Abstract
Myopathy is the major adverse effect of statins. However, the underlying mechanism of statin-induced skeletal muscle atrophy, one of statin-induced myopathy, remains to be elucidated. Myostatin is a negative regulator of skeletal muscle mass and functions. Whether myostatin is involved in statin-induced skeletal muscle atrophy remains unknown. In this study, we uncovered that simvastatin administration increased serum myostatin levels in mice. Inhibition of myostatin with follistatin, an antagonist of myostatin, improved simvastatin-induced skeletal muscle atrophy. Simvastatin induced myostatin expression not only in skeletal muscle but also in brown adipose tissue (BAT). Mechanistically, simvastatin inhibited the phosphorylation of forkhead box protein O1 (FOXO1) in C2C12 myotubes, promoting the nuclear translocation of FOXO1 and thereby stimulating the transcription of myostatin. In differentiated brown adipocytes, simvastatin promoted myostatin expression mainly by inhibiting the expression of interferon regulatory factor 4 (IRF4). Moreover, the stimulative effect of simvastatin on myostatin expression was blunted by geranylgeranyl diphosphate (GGPP) supplementation in both myotubes and brown adipocytes, suggesting that GGPP depletion was attributed to simvastatin-induced myostatin expression. Besides, the capacities of statins on stimulating myostatin expression were positively correlated with the lipophilicity of statins. Our findings provide new insights into statin-induced skeletal muscle atrophy.

Graphical headlights
1. Simvastatin induces skeletal muscle atrophy via increasing serum myostatin levels in mice;
2. Simvastatin promotes myostatin expression in both skeletal muscle and brown adipose tissue through inhibiting GGPP production;
3. The stimulating effect of statins on myostatin expression is positively correlated with the lipophilicity of statins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Statins, inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, are the most widely used lipid-lowering drugs worldwide with undesirable statin-associated muscle symptoms (SAMS) (Weintraub 2017; Ward et al. 2019). Proposed mechanisms of SAMS include mitochondrial dysfunction (Schirris et al. 2015), oxidative stress (Ahmadi et al. 2018), genetic susceptibility polymorphisms (Needham and Mastaglia 2014), immune-mediated necrotizing myopathy (Selva-O'Callaghan et al. 2018), and inhibited isoprenylation of small G-proteins caused by impaired mevalonate metabolism (Johnson et al. 2004). The lipophilicity of statins is also thought as a factor of SAMS. Both hydrophilic and lipophilic statins are selectively taken up in hepatocytes, but lipophilic statins are more easily to penetrate to extrahepatic cells to cause side effects (Germershausen et al. 1989; McKenney 2003; Cao et al. 2009; Bonifacio et al. 2016). However, it is incomprehensive to fully understand the underlying mechanisms of SAMS.
Myostatin (MSTN), namely growth differentiation factor 8 (GDF8), as a member of the transforming growth factor β (TGF-β) superfamily, is dominantly expressed in skeletal muscle, and is also expressed in other tissues, such as brown adipose tissue (BAT) (Kong et al. 2018) and the heart (Barallobre-Barreiro et al. 2016). Myostatin functions as a negative regulator of muscle mass via interacting with the activin receptor IIB in an autocrine or paracrine manner, and plays a critical role in a series of muscle wasting diseases, including oculopharyngeal muscular dystrophy (Harish et al. 2019), cardiac cachexia (Heineke et al. 2010), cancer cachexia (Gallot et al. 2014), and spinal muscular atrophy (Zhou et al. 2020). Atrogin 1/MAFbx, a specific E3 ubiquitin ligase mediating muscle protein degradation, mediates statin-induced muscle toxicity, suggesting that statin-induced muscle toxicity is a muscle-wasting disease (Hanai et al. 2007). Importantly, emerging evidences suggest that myostatin may be involved in statin-induced muscle toxicity. Simvastatin treatment could induce increased myostatin mRNA expression in rat skeletal muscle (Goodman et al. 2015), and myostatin mRNA expression in vastus lateralis muscle biopsies obtained from statin myalgic subjects is upregulated around 2-fold (Mallinson et al. 2015), yet now, the role of myostatin in statin-induced skeletal muscle atrophy remains unclear.
Geranylgeranyl diphosphate (GGPP) and farnesyl diphosphate (FPP), two isoprenoids derived from mevalonate pathway, are responsible for protein geranylgeranylation and farnesylation respectively (Wang, Casey 2016). Impaired isoprenoid production is of interest in the study of statin toxicity. GGPP-mediated geranylgeranylation of small GTP-binding proteins plays a central role in statin-induced muscle damage (Cao et al. 2009). In addition, simvastatin induces TGF-β expression in cultured heart cells via inhibiting the geranylgeranylation of RhoA GTPase (Park, Galper 1999). Thus, as a member of TGF-β superfamily, whether myostatin is correlated with GGPP/FPP is also needed to be clarified.
This study aimed to investigate the effect of statins on myostatin and the role of myostatin played in simvastatin-induced skeletal muscle atrophy. The sources of increased serum myostatin and the mechanism though which myostatin was regulated by statins were exploited. The capacities of eight statins on stimulating myostatin expression were compared. By using simvastatin as a tool, we found that simvastatin stimulated myostatin expression in both skeletal muscle and BAT. The stimulative effect of simvastatin on myostatin expression was a result of GGPP depletion in both myotubes and brown adipocytes. Moreover, the capacities of statins on stimulating myostatin expression were positively correlated with the lipophilicity of statins. This study gives a new insight into statin-induced myopathy, and may provide a potential therapeutic strategy for patients with statin-induced skeletal muscle atrophy.
Materials and methods
Cell culture and reagents
The mouse myoblast C2C12 cell line was purchased from American Type Culture Collection (ATCC, USA), and the cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, KeyGEN BioTECH, China) with 10% fetal bovine serum (FBS, Gibco, USA), 1% penicillin-streptomycin. The condition of incubator is 5% CO2, 37 °C. For differentiating into myotubes, C2C12 myoblasts were switched to the medium with 2% horse serum (Gibco, USA) for 6–10 days.
Mouse embryonic fibroblast C3H10T1/2 cell line was purchased from Shanghai Model Cell Institute. The cells were maintained in DMEM (KeyGEN BioTECH, China) with 10% new bovine serum (NBS, Gibco, USA), 1% penicillin-streptomycin. The condition of incubator is 5% CO2, 37 °C. When C3H10T1/2 cells reach 90% confluence, cells were switched to medium containing 1 μM dexamethasone (Sigma-Aldrich, USA), 0.5 mM isobutylmethylxanthine (Sigma-Aldrich, USA), 5 mg/ml insulin (Sigma-Aldrich, USA), and 1 μM rosiglitazone (Sigma-Aldrich, USA) for 1 day. Then, cells were switched to medium containing 5 mg/ml insulin and 1 μM rosiglitazone for 2 days. The cells were finally switched to medium containing 1 μM rosiglitazone until the differentiation completed.
Animal experiments
Animal care and surgery protocols were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Animal Care Committee of China Pharmaceutical University. To investigate the effect of simvastatin on serum myostatin, male C57BL/6J mice (20 ± 2 g) from Laboratory Animal Center of Yangzhou University (Yangzhou, China) were fed adaptively for 1 week. Mice were housed under standard laboratory conditions, fed with standard diet and tap water and libitum. Then, mice were divided into two groups. One group was administrated with simvastatin (80 mg/kg/day) by gavage for 2 months (n = 14). Control group was administrated with 0.5% CMC-Na by gavage correspondently (n = 10). After administration for 2 months, mice were anesthetized; then, blood and tissues were harvested for experiments. Gastrocnemius muscle was striped and weighed. Tissues were stored at −80 °C for other experiments.
To investigate the role of myostatin in simvastatin-induced skeletal muscle atrophy, male C57BL/6J mice (20 ± 2 g) from Laboratory Animal Center of Yangzhou University (Yangzhou, China) were fed adaptively for 1 week. Mice were housed under standard laboratory conditions, fed with standard diet and tap water and libitum. Then, mice were divided into four groups. Control group was administrated 0.5% CMC-Na by gavage (n = 10). Follistatin group was administrated with follistatin (12 μg/kg/day) via intraperitoneal injection for 2 months (n = 10). Simvastatin group was administrated with simvastatin (80 mg/kg/day) by gavage for 2 months (n = 10). Simvastatin + follistatin group was administrated with simvastatin (80 mg/kg/day) by gavage plus follistatin (12 μg/kg/day) via intraperitoneal injection for 2 months (n = 10). After administration for 2 months, mice were anesthetized; then, blood and tissues were harvested for experiments. Gastrocnemius muscle was striped and weighed. Tissues were stored at −80 °C for other experiments.
Determination of serum creatine kinase activity
Serum creatine kinase activity was measured by the Creatine Kinase Activity Assay Kit (Sigma-Aldrich, MAK116) according to the protocol provided by the manufacturer.
Grip strength test
Grip Strength Meter (YLS-13A, Shandong, China) was used to measure grip strength of limbs. The test was repeated five times in a row over the same period. The average of five times tests was considered the maximum grip strength of each mouse.
Myotube diameter analysis
Nikon inverted microscope was used to capture myotube images. National Institutes of Health (NIH) ImageJ 1.63 software was used to measure diameter. For a single myotube, the mean value of more than 3 measurements is used as the diameter of this single myotube. For each well, diameters of 200 myotubes were detected, and the mean value was recorded as myotube diameter.
Quantitative real-time PCR
RNA isolater Total RNA Extraction Reagent (Vazyme, R401–01) was used to extract total RNA from gastrocnemius muscle, BAT and cells. The mRNA expression of FBXO32, MuRF1, IRF4, and MSTN was detected by real-time PCR (RT-qPCR) with primers as showed in Table 1. The ratio of the mRNA interested to GAPDH was calculated and expressed as mean ± standard error of mean (SEM).
Western blot analysis
RIPA buffer was used to extract total protein from gastrocnemius muscle, BAT, and cells, and western blot was performed as previously described (Zhao et al. 2013). p-Smad2 (Ser465/Ser467), p-Smad3 (Ser423/425), Smad2/3, Akt, p-Akt (Ser473), mTOR, p-mTOR (Ser2448), p-p70S6K (Thr389), p70S6K, myostatin, p-FOXO1 (Ser253), FOXO1, IRF4 primary antibodies were purchased from Cell Signaling Technology, Inc. (MA, USA). GAPDH primary antibody was obtained from ABclonal (Wuhan, China). Atrogin 1/MAFbx, MuRF1 primary antibodies were purchased from ProteinTech Group (Wuhan, China). HRP-conjugated AffiniPure Goat Anti-Mouse IgG (H + L) and HRP-conjugated AffiniPure Goat Anti-Rabbit IgG (H + L) (ProteinTech Group, Wuhan, China) were used as the secondary antibodies.
Hematoxylin and eosin staining and immunohistochemical analysis
Gastrocnemius muscle and BAT were fixed in 4% paraformaldehyde (PFA) at 4 °C overnight. Then, tissues were embedded in paraffin wax. Paraffin sections (3 μm) were cut off and placed on a glass slide. The slides were fixed using 4% PFA, followed by a series of experimental procedures, including deparaffinization, rehydration, and antigen retrieval. The slides were blocked with 10% goat serum (Beyotime, Jiangsu, China) and then incubated with myostatin primary antibody, with HRP-tagged goat anti-rabbit as a secondary antibody. Representative images were captured using Nikon inverted microscope.
Determination of serum myostatin
An ELISA kit purchased from R&D Systems (DGDF80; Minnesota, USA) was adopted to detect serum myostatin levels according to the protocol provided by the manufacturer.
Immunofluorescence staining of FOXO1
In brief, C2C12 myoblasts were fixed with 4% PFA solution and punched with 0.5% Triton X-100 (Sigma-Aldrich, T8787) at room temperature. The cells were blocked with 5% bovine serum albumin (BSA) at room temperature and incubated with FOXO1 primary antibody (Cell Signaling Technology, C29H4) overnight at 4 °C. Then, the cells were incubated with the secondary antibody (IgG-FITC) for 1 h at room temperature in dark, followed with 4′, 6-diamidino-2-phenylindole (DAPI) staining for 15 min in dark. Images were captured under the confocal microscopy within 6 h. All solutions mentioned in this experiment were prepared with PBS.
Nuclear and cytoplasmic extraction
The extraction of nuclear and cytoplasmic protein of C2C12 myoblasts was achieved using a nuclear and cytoplasmic extraction kit (Thermo Scientific, 78,835), according to the protocol provided by the manufacturer.
Plasmids and plasmid transfection
IRF4 expression plasmid and IRF4 interfering plasmid were purchased from GenePharma Co., Ltd. (Shanghai, China). C3H10T1/2 brown adipocytes were transfected with plasmids using Lipofectamine 3000 (Invitrogen, L3000015) according to the protocol provided by the manufacturer. Further experiments were performed after 48 h of plasmids transfection in C3H10T1/2 brown adipocytes.
Statistical analysis
Data were analyzed using the GraphPad Prism 8 software (San Diego, CA, USA). All data were expressed as the means ± standard error of mean (SEM). Student’s t test and one-way ANOVA were used to calculate statistical significance. A value of p < 0.05 meant significant; values of p < 0.01, p < 0.001 and p < 0.0001 meant highly significant; ns meant no significance.
Results
Simvastatin administration induces skeletal muscle atrophy and increases serum myostatin levels in C57BL/6J mice
In order to establish statin-induced skeletal muscle atrophy model, simvastatin, the most commonly chosen statin in the studies of statin-induced myopathy, was used (Panajatovic et al. 2019; Ramesh et al. 2019). After gavage of simvastatin (80 mg/kg/day) for 8 weeks (Song et al. 2018; Ren et al. 2020), there seems to be a decreased trend of body weight (Fig. 1a). Serum creatine kinase, a marker of skeletal muscle damage, was increased by around 20% (Fig. 1b). Simvastatin administration significantly impaired skeletal muscle function by using grip strength test (Fig. 1c). Compared with control group, the mass of gastrocnemius muscle was decreased by around 6.5%, and hematoxylin and eosin (HE) staining showed shrunken muscle fibers in simvastatin treatment group (Fig. 1d and e). As showed in Fig. 1f and g, the mRNA expression of both atrophic markers, Atrogin 1/MAFbx and MuRF 1, was upregulated in gastrocnemius muscle of mice with simvastatin administration. The protein level of Atrogin 1/MAFbx and MuRF 1 was also significantly increased (Fig. 1h). Next, we employed C2C12 myotubes to investigate the prosarcopenic effect of simvastatin in vitro. As shown in Fig. S1a, simvastatin caused an obvious reduction of myotube diameter in a concentration-dependent manner. Both atrophic markers Atrogin 1/MAFbx and MuRF 1 were upregulated by simvastatin treatment at both mRNA and protein level (Fig. S1b-d). Collectively, these results show that simvastatin could induce skeletal muscle atrophy in vivo and in vitro.
Simvastatin administration induces skeletal muscle atrophy and increased serum myostatin levels. Male C57BL/6J mice (20 ± 2 g) were randomly grouped (control group, n = 10; simvastatin group, n = 14). After simvastatin (80 mg/kg/day) administration for 8 weeks, mice were analyzed as follows. a Body weight. b Serum creatine kinase levels. c Holding power. d Weight of gastrocnemius muscles. e HE staining of gastrocnemius muscle sections (scale bar = 50 μm). f-g Total RNAs were extracted from gastrocnemius muscle tissues, the mRNA expression of two atrophic markers FBXO32 (Atrogin 1) and MuRF1 was detected by RT-qPCR (n = 3). h For each group, protein samples from the gastrocnemius muscle tissues of 3 mice were prepared, and western blot was used to detect the expression of proteins as indicated. Protein amount was quantified using ImageJ (n = 3). i Serum myostatin levels were detected using an ELISA kit. j-l Correlation analysis of serum myostatin levels and body weight (j), gastrocnemius mass (k), grip strength of limbs (l) in simvastatin group. m For each group, protein samples from the gastrocnemius muscle tissues of 3 mice were prepared, and western blot was used to detect the expression of proteins as indicated. Protein amount was quantified using ImageJ (n = 3). Data represent the mean ± SEM. Statistical analysis was done with one-way ANOVA. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
It was reported that myostatin was implicated in statin-induced myopathy (Mallinson et al. 2015); we next sought to investigate whether simvastatin could promote myostatin expression. As a secreted cytokine, serum myostatin levels were detected. As showed in Fig. 1i, simvastatin caused an increase of serum myostatin in mice compared with control group. We observed that these mice with higher serum myostatin levels presented reduced body weight, smaller gastrocnemius muscle and obviously decreased grip strength of limbs. Regression analysis showed that serum myostatin levels were negatively correlated with body weight (Fig. 1j), gastrocnemius mass (Fig. 1k), and grip strength of limbs (Fig. 1l) in simvastatin administration group. Besides, in atrophic gastrocnemius, myostatin/Smad signaling pathway was activated and the anabolic pathway, Akt/mTOR signaling pathway was inhibited (Fig. 1m). This was consistent with previous studies that statins activated TGF-β/Smad signaling pathway and inhibited Akt/mTOR signaling (Vecerova et al. 2012; Porreca et al. 2002; Bonifacio et al. 2017; Mallinson et al. 2009; Bonifacio et al. 2015; Mullen et al. 2011). Taken together, these results indicate that simvastatin-induced skeletal muscle atrophy is correlated with increased serum myostatin levels in C57BL/6J mice.
Myostatin inhibition by follistatin improves simvastatin-caused skeletal muscle atrophy
To investigate whether increased serum myostatin levels were a reason for simvastatin-induced skeletal muscle symptoms, we employed follistatin, the natural antagonist of myostatin, which could binds and neutralizes myostatin to inhibit myostatin activity (Singh et al. 2014). Intraperitoneal injection of follistatin (12 μg/kg/d) was chosen based on previous studies (Jeong et al. 2013; Dasarathy et al. 2011). The mice were randomly divided into 4 groups, including control group, follistatin (12 μg/kg/d) group, simvastatin (80 mg/kg/d) group, and follistatin (12 μg/kg/d) + simvastatin (80 mg/kg/d) group. After 2-month administration of follistatin, simvastatin, and follistatin + simvastatin, it was seen that simvastatin increased serum myostatin levels and follistatin did not influence serum myostatin levels (Fig.2a–b). Interestingly, although follistatin did not prevent simvastatin-induced elevation of serum CK levels, simvastatin-caused loss of body weight was greatly improved (Fig. 2c–d). Besides, follistatin also did not obviously improve grip strength, but significantly reversed mass loss of skeletal muscle caused by simvastatin (Fig. 2e–g). The induced expression of two atrophic markers Atrogin 1/MAFbx and MuRF 1 by simvastatin was also reversed by follistatin at both mRNA and protein level (Fig. 2h–j). Moreover, simvastatin-induced activation of Smad signaling pathway was also suppressed by follistatin while inhibited anabolic pathway was recovered by follistatin (Fig. 2j). Taken together, these results suggest that myostatin mediates simvastatin-induced skeletal muscle atrophy.
Myostatin inhibition by follistatin improves simvastatin-caused skeletal muscle atrophy. Male C57BL/6J mice (20 ± 2 g) were randomly grouped (n = 10). After administration of follistatin (12 μg/kg/day), simvastatin (80 mg/kg/day), and follistatin combined with simvastatin for 8 weeks, mice were analyzed as follows. a Serum myostatin levels. b Body weight. c Serum CK activity. d Grip strength. e Weight of gastrocnemius muscle. f HE staining of gastrocnemius muscle sections (scale bar = 50 μm). g-h Total RNAs were extracted from gastrocnemius muscle tissues, the mRNA expression of two atrophic markers FBXO32 (Atrogin 1/MAFbx) and MuRF1 was detected by RT-qPCR (n = 3). i For each group, protein samples from the gastrocnemius muscle tissues of 3 mice were prepared, and western blot was used to detect the expression of proteins as indicated. Protein amount was quantified using ImageJ (n = 3). Data represent the mean ± SEM. Statistical analysis was done with one-way ANOVA. *p < 0.05; **p < 0.01; ***p < 0.001
Simvastatin induces myostatin expression in skeletal muscle and BAT
Previous studies reported that myostatin mainly expressed in muscle tissues; however, some studies demonstrated that other organs also expressed a certain amount of myostatin, such as the heart, BAT (Biesemann et al. 2014; Kong et al. 2018). Thus, we analyzed the protein expression of myostatin in the heart, liver, spleen, lung, kidney, white adipose tissue (WAT), BAT, and gastrocnemius muscle of simvastatin-treated mice. As showed in Fig. 3a, myostatin was widely expressed in all of the detected tissues. However, the level of myostatin in BAT was most abundant except gastrocnemius muscle. Thus, we hypothesized that increased serum myostatin mainly derived from gastrocnemius muscle and BAT. As expected, compared with control group, mRNA and protein level of myostatin in the gastrocnemius muscle of simvastatin-treated mice were significantly upregulated (Fig. 3b, c, and f), and simvastatin also induced a significant increase of myostatin in BAT (Fig. 3d, e, and g), but with no significant effect on myostatin expression in other organs (Fig. S2a-f). This was consistent with a previous study that BAT functioned as a myostatin-secreting organ to regulate systemic insulin sensitivity and skeletal muscle function (Steculorum et al. 2016; Kong et al. 2018). Taken together, these findings illustrate that simvastatin induces myostatin expression in skeletal muscle and BAT.
Simvastatin induces myostatin expression in skeletal muscle and BAT. Male C57BL/6J mice (20 ± 2 g) were randomly grouped (control group, n = 10; simvastatin group, n = 14). After simvastatin (80 mg/kg/day) administration for 8 weeks, the mice were subjected to a series of analyses as indicated below. a Expression of myostatin in main organs of mice which had increased serum myostatin levels (n = 3). b Total RNA was extracted from gastrocnemius muscle tissues; myostatin expression at mRNA level was detected by RT-qPCR (control group, n = 10; simvastatin group, n = 14). c For each group, protein samples from the gastrocnemius muscle tissues of 3 mice were prepared, and western blot was used to detect the expression of proteins as indicated. Protein amount was quantified using ImageJ (n = 3). d Total RNA was extracted from BAT; myostatin expression at mRNA level was detected by RT-qPCR (control group, n = 10; simvastatin group, n = 14). e For each group, protein samples from the BAT of 3 mice were prepared, and western blot was used to detect the expression of proteins as indicated. Protein amount was quantified using ImageJ (n = 3). f Representative immunohistochemical staining picture of myostatin of gastrocnemius muscle (scale bar = 100 μm) (n = 3). g Representative immunohistochemical staining picture of myostatin of BAT (scale bar = 100 μm) (n = 3). Data represent the mean ± SEM. Statistical analysis was done with one-way ANOVA. **p < 0.01; ***p < 0.001; ****p < 0.0001
Simvastatin induces myostatin expression via promoting FOXO1 nuclear translocation in C2C12 myotubes
Myostatin was transcriptionally regulated by transcription factor FOXO1 (Allen, Unterman 2007; Allen and Du 2008). FOXO1, a downstream factor of AKT, could be phosphorylated by AKT at Ser253 site to an inactivated form and then move out to nucleus (Manning and Toker 2017). We hypothesized that simvastatin induced myostatin expression via promoting FOXO1 nuclear translocation in skeletal muscle. As showed in Fig. 4a and b, simvastatin promoted myostatin expression in a dose-dependent manner at both mRNA and protein level in C2C12 myotubes. Meanwhile, the expression of p-AKT (Ser473) and p-FOXO1 (Ser253) was also significantly inhibited by simvastatin (Fig. 4c). The inhibitory effects of simvastatin on AKT and FOXO1 phosphorylation were also confirmed in gastrocnemius muscle and in consistent with published study (Mallinson et al. 2009) (Fig. 4d). Next, immunofluorescent staining was performed to observe the subcellular localization of FOXO1 in C2C12 myoblasts. As showed in Fig. 4e, simvastatin treatment significantly induced an accumulation of FOXO1 in nucleus, which was consistent with western blot results (Fig. 4f). Moreover, AS1842856, a specific inhibitor of FOXO1, also attenuated the stimulative effect of simvastatin on myostatin expression at both mRNA and protein level (Fig. 4g and h). In conclusion, these findings indicate that simvastatin induces myostatin expression via promoting FOXO1 nuclear translocation in C2C12 myotubes.
Simvastatin induces myostatin expression via promoting FOXO1 nuclear translocation in C2C12 myotubes. a C2C12 myotubes were treated with simvastatin (2 μM, 10 μM, 50 μM) for 24 h, total RNA was collected, and RT-qPCR was performed to detect RNA expression of myostatin (n = 3). b C2C12 myotubes were treated with simvastatin (2 μM, 10 μM, 50 μM) for 24 h, total protein was collected, and western blot was performed to detect myostatin expression (n = 3). c C2C12 myotubes were treated with simvastatin (2 μM, 10 μM, 50 μM) for 24 h; total AKT, p-AKT (Ser 473), total FOXO1, and p-FOXO1 (Ser253) were detected by western blot (n = 3). d For each group, protein samples from the gastrocnemius muscle tissues of 3 mice were prepared, and western blot was used to detect the expression of p-FOXO1, FOXO1. Protein amount was quantified using ImageJ (n = 3). e C2C12 myoblasts were treated with simvastatin (2 μM, 10 μM, 50 μM) for 24 h, FOXO1 was stained, and images were captured under the confocal microscopy. Scale bar = 20 μM (n = 3). f C2C12 myoblasts were treated with simvastatin (2 μM, 10 μM, 50 μM) for 24 h; nuclear and cytoplasmic extraction was performed. Total FOXO1 and p-FOXO1 in nucleus and cytosol were detected by western blot (n = 3). g C2C12 myotubes were respectively treated with 10 μM simvastatin, 30 nM AS1842856, 10 μM simvastatin + 30 nM AS1842856 for 24 h, total RNA was collected, and RT-qPCR was performed to detect RNA expression of myostatin (n = 3). h C2C12 myotubes were respectively treated with 10 μM simvastatin, 30 nM AS1842856, 10 μM simvastatin + 30 nM AS1842856 for 24 h, total protein was collected, and western blot was performed to detect myostatin expression. Protein amount was quantified using ImageJ (n = 3). Data represent the mean ± SEM. Statistical analysis was done with one-way ANOVA. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
Simvastatin induces myostatin expression via suppression of IRF4 expression in brown adipocytes
Next, we investigated the mechanism through which simvastatin induced myostatin production in BAT. C3H10T1/2 cells could differentiate into brown adipocytes under induction (Xue et al. 2014). After induction, uncoupling protein 1 (UCP1) was significantly increased at both mRNA and protein level in C3H10T1/2 cells, suggesting that it had already been successfully induced into brown adipocytes (Fig. S3a and S3b). Myostatin expression was increased a dose-dependent manner at both mRNA and protein level in brown adipocytes upon simvastatin treatment (Fig. 5a and b). Next, whether simvastatin induced myostatin expression also via activating FOXO1 in brown adipocytes was investigated. Unexpectedly, simvastatin had no effect on FOXO1 phosphorylation in brown adipocytes (Fig. 5c), suggesting that there were other transcription factors mediated simvastatin-induced myostatin expression in brown adipocytes. Interferon regulatory factor 4 (IRF4) was reported to play a key role in thermogenic function of BAT via functioning as a transcriptional partner of PGC-1α (Kong et al. 2014). It was demonstrated that IRF4 was a negative regulator of myostatin expression in BAT (Kong et al. 2018). Hence, we hypothesized that simvastatin stimulated myostatin expression via suppressing IRF4 expression in brown adipocytes. As showed in Fig. 5d and e, simvastatin significantly decreased both mRNA and protein expression of IRF4 in brown adipocytes. Downregulation of IRF4 by simvastatin was also confirmed in BAT of C57BL/6J mice (Fig. 5f and g). Knockdown of IRF4 using shRNA targeting IRF4 in brown adipocytes (Fig. S3c and S3d) mimicked the stimulative effect of simvastatin on myostatin expression at both mRNA (Fig. S3e) and protein level (Fig. S3f). In contrast, overexpression of IRF4 (Fig. 5h and i) attenuated the stimulative effect of simvastatin on myostatin expression (Fig. 5j and k). In order to verify the endocrine relevance between BAT and skeletal muscle, C2C12 myotubes was cultured with the conditioned medium collected from brown adipocytes with IRF4 knockdown. Myostatin level in conditioned medium was determined by myostatin ELISA kit, and the result showed that secreted myostatin was increased after IRF4 knockdown in brown adipocytes (Fig. S4a). Moreover, although conditioned medium did not harm C2C12 myotubes to the extent of increased Atrogin 1/MAFbx and MuRF-1 expression, the phosphorylation of Smad 2/3 was obviously increased, suggesting that Smad signaling pathway was activated by conditioned medium (Fig. S4b). Taken together, these results suggest that simvastatin promoted myostatin expression via inhibiting IRF4 expression in BAT.
Simvastatin induces myostatin expression via suppression of IRF4 expression in brown adipocytes. a Brown adipocytes were treated with simvastatin (2 μM, 10 μM, 50 μM) for 24 h, total RNA was collected, and RT-qPCR was performed to detect RNA expression of myostatin (n = 3). b Brown adipocytes were treated with simvastatin (2 μM, 10 μM, 50 μM) for 24 h, total protein was collected, and western blot was performed to detect myostatin expression (n = 3). c-d Brown adipocytes were treated with simvastatin (2 μM, 10 μM, 50 μM) for 24 h, total protein was collected, and western blot was performed to detect p-FOXO1 (Ser253), total FOXO1 expression (c), and IFR4 expression (d) (n = 3). e Brown adipocytes were treated with simvastatin (2 μM, 10 μM, 50 μM) for 24 h, total RNA was collected, and RT-qPCR was performed to detect RNA expression of IRF4 (n = 3). f Total RNA was extracted from BAT; IRF4 expression at mRNA level was detected by RT-qPCR (control group, n = 10; simvastatin group, n = 14). g For each group, protein samples from the BAT of 3 mice were prepared, and western blot was used to detect the expression of IRF4. Protein amount was quantified using ImageJ (n = 3). h-i Brown adipocytes (day 6 post-induction) were pre-transfected with IRF4-expression plasmid for 4 h and cultured for 24–48 h with fresh complete medium. Total RNA and total protein samples were harvested. mRNA expression (h) and protein expression (i) of myostatin were detected by RT-qPCR and western blot, respectively. Protein amount was quantified using ImageJ (n = 3). j-k Brown adipocytes (day 6 post-induction) were pre-transfected with IRF4-expression plasmid for 4 h and cultured for 24–48 h with fresh complete medium. Then, treated simvastatin (10 μM) for another 24 h. Total RNA and total protein samples were harvested. mRNA expression (j) and protein expression (k) of myostatin were detected by RT-qPCR and western blot, respectively. Protein amount was quantified using ImageJ (n = 3). Data represent the mean ± SEM. Statistical analysis was done with one-way ANOVA. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
Simvastatin-induced myostatin expression is dependent on GGPP depletion in both myotubes and brown adipocytes
As inhibitors of HMG-CoA reductase, statins inhibit the production of mevalonate, a precursor for cholesterol biosynthesis. GGPP and FPP, by-products of cholesterol, are also reduced by statins. Whether the stimulative effect of simvastatin on myostatin expression could be attributed to GGPP or FPP depletion is still unknown. To investigate the relationship between simvastatin-induced myostatin expression and GGPP/FPP, C2C12 myotubes were treated with simvastatin or simvastatin supplemented with mevalonate, GGPP, or FPP. The results showed that the stimulative effect of simvastatin on myostatin expression was significantly attenuated by mevalonate or GGPP supplementation while FPP showed weak effect (Fig. 6a and b). Mevalonate and GGPP also reversed simvastatin-induced inhibition of AKT phosphorylation and FOXO1 phosphorylation (Fig. 6b). Moreover, the nuclear translocation of FOXO1 induced by simvastatin was also significantly blocked by mevalonate and GGPP supplementation (Fig. 6c and d). In brown adipocytes, mevalonate and GGPP showed similar inhibitory effect on simvastatin-induced myostatin expression at both mRNA and protein level (Fig. 6e and g). Mechanistically, this effect should own to the recovery of IRF4 expression (Fig. 6f and f). Collectively, these findings suggest that simvastatin-induced myostatin expression is a result of GGPP depletion in both myotubes and brown adipocytes.
GGPP prevents simvastatin-induced myostatin expression in C2C12 myotubes and C3H10T1/2 brown adipocytes. a C2C12 myotubes were treated with simvastatin or simvastatin supplemented with mevalonate, GGPP, and FPP respectively for 24 h. Total RNA was collected and mRNA expression of myostatin (MSTN) was detected by RT-qPCR (n = 3). b C2C12 myotubes were treated with simvastatin or simvastatin supplemented with mevalonate, GGPP, and FPP respectively for 24 h. Protein samples were collected; the expression of p-AKT (Ser473), p-FOXO1 (Ser253), and myostatin was detected by western bolt. Protein amount was quantified using ImageJ (n = 3). c C2C12 myoblasts were treated with simvastatin or simvastatin supplemented with mevalonate, GGPP, and FPP respectively for 24 h; FOXO1 was stained; and images were captured under the confocal microscopy. Scale bar = 20 μM (n = 3). d C2C12 myoblasts were treated with simvastatin or simvastatin supplemented with mevalonate, GGPP, and FPP respectively for 24 h; cytosol and nucleus were extracted. The expression of p-FOXO1 and FOXO1 was detected by western blot. Protein amount was quantified using ImageJ (n = 3). e-f C3H10T1/2 brown adipocytes were treated with simvastatin or simvastatin supplemented with mevalonate, GGPP, and FPP respectively for 24 h. Total RNA was extracted; the mRNA expression of myostatin (MSTN) and IRF4 was analyzed by RT-qPCR (n = 3). g C3H10T1/2 brown adipocytes were treated with simvastatin or simvastatin supplemented with mevalonate, GGPP, and FPP respectively for 24 h. Myostatin and IRF4 protein expression were detected by western blot. Protein amount was quantified using ImageJ (n = 3). Data represent the mean ± SEM. Statistical analysis was done with one-way ANOVA. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
The stimulative effect of statins on myostatin expression is positively correlated with their lipophilicity
Statins could be classified into hydrophilic statins and lipophilic statins according to their lipophilicity. In order to investigate whether their effects on stimulating myostatin expression should be different, seven available statins including simvastatin, lovastatin, pitavastatin, fluvastatin, atorvastatin, rosuvastatin, pravastatin, and the precursor of pravastatin, mevastatin were studied (Table 2). As showed in Fig. 7a, the lipophilicity of statins can be concluded based on previous studies (Rageh et al. 2017; Serajuddin et al. 1991; Schachter 2005; Fong 2014). Then, the capacities of these statins on inducing myostatin expression were investigated in C2C12 myotubes and C3H10T1/2 brown adipocytes. As showed in Fig. 7b, almost all statins could increase myostatin expression in C2C12 myotubes, and lipophilic statins seemed possess more obvious effects than hydrophilic statins, especially simvastatin. The phosphorylation of FOXO1 was also significantly inhibited by lipophilic statins, while hydrophilic statins has no obvious effect on it (Fig. 7b). In brown adipocytes, lipophilic statins induced myostatin expression and inhibited IRF4 expression almost in a lipophilicity-dependent manner (Fig. 7c). Taken together, the results suggest that statins could stimulate myostatin expression in myotubes and brown adipocytes, and the effect is likely positively correlated with the lipophilicity of statins (Fig. 8).
The stimulative effect of statins on myostatin expression is positively correlated with the lipophilicity of statins. a Tested statins were listed according to their lipophilicity. b C2C12 myotubes were treated with 10 μM various statins for 24 h, protein samples were harvested, and the expression of myostatin, p-FOXO1, and total FOXO1 was detected by western blot. Protein amount was quantified using ImageJ (n = 3). c C3H10T1/2 brown adipocytes were treated with 10 μM various statins for 24 h, protein samples were harvested, and myostatin, IRF4 expression were detected by western blot. Protein amount was quantified using ImageJ (n = 3). Data represent the mean ± SEM. Statistical analysis was done with one-way ANOVA. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
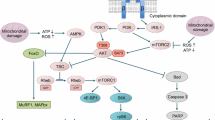
In muscle fibers, statins directly inhibit the production of GGPP, resulting in the dephosphorylation of AKT and FOXO1. Dephosphorylation of FOXO1 promotes the nuclear translocation of FOXO1, leading to increased transcription of myostatin. In brown adipocytes, statins suppress IRF4 expression via inhibiting GGPP production. Decreased expression of IRF4, a transcription repressor of myostatin, leads to increased transcription of myostatin.
Discussion
The present study demonstrated that simvastatin induced skeletal muscle atrophy via stimulating myostatin expression in both skeletal muscle and BAT through different mechanisms. In brief, simvastatin promoted myostatin expression via inhibiting phosphorylation of FOXO1 then promoting its nuclear translocation in skeletal muscle, while simvastatin induced myostatin expression in BAT via suppressing the expression of IRF4. Furthermore, the stimulative effect of simvastatin on myostatin expression could be attributed to GGPP depletion in both skeletal muscle and BAT. Moreover, the capacity of statins on stimulating myostatin expression is positively correlated with the lipophilicity of statins.
Statin-induced myopathy is a widely known side effect of statins, but its underlying mechanism remains enigmatic. Up to now, several possible mechanisms have been proposed, such as mitochondrial complex III Inhibition (Schirris et al. 2015), oxidative stress (Ahmadi et al. 2018), and induction of muscle-specific ubiquitin ligase Atrogin 1/MAFbx (Hanai et al. 2007). However, whether myostatin is involved in statin-induced skeletal muscle damage remains undetermined. Present study showed that simvastatin significantly induced myostatin expression in skeletal muscle and BAT and increased serum myostatin levels. Myostatin inhibition attenuated simvastatin-induced skeletal muscle atrophy, but did not effectively improve grip strength. This could be explained by inhibited mitochondrial function upon simvastatin treatment, such as damage of the mitochondrial respiratory chain, increased production of ROS (du Souich et al. 2017). Myostatin inhibition may mainly suppress myostatin/Smad signaling pathway-mediated protein downregulation, and does not benefit mitochondrial functions.
In our study, in addition to skeletal muscle, BAT also contributed to increased serum myostatin levels by simvastatin administration. Despite BAT mainly exist in newborns and widely absent in adults, emerging literatures emphasize that BAT plays an important role in physiology and pathology of human beings (Tahara et al. 2020; Deshmukh et al. 2019; Yoneshiro et al. 2019). Consistently, a previous study just demonstrated that BAT was a myostatin secreting organ to regulate skeletal muscle function (Kong et al. 2018). We did not observe a change of myostatin expression in the heart, liver, spleen, lung, kidney, and WAT after simvastatin administration. Although myostatin expresses in almost all of these tested organs, only skeletal muscle and BAT have been demonstrated to secrete myostatin into circulatory system to regulate systemic metabolism and osteoclast differentiation (Dankbar et al. 2015; Steculorum et al. 2016; Kong et al. 2018). This is possibly because basic myostatin expression is higher in skeletal muscle and BAT than other organs. In addition, statins selectively function in the liver largely because of efficient first-pass uptake. Lipophilic statins are taken up primarily via passive diffusion, while the uptake of hydrophilic statins is achieved by active carrier-mediated processes (Schachter 2005), but excessive lipophilic statins could enter other organs via passive diffusion, especially organs with abundant blood vessels including skeletal muscle and BAT. Moreover, drug transporters including human organic anion transporting polypeptide 2B1 (OATP2B1), multidrug resistance-associated protein (MRP) 1, 4, and 5, are also factors determining the distribution of statins in skeletal muscle (Knauer et al. 2010). Thus, simvastatin-induced myostatin expression in skeletal muscle and BAT was a comprehensive outcome of basic myostatin expression in organs and pharmacokinetic distribution of simvastatin. In addition, statins induced myostatin expression almost in a lipophilicity-dependent manner in both myotubes and brown adipocytes. This is possibly because intracellular concentration of statins is also positively correlated with lipophilicity.
GGPP is a by-product of cholesterol synthesis in mevalonate pathway. Statin treatment inevitably inhibits the production of GGPP when exerts its cholesterol lowering function. Our results suggested that statin-induced myostatin expression could be attributed to statin-caused GGPP depletion in both myotubes and brown adipocytes. It is interesting that GGPP depletion by statin induced myostatin in myotubes and brown adipocytes via different downstream mechanisms. Simvastatin promoted myostatin expression in myotubes by promoting the nuclear translocation of FOXO1, and increased myostatin expression in brown adipocytes by inhibiting the expression of IRF4 FOXO1 played a vital role in muscle functions, including skeletal muscle atrophy (Stitt et al. 2004; Milan et al. 2015), cardiomyocyte proliferation, and myocardial growth (Evans-Anderson et al. 2008), while IRF4 reportedly played a more important role in brown adipocytes, including regulation of myostatin expression (Kong et al. 2014; Kong et al. 2018). IRF4 inhibition by simvastatin has also been demonstrated in immune cells. Coincidently, the geranylgeranyl transferase inhibitor mimicked the inhibitory effect of simvastatin on IRF4 expression in Th17 cells, which indicated the role of GGPP in simvastatin-caused IRF4 downregulation (Zhang et al. 2011; Nam et al. 2016). Hence, these studies support our conclusion to some extent.
Increased risk of hyperglycemia is another adverse effect of statins (Sattar et al. 2010). It was reported that increased myostatin caused insulin resistance (Steculorum et al. 2016), and GGPP depletion contributed to statin-caused diabetes (Shen et al. 2019). Thus, it could be speculated that statin-induced myostatin production is likely a potential mechanism of statin-caused diabetes. Whether myostatin is involved in statin-induced diabetes will be also investigated. Moreover, GGPP is responsible for geranylgeranylation of proteins. Small GTPase proteins, such as Rho proteins, are the most common substrate for geranylgeranylation (Wang and Casey 2016). It is likely that these small GTPase proteins mediate the effect of GGPP depletion on myostatin expression. More work on this part is needed in our future study. Furthermore, monoclonal antibody of myostatin should have been used in the in vivo experiment, because follistatin is not a specific antagonist. Follistatin also inhibits activin and growth differentiation factor 11 (GDF11) (Harrington et al. 2006; Suh et al. 2020). Although follistatin alone did not cause skeletal muscle hypertrophy, the role of activin and GDF11 in the treatment of simvastatin-induced skeletal muscle atrophy by follistatin could not be ruled out.
Conclusion
In summary, our study reveals the stimulative effect of statins on myostatin expression and the role of myostatin played in statin-induced skeletal muscle atrophy. Our study uncovers the mechanisms by which statins regulate myostatin expression, and offers a suggestion on the choice of statins for hyperlipidaemia patients.
Abbreviations
- CK:
-
Creatine kinase
- HMG-CoA:
-
3-hydroxy-3-methyl-glutaryl-coenzyme A
- SAMS:
-
Statin-associated muscle symptoms
- GDF8:
-
Growth differentiation factor 8
- TGF-β:
-
Transforming growth factor β
- BAT:
-
Brown adipose tissue
- FOXO1:
-
Forkhead box protein O1
- IRF4:
-
Interferon regulatory factor 4
- UCP1:
-
Uncoupling protein 1
- GGPP:
-
Geranylgeranyl diphosphate
- FPP:
-
Farnesyl pyrophosphate
- FBS:
-
Fetal bovine serum
- NBS:
-
New bovine serum
- PFA:
-
Paraformaldehyde
- BSA:
-
Bovine serum albumin
- DAPI:
-
4′, 6-diamidino-2-phenylindole
References
Ahmadi Y, Ghorbanihaghjo A, Naghi-Zadeh M, Yagin NL. Oxidative stress as a possible mechanism of statin-induced myopathy. Inflammopharmacology. 2018;26(3):667–74. https://doi.org/10.1007/s10787-018-0469-x.
Allen DL, Du M. Comparative functional analysis of the cow and mouse myostatin genes reveals novel regulatory elements in their upstream promoter regions. Comp Biochem Physiol Part B, Biochem Mol Biol. 2008;150(4):432–9. https://doi.org/10.1016/j.cbpb.2008.05.002.
Allen DL, Unterman TG. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol. 2007;292(1):C188–99. https://doi.org/10.1152/ajpcell.00542.2005.
Barallobre-Barreiro J, Gupta SK, Zoccarato A, Kitazume-Taneike R, Fava M, Yin X, et al. Glycoproteomics reveals decorin peptides with anti-myostatin activity in human atrial fibrillation. Circulation. 2016;134(11):817–32. https://doi.org/10.1161/circulationaha.115.016423.
Biesemann N, Mendler L, Wietelmann A, Hermann S, Schafers M, Kruger M, et al. Myostatin regulates energy homeostasis in the heart and prevents heart failure. Circ Res. 2014;115(2):296–310. https://doi.org/10.1161/circresaha.115.304185.
Bonifacio A, Mullen PJ, Mityko IS, Navegantes LC, Bouitbir J, Krähenbühl S. Simvastatin induces mitochondrial dysfunction and increased atrogin-1 expression in H9c2 cardiomyocytes and mice in vivo. Arch Toxicol. 2016;90(1):203–15. https://doi.org/10.1007/s00204-014-1378-4.
Bonifacio A, Sanvee GM, Bouitbir J, Krahenbuhl S. The AKT/mTOR signaling pathway plays a key role in statin-induced myotoxicity. Biochim Biophys Acta. 2015;1853(8):1841–9. https://doi.org/10.1016/j.bbamcr.2015.04.010.
Bonifacio A, Sanvee GM, Brecht K, Kratschmar DV, Odermatt A, Bouitbir J et al. IGF-1 prevents simvastatin-induced myotoxicity in C2C12 myotubes. 2017;91(5):2223–34. https://doi.org/10.1007/s00204-016-1871-z.
Cao P, Hanai J, Tanksale P, Imamura S, Sukhatme VP, Lecker SH. Statin-induced muscle damage and atrogin-1 induction is the result of a geranylgeranylation defect. FASEB J. 2009;23(9):2844–54. https://doi.org/10.1096/fj.08-128843.
Dankbar B, Fennen M, Brunert D, Hayer S, Frank S, Wehmeyer C, et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med. 2015;21(9):1085–90. https://doi.org/10.1038/nm.3917.
Dasarathy S, McCullough AJ, Muc S, Schneyer A, Bennett CD, Dodig M, et al. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol. 2011;54(5):915–21. https://doi.org/10.1016/j.jhep.2010.08.032.
Deshmukh AS, Peijs L, Beaudry JL, Jespersen NZ, Nielsen CH, Ma T, et al. Proteomics-based comparative mapping of the secretomes of human brown and white adipocytes reveals EPDR1 as a novel batokine. Cell Metab. 2019;30(5):963–75.e7. https://doi.org/10.1016/j.cmet.2019.10.001.
du Souich P, Roederer G, Dufour R. Myotoxicity of statins: mechanism of action. Pharmacol Ther. 2017;175:1–16. https://doi.org/10.1016/j.pharmthera.2017.02.029.
Evans-Anderson HJ, Alfieri CM, Yutzey KE. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res. 2008;102(6):686–94. https://doi.org/10.1161/circresaha.107.163428.
Fong CW. Statins in therapy: understanding their hydrophilicity, lipophilicity, binding to 3-hydroxy-3-methylglutaryl-CoA reductase, ability to cross the blood brain barrier and metabolic stability based on electrostatic molecular orbital studies. Eur J Med Chem. 2014;85:661–74. https://doi.org/10.1016/j.ejmech.2014.08.037.
Gallot YS, Durieux AC, Castells J, Desgeorges MM, Vernus B, Plantureux L, et al. Myostatin gene inactivation prevents skeletal muscle wasting in cancer. Cancer Res. 2014;74(24):7344–56. https://doi.org/10.1158/0008-5472.can-14-0057.
Germershausen JI, Hunt VM, Bostedor RG, Bailey PJ, Karkas JD, Alberts AW. Tissue selectivity of the cholesterol-lowering agents lovastatin, simvastatin and pravastatin in rats in vivo. Biochem Biophys Res Commun. 1989;158(3):667–75. https://doi.org/10.1016/0006-291x(89)92773-3.
Goodman CA, Pol D, Zacharewicz E, Lee-Young RS, Snow RJ, Russell AP, et al. Statin-induced increases in atrophy gene expression occur independently of changes in PGC1alpha protein and mitochondrial content. PLoS One. 2015;10(5):e0128398. https://doi.org/10.1371/journal.pone.0128398.
Hanai J, Cao P, Tanksale P, Imamura S, Koshimizu E, Zhao J, et al. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest. 2007;117(12):3940–51. https://doi.org/10.1172/jci32741.
Harish P, Malerba A, Lu-Nguyen N, Forrest L, Cappellari O, Roth F, et al. Inhibition of myostatin improves muscle atrophy in oculopharyngeal muscular dystrophy (OPMD). J Cachexia Sarcopenia Muscle. 2019;10(5):1016–26. https://doi.org/10.1002/jcsm.12438.
Harrington AE, Morris-Triggs SA, Ruotolo BT, Robinson CV, Ohnuma S, Hyvönen M. Structural basis for the inhibition of activin signalling by follistatin. EMBO J. 2006;25(5):1035–45. https://doi.org/10.1038/sj.emboj.7601000.
Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, et al. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010;121(3):419–25. https://doi.org/10.1161/circulationaha.109.882068.
Jeong J, Conboy MJ, Conboy IM. Pharmacological inhibition of myostatin/TGF-β receptor/pSmad3 signaling rescues muscle regenerative responses in mouse model of type 1 diabetes. Acta Pharmacol Sin. 2013;34(8):1052–60. https://doi.org/10.1038/aps.2013.67.
Johnson TE, Zhang X, Bleicher KB, Dysart G, Loughlin AF, Schaefer WH, et al. Statins induce apoptosis in rat and human myotube cultures by inhibiting protein geranylgeranylation but not ubiquinone. Toxicol Appl Pharmacol. 2004;200(3):237–50. https://doi.org/10.1016/j.taap.2004.04.010.
Knauer MJ, Urquhart BL, Meyer zu Schwabedissen HE, Schwarz UI, Lemke CJ, Leake BF, et al. Human skeletal muscle drug transporters determine local exposure and toxicity of statins. Circ Res. 2010;106(2):297–306. https://doi.org/10.1161/circresaha.109.203596.
Kong X, Banks A, Liu T, Kazak L, Rao RR, Cohen P, et al. IRF4 is a key thermogenic transcriptional partner of PGC-1α. Cell. 2014;158(1):69–83. https://doi.org/10.1016/j.cell.2014.04.049.
Kong X, Yao T, Zhou P, Kazak L, Tenen D, Lyubetskaya A, et al. Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab. 2018;28(4):631–43.e3. https://doi.org/10.1016/j.cmet.2018.07.004.
Mallinson JE, Constantin-Teodosiu D, Sidaway J, Westwood FR, Greenhaff PL. Blunted Akt/FOXO signalling and activation of genes controlling atrophy and fuel use in statin myopathy. J Physiol. 2009;587(1):219–30. https://doi.org/10.1113/jphysiol.2008.164699.
Mallinson JE, Marimuthu K, Murton A, Selby A, Smith K, Constantin-Teodosiu D, et al. Statin myalgia is not associated with reduced muscle strength, mass or protein turnover in older male volunteers, but is allied with a slowing of time to peak power output, insulin resistance and differential muscle mRNA expression. J Physiol. 2015;593(5):1239–57. https://doi.org/10.1113/jphysiol.2014.285577.
Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169(3):381–405. https://doi.org/10.1016/j.cell.2017.04.001.
McKenney JM. Pharmacologic characteristics of statins. Clin Cardiol. 2003;26(4 Suppl 3):Iii32–8. https://doi.org/10.1002/clc.4960261507.
Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. 2015;6:6670. https://doi.org/10.1038/ncomms7670.
Mullen PJ, Zahno A, Lindinger P, Maseneni S, Felser A, Krahenbuhl S, et al. Susceptibility to simvastatin-induced toxicity is partly determined by mitochondrial respiration and phosphorylation state of Akt. Biochim Biophys Acta. 2011;1813(12):2079–87. https://doi.org/10.1016/j.bbamcr.2011.07.019.
Nam S, Kang K, Cha JS, Kim JW, Lee HG, Kim Y, et al. Interferon regulatory factor 4 (IRF4) controls myeloid-derived suppressor cell (MDSC) differentiation and function. J Leukoc Biol. 2016;100(6):1273–84. https://doi.org/10.1189/jlb.1A0215-068RR.
Needham M, Mastaglia FL. Statin myotoxicity: a review of genetic susceptibility factors. Neuromusc Disord: NMD. 2014;24(1):4–15. https://doi.org/10.1016/j.nmd.2013.09.011.
Panajatovic MV, Singh F, Roos NJ, Duthaler U, Handschin C, Krahenbuhl S et al. PGC-1alpha plays a pivotal role in simvastatin-induced exercise impairment in mice. 2019;e13402. https://doi.org/10.1111/apha.13402.
Park HJ, Galper JB. 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors up-regulate transforming growth factor-beta signaling in cultured heart cells via inhibition of geranylgeranylation of RhoA GTPase. Proc Natl Acad Sci U S A. 1999;96(20):11525–30. https://doi.org/10.1073/pnas.96.20.11525.
Porreca E, Di Febbo C, Baccante G, Di Nisio M, Cuccurullo F. Increased transforming growth factor-beta(1) circulating levels and production in human monocytes after 3-hydroxy-3-methyl-glutaryl-coenzyme a reductase inhibition with pravastatin. J Am Coll Cardiol. 2002;39(11):1752–7. https://doi.org/10.1016/s0735-1097(02)01857-0.
Rageh AH, Atia NN, Abdel-Rahman HM. Lipophilicity estimation of statins as a decisive physicochemical parameter for their hepato-selectivity using reversed-phase thin layer chromatography. J Pharm Biomed Anal. 2017;142:7–14. https://doi.org/10.1016/j.jpba.2017.04.037.
Ramesh M, Campos JC, Lee P, Song Y, Hernandez G, Sin J, et al. Mitophagy protects against statin-mediated skeletal muscle toxicity. FASEB J. 2019;33(11):11857–69. https://doi.org/10.1096/fj.201900807RR.
Ren L, Xuan L, Han F, Zhang J, Gong L, Lv Y, et al. Vitamin D supplementation rescues simvastatin induced myopathy in mice via improving mitochondrial cristae shape. Toxicol Appl Pharmacol. 2020;401:115076. https://doi.org/10.1016/j.taap.2020.115076.
Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet (London, England). 2010;375(9716):735–42. https://doi.org/10.1016/s0140-6736(09)61965-6.
Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19(1):117–25. https://doi.org/10.1111/j.1472-8206.2004.00299.x.
Schirris TJ, Renkema GH, Ritschel T, Voermans NC, Bilos A, van Engelen BG, et al. Statin-induced myopathy is associated with mitochondrial complex III inhibition. Cell Metab. 2015;22(3):399–407. https://doi.org/10.1016/j.cmet.2015.08.002.
Selva-O'Callaghan A, Alvarado-Cardenas M, Pinal-Fernández I, Trallero-Araguás E, Milisenda JC, Martínez M, et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert Rev Clin Immunol. 2018;14(3):215–24. https://doi.org/10.1080/1744666x.2018.1440206.
Serajuddin AT, Ranadive SA, Mahoney EM. Relative lipophilicities, solubilities, and structure-pharmacological considerations of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors pravastatin, lovastatin, mevastatin, and simvastatin. J Pharm Sci. 1991;80(9):830–4. https://doi.org/10.1002/jps.2600800905.
Shen L, Gu Y, Qiu Y, Cheng T, Nie A, Cui C, et al. Atorvastatin targets the islet mevalonate pathway to dysregulate mTOR signaling and reduce beta-cell functional mass. Diabetes. 2019;69:48–59. https://doi.org/10.2337/db19-0178.
Singh R, Braga M, Pervin S. Regulation of brown adipocyte metabolism by myostatin/follistatin signaling. Front Cell Dev Biol. 2014;2:60. https://doi.org/10.3389/fcell.2014.00060.
Song M, Chen FF, Li YH, Zhang L, Wang F, Qin RR, et al. Trimetazidine restores the positive adaptation to exercise training by mitigating statin-induced skeletal muscle injury. J Cachexia Sarcopenia Muscle. 2018;9(1):106–18. https://doi.org/10.1002/jcsm.12250.
Steculorum SM, Ruud J, Karakasilioti I, Backes H, Engstrom Ruud L, Timper K, et al. AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell. 2016;165(1):125–38. https://doi.org/10.1016/j.cell.2016.02.044.
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14(3):395–403. https://doi.org/10.1016/s1097-2765(04)00211-4.
Suh J, Kim NK, Lee SH, Eom JH, Lee Y, Park JC et al. GDF11 promotes osteogenesis as opposed to MSTN, and follistatin, a MSTN/GDF11 inhibitor, increases muscle mass but weakens bone. 2020;117(9):4910–20. https://doi.org/10.1073/pnas.1916034117.
Tahara A, Tahara N, Maeda-Ogata S, Bekki M, Sugiyama Y, Honda A, et al. Brown adipose tissue activation in severe heart failure. Eur Heart J. 2020;41(25):2415. https://doi.org/10.1093/eurheartj/ehaa241.
Vecerova L, Strasky Z, Rathouska J, Slanarova M, Brcakova E, Micuda S, et al. Activation of TGF-beta receptors and Smad proteins by atorvastatin is related to reduced atherogenesis in ApoE/LDLR double knockout mice. J Atheroscler Thromb. 2012;19(2):115–26. https://doi.org/10.5551/jat.8185.
Wang M, Casey PJ. Protein prenylation: unique fats make their mark on biology. Nat Rev Mol Cell Biol. 2016;17(2):110–22. https://doi.org/10.1038/nrm.2015.11.
Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124(2):328–50. https://doi.org/10.1161/circresaha.118.312782.
Weintraub WS. Perspective on trends in statin use. JAMA Cardiol. 2017;2(1):11–2. https://doi.org/10.1001/jamacardio.2016.4710.
Xue R, Wan Y, Zhang S, Zhang Q, Ye H, Li Y. Role of bone morphogenetic protein 4 in the differentiation of brown fat-like adipocytes. Am J Physiol Endocrinol Metab. 2014;306(4):E363–72. https://doi.org/10.1152/ajpendo.00119.2013.
Yoneshiro T, Wang Q, Tajima K, Matsushita M, Maki H, Igarashi K, et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature. 2019;572(7771):614–9. https://doi.org/10.1038/s41586-019-1503-x.
Zhang X, Tao Y, Troiani L, Markovic-Plese S. Simvastatin inhibits IFN regulatory factor 4 expression and Th17 cell differentiation in CD4+ T cells derived from patients with multiple sclerosis. J Immunol (Baltimore, Md : 1950). 2011;187(6):3431–7. https://doi.org/10.4049/jimmunol.1100580.
Zhao R, Sun L, Lin S, Bai X, Yu B, Yuan S, et al. The saponin monomer of dwarf lilyturf tuber, DT-13, inhibits angiogenesis under hypoxia and normoxia via multi-targeting activity. Oncol Rep. 2013;29(4):1379–86. https://doi.org/10.3892/or.2013.2272.
Zhou H, Meng J, Malerba A, Catapano F, Sintusek P, Jarmin S, et al. Myostatin inhibition in combination with antisense oligonucleotide therapy improves outcomes in spinal muscular atrophy. J Cachexia Sarcopenia Muscle. 2020;11(3):768–82. https://doi.org/10.1002/jcsm.12542.
Funding
This work was supported by the National Key R&D Program of China (2019YFC1711000), National Natural Science Foundation of China (81722048), and “Double First-Class” University project (CPU2018GY09).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical standards
The manuscript does not contain clinical studies or participant data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Zheng, ZG., Meng, L. et al. Statins induce skeletal muscle atrophy via GGPP depletion-dependent myostatin overexpression in skeletal muscle and brown adipose tissue. Cell Biol Toxicol 37, 441–460 (2021). https://doi.org/10.1007/s10565-020-09558-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-020-09558-w