ED. Transforming growth factor-β signaling during epithelial-mesenchymal transformation: implications for embryogenesis and tumor metastasis. Cells Tissues Organs. 2005;179(1–2):11–23.
Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24(50):7443–54.
Malage RD. Studies on regulation of TGF-β and markers of epithelial to mesenchymal transition in breast cancer cells. Dissertation, Jawaharlal Nehru University. 2008;110067.
Chen D, Wilkinson CRM, Watt S, Penkett CJ, Toone WM, Jones N, et al. Multiple pathways differentially regulate global oxidative stress responses in fission yeast. Mol Biol Cell. 2008;19(1):308–17.
Moustakas A, Heldin C-HH. The regulation of TGFbeta signal transduction. Development. 2009;136(22):3699–714.
Lin YS, Tsai PH, Kandaswami CC, Cheng CH, Ke FC, Lee PP, et al. Effects of dietary flavonoids, luteolin, and quercetin on the reversal of epithelial-mesenchymal transition in A431 epidermal cancer cells. Cancer Sci. 2011;102(10):1829–39.
Deng XH, Song HY, Zhou YF, Yuan GY, Zheng FJ. Effects of quercetin on the proliferation of breast cancer cells and expression of survivin in vitro. Exp Ther Med. 2013;6(5):1155–8.
Salem-Alrawaiq N, Abdullah A. A review of flavonoid quercetin: metabolism, bioactivity and antioxidant properties. Int J PharmTech Res. 2014;6(3):933–41.
Uygur B, Wu W-S. SLUG promotes prostate cancer cell migration and invasion via CXCR4/CXCL12 axis. Mol Cancer BioMed Central Ltd; 2011;10(1):139.
Zhang K, Chen D, Jiao X, Zhang S, Liu X, Cao J, et al. Slug enhances invasion ability of pancreatic cancer cells through upregulation of matrix metalloproteinase-9 and actin cytoskeleton remodeling. Lab Investig. Nature Publishing Group2011;91(3):426–38.
Kurrey NK, Amit K, Bapat SA. Snail and slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol Oncol. 2005;97(1):155–65.
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis Ben Gurion University of the Negev. Cell. 2004;117:927–39.
Bhat FA, Sharmila G, Balakrishnan S, Arunkumar R, Elumalai P, Suganya S, et al. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. Elsevier Inc.2014;25(11):1132–9.
Kim H, Seo EM, Sharma AR, Ganbold B, Park J, Sharma G, et al. Regulation of Wnt signaling activity for growth suppression induced by quercetin in 4T1 murine mammary cancer cells. Int J Oncol. 2013;43(4):1319–25.
Novo MCT, Osugui L, dos Reis VO, Longo-Maugeri IM, Mariano M, Popi AF. Blockage of Wnt/β-catenin signaling by quercetin reduces survival and proliferation of B-1 cells in vitro. Immunobiology Elsevier GmbH.; 2015;220(1):60–67.
Maso V, Calgarotto AKA, Franchi GCA, Nowill AED, Filho PLA, Vassallo J, et al. Multitarget effects of quercetin in leukemia. Cancer Prev Res (Phila). 2014;7(12):1240–50.
Zavadil J, Haley J, Kalluri R, Muthuswamy SK, Thompson E. Epithelial-mesenchymal transition. Cancer Res. American Society for Investigative Pathology2008;68(23):9574–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Meghna M. Baruah and Neeti Sharma combined first author.
Electronic supplementary material
Supplementary Table 1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Baruah, M.M., Khandwekar, A.P. & Sharma, N. Quercetin modulates Wnt signaling components in prostate cancer cell line by inhibiting cell viability, migration, and metastases. Tumor Biol. 37, 14025–14034 (2016). https://doi.org/10.1007/s13277-016-5277-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5277-6