Abstract
Nuphar alkaloids, originally isolated from water lilies, induce apoptosis in mammalian cells in less than 1 h, making them possibly the fastest known inducers. However, the mechanism by which this rapid apoptosis occurs remains unknown. We have investigated canonical aspects of apoptosis to determine how the nuphar alkaloid, (+)-6-hydroxythiobinupharidine (6HTBN), induces apoptosis. 6HTBN induced rapid apoptosis in various leukemia, lymphoma, and carcinoma cell lines, suggesting that its mechanism is cell-type independent. It also circumvented resistance of patient-derived chronic lymphocytic leukemia cells generated by co-culture on survival-promoting stroma. Intriguingly, 6HTBN failed to induce apoptosis in platelets. The mechanism of apoptosis involves activation of caspase 9 and caspase 3, but not caspase 8 as previously reported. The release of cytochrome c from mitochondria occurred even in the absence of BAX/BAK and in cells that retained mitochondrial membrane potential. These results suggest a novel mechanism of apoptosis that has previously not been reported. The molecular target of the nuphar alkaloids remains to be determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nuphar alkaloids isolated from the yellow water lilies Nuphar pumilum, Nuphar japonicum, and Nuphar lutea have been shown to induce apoptosis in mammalian cells in less than an hour, making them possibly the fastest inducers of apoptosis known (Achmatowicz and Bellen 1962; Matsuda et al. 2006). We and others have recently synthesized both the natural dimeric nuphar alkaloids, and a series of non-natural derivatives, including monomeric analogs which also induce apoptosis extremely rapidly (Jansen and Shenvi 2013; Korotkov et al. 2015; Li et al. 2016; Li et al. 2017; Lacharity et al. 2017). Structure activity analysis has demonstrated the critical requirement for the aminal hydroxyl group for apoptosis. In the current studies, we have used (+)-6-hydroxythiobinupharidine (6-HTBN; Fig. 1), which has one aminal hydroxyl group, albeit the majority of other nuphar alkaloids synthesized also induce rapid apoptosis.
The mechanism responsible for the rapid apoptosis remains elusive. Matsuda et al. (2006) suggested that nuphar alkaloids induce apoptosis through the extrinsic pathway and activation of caspase 8; this is contradicted by our results presented here. Ozer et al. (2009) proposed that nuphar alkaloids inhibit NF-kB, though reanalysis of that paper suggests the inhibition of NF-kB was more likely a consequence rather than a cause of apoptosis. Tada et al. (2016) proposed that the sulfur of dihydroxylated nuphar dimers can react as electrophiles by undergoing a retrodimerization process in cellular environments, but this cannot explain the acute apoptosis induced by all the nuphar derivatives such as monohydroxylated (i.e., 6-HTBN) or monomeric variants (Korotkov et al. 2015; Li et al. 2016). We report here a novel mechanism of apoptosis involving caspase 9 and caspase 3, but surprisingly, release of cytochrome c from the mitochondria is both independent of BAX and BAK and occurs without disrupting the mitochondrial membrane potential. These data, along with the unprecedented rate of cell death, may indicate a new biological target and mechanism of apoptosis.
Materials and methods
Reagents
6-HTBN is one of the nuphar alkaloids previously isolated from water lilies; for these studies, it was synthesized as previously reported (Korotkov et al. 2015). ABT-737 was provided by AbbVie (North Chicago, IL). Vinblastine was purchased from Sigma Chemical Co. The caspase inhibitors DEVD-fluoromethyl ketone, LEHD-fluoromethyl ketone, and QVD (quinoline-Val-Asp-difluorophenoxymethyl ketone) were obtained from Cayman Chemicals (Ann Arbor, MI).
Cell culture
Cell lines were obtained from either the American Type Culture Collection or the Developmental Therapeutics Program, National Cancer Institute, Bethesda, MD. Jurkat clones C3 and C4, which lack both BAX and BAK, were kindly provided by Dr. Hanna Rabinowich, Pittsburgh (Han et al. 2004). L4.5 cells are a derivative of murine L929 fibroblasts expressing CD154 (CD40L) and were obtained from Sonia Neron at Hema-Quebec (Quebec, Canada) (Neron et al. 1996). Cells were routinely maintained in RPMI1640 media with 10% fetal bovine serum, antibiotics, and antimycotic. All cells were used within 3 months of thawing from frozen stocks.
Blood was obtained from patients with chronic lymphocytic leukemia (CLL), and peripheral blood mononuclear cells were purified using Ficoll-Paque as previously described (Bates et al. 2011). For stroma co-culture experiments, CLL cells were plated on a confluent monolayer of CD154+ stroma cells for 24 h and then incubated with 6-HTBN (Soderquist et al. 2013).
Platelets were isolated from non-cancer bearing individuals by mixing 10 mL of blood with 1 mL sodium citrate (3% w/v). This mixture was centrifuged for 10 min at 1000 rpm, and the top layer containing the platelet-rich plasma was collected. Purified platelets were plated in RPMI-1640 plus 10% serum and treated immediately with drugs.
To assess chromatin condensation, a hallmark of apoptosis, cells were incubated with 2 μg/mL Hoechst 33342 for 15 min at 37 °C and visualized with a fluorescent microscope. At least 200 cells were scored from each sample, and data were expressed as the percentage of cells with condensed chromatin.
Immunoblot analysis
Cells were lysed directly in urea sample buffer (4 M urea, 10% β-mercaptoethanol, 6% SDS, 125 mM Tris (pH 6.8), 0.01% bromophenol blue, and protease/phosphatase cocktail inhibitor). Alternately, cells were lysed in 10 mM Hepes (pH 7.5), 300 mM KCl, 1% NP-40, and protease/phosphatase inhibitor mixture and then combined with an equal volume 2× urea buffer (8 M urea, 100 mM Tris (pH 6.8), 10% β-mercaptoethanol, 4% SDS, and 0.01% bromophenol blue). Lysates were then boiled for 5 min. Proteins were subsequently separated by SDS-PAGE and transferred to polyvinylidene difluoride membrane (Millipore). All membranes were blocked in 5% non-fat milk in Tris-buffered saline, 0.05% Tween 20, and then incubated in 5% BSA or 5% non-fat milk with the appropriate primary antibody overnight. Subsequently, membranes were washed in Tris-buffered saline, 0.05% Tween 20, and incubated with secondary antibody conjugated to horseradish peroxidase. Proteins were visualized by enhanced chemiluminescence (Amersham). Actin, MEK1/2, and Tom20 were used as loading controls.
Antibodies were obtained from the following sources: Cell Signaling: caspase 8 (9746), GAPDH (5174), MEK1/2 (9122), PARP (9532), and Tom20 (42406); BD Biosciences: cytochrome c (556433) and gelsolin (610412); and Sigma: Actin-HRP (A3854). Secondary antibodies were purchased from Bio-Rad (Hercules, CA).
Digitonin permeabilization (cytochrome c release)
Separation of membrane/organelle fraction from cytosol was performed based on previously published methods (Single et al. 1998; Ganju and Eastman 2003). Cells were incubated with digitonin buffer (8.75 μg digitonin/106 cells, 75 mM NaCl, 1 mM NaH2PO4, 8 mM Na2HPO4, 250 mM sucrose and protease/phosphatase inhibitor mixture) for 2 min on ice. Cells were then centrifuged at 12,500 rpm for 1 min at 4 °C. The supernatant was transferred and supplemented with an equal volume of 2× urea buffer. The pellet was dissolved in an equal volume of digitonin buffer and 2× urea buffer. The samples were then boiled for 5 min prior to western blotting.
Flow cytometry detection of JC-1
Perelman et al. (2012) demonstrated that excitation at 405 nm gave better resolution of JC-1 monomers and aggregates than the more normally used 488 nm excitation. Following further analysis of the various excitation and emission wavelengths, we selected FL10 (405 nm excitation 550/40 nm emission) for optimal detection of monomers, and FL2 (488 nm excitation 575/30 nm emission) for aggregates.
Results
Rapid apoptosis occurs in almost all cells
Our prior experiments with the nuphar alkaloids were performed primarily in U937 human leukemia cells. We have extended this analysis to additional cell lines and find the high rate, and potency of nuphar-induced apoptosis is similar across the panel. Apoptosis was scored both by chromatin condensation and cleavage of the caspase substrate poly(ADP-ribose) polymerase (PARP) (Fig. 2a, b). The nuphar alkaloid 6-HTBN at 10 μM induced cleavage of PARP within 1 h and chromatin condensation within 1–2 h. Lower concentrations exhibited slightly slower induction of apoptosis, though even 1.25 μM 6-HTBN induced extensive chromatin condensation by 24 h. The cell lines studied include U937, a histiocytic lymphoma, and NB4, and acute myelocytic leukemia. The sensitivity of a Burkett’s lymphoma line Raji is also shown in Fig. 2b.
Concentration and time-dependent apoptosis induced by nuphar alkaloid. The indicated cell lines (NB4, U937, HeLa, and Raji) were incubated with 0–10 μM 6-HTBN. Cells from one CLL patients (CLL59) were similarly incubated with 6-HTBN before or after co-culture with L4.5 cells. Cells were scored a for chromatin condensation or b for cleavage of PARP
We also demonstrated that chronic lymphocytic leukemia (CLL) cells freshly isolated from blood of patients also undergo rapid 6-HTBN-induced apoptosis (Fig. 2). In a patient, CLL cells also reside in the lymph node and bone marrow where they are much more resistant to most drugs due to upregulation of various members of the BCL2 anti-apoptotic family, most notably BCLX and MCL1. This resistance can be mimicked in vitro by co-culturing CLL cells with a stromal cell line expressing CD154, the ligand for CD40 on the CLL cells (Soderquist et al. 2013; Soderquist et al. 2014). 6-HTBN is just as potent at inducing apoptosis in these co-cultured cells (Fig. 2).
The rapid apoptosis was not restricted to hematopoietic cells as many carcinomas also respond rapidly to similar concentrations of 6-HTBN. As an example, rapid PARP cleavage was observed in HeLa cells (Fig. 2b) and HCT116 cells as discussed below (Fig. 5). Intriguingly, we found that platelets were completely resistant to 6-HTBN; in this case, apoptosis was measured as cleavage of gelsolin (Fig. 3). Platelets rely on BCLX for survival and a positive control for gelsolin cleavage is shown upon incubation with the BCL2/BCLX inhibitor ABT-737 with or without the microtubule inhibitor vinblastine. Platelets lack nuclei so would presumably be resistant to many other drugs including those that target DNA.
Resistance of human platelets to 6-HTBN. Freshly isolated platelets were incubated with 0–10 μM 6-HTBN for 6 or 24 h; then, lysates analyzed for cleavage of gelsolin. As a positive control, platelets were incubated with ABT-737 (with or without vinblastine which has no impact in this experiment) for 6 h
6-HTBN activates the intrinsic pathway of apoptosis
It was previously reported that caspase 8 is required for nuphar-induced apoptosis (Matsuda et al. 2006). Indeed, upon incubation of Jurkat cells with 6-HTBN, caspase 8 cleavage is rapidly induced (Fig. 4a). However, caspase 8 can also be cleaved as a consequence of the intrinsic pathway of apoptosis downstream of caspase 9 and caspase 3. Accordingly, we assessed the efficacy of 6-HTBN to induce apoptosis in a Jurkat derivative that lacks caspase 8. These cells were as sensitive to 6-HTBN as the Jurkat wild-type cells. In addition, we assessed the efficacy of caspase 9 (z-LEHD-fmk) and caspase 3 (z-DEVD-fmk) inhibitors to prevent apoptosis. Both inhibitors effectively prevented PARP cleavage as well as caspase 8 cleavage, while the combination of inhibitors had greater efficacy. It is worth noting that it required 40 μM LEHD-fmk to prevent PARP cleavage, whereas the prior experiments used 20 μM LEHD-fmk and thereby concluded that caspase 9 was not required for 6-HTBN-induced apoptosis (Matsuda et al. 2006). Our results demonstrate that the caspase 9/3 pathway is required for apoptosis.
6-HTBN-mediated apoptosis is dependent on caspase 3 and 9, but not caspase 8. a Jurkat wild type (left) and a derivative lacking caspase 8 (right) were incubated with 5 μM 6-HTBN for 2 h in the presence of the indicated concentration of caspase 3 inhibitor DEVD or caspase 9 inhibitor LEHD and then analyzed for PARP cleavage and caspase 8 cleavage. While caspase 8 is cleaved in the Jurkat wt cells, this is likely a consequence of caspase 3 activity as it is inhibited by both DEVD and LEHD. b The caspase 8 negative Jurkat cells were incubated with 6-HTBN, then fractionated into (1) pellet which includes mitochondria and (2) cytosol which contains cytochrome c released from mitochondria. The incubation also contained the pan-caspase inhibitor QVD to prevent loss of cytochrome c and to demonstrate that the release of cytochrome c occurs independently of caspases. The blots shown are derived from one exposure of a single western blot, but intervening lanes that are not relevant to this paper were excised where indicated
Caspases are activated as a consequence of cytochrome c release from the mitochondria. Upon incubation of the caspase 8-defective Jurkat cells with 6-HTBN, cytochrome c was rapidly released and detected in the cytosol (Fig. 4b). These experiments were performed in the presence of the pan-caspase inhibitor QVD which preserves the released cytochrome c in the cytosol and also demonstrates that this release is not a consequence of caspase activity.
Cytochrome c release occurs independently of BAX and BAK
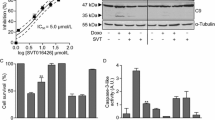
Cytochrome c is usually released through a mitochondrial pore formed by oligomerization of the pro-apoptotic proteins BAX and/or BAK. The absence of these proteins usually makes cells completely resistant to inducers of the intrinsic pathway of apoptosis. Jurkat cells constitutively lack BAX. We used a Jurkat derivative that also lacks BAK (Han et al. 2004). These cells are completely resistant to the BCL2/BCLX inhibitor ABT-737 but still underwent PARP cleavage when incubated with 6-HTBN (Fig. 5a, b). Furthermore, 6-HTBN still induced release of cytochrome c from the mitochondria of these BAX/BAK-deficient cells (Fig. 5c). A confirmatory experiment was performed in an HCT116 derivative that also lacks BAX and BAK; 6-HTBN again induced apoptosis in a BAX/BAK–independent manner (Fig. 5d).
6-HTBN-mediated apoptosis is independent of BAX and BAK. a Jurkat wild-type cells and two clonal derivatives lacking BAX and BAK (c3 and c4) were incubated with 0–10 μM ABT-737 for 6 or 8 h and then assayed for PARP cleavage to demonstrate the resistance afforded by the lack of BAX and BAK. b The Jurkat c4 cell line was incubated with 0–10 μM 6-HTBN for 0–2 h and then assayed for PARP cleavage. The Jurkat c3 cell line gave identical results. c Jurkat c4 cell line was incubated with 10 μM 6-HTBN for 1 h and then fractionated to assess the translocation of cytochrome c from pellet (containing mitochondria) to cytosol. The caspase inhibitor QVD was included in the indicated samples as this preserves cytochrome c once released from mitochondria but prevents PARP cleavage. Much of the cleaved PARP also appears in the cytosolic fraction. MEK1/2 and Tom20 are controls for cytosol and mitochondria respectively. The Jurkat c3 cell line gave identical results. d HCT116 wild-type cells and a derivative deleted for both BAX and BAK (DKO) were incubated with 6-HTBN for 1 h and analyzed for PARP cleavage. WCL whole cell lysate
An alternate pathway for the release of cytochrome c can occur if mitochondria lose their inner membrane potential resulting in swelling and rupture of the outer mitochondrial membrane. We assessed the mitochondrial membrane potential using JC-1 fluorescence and flow cytometry (Fig. 6). JC-1 forms red fluorescent oligomers in polarized mitochondria, but green monomers following depolarization. We also used wavelengths that better resolve the green shift of the monomers (Perelman et al. 2012). Incubation with carbonyl cyanide m-chlorophenylhydrazone (CCCP) acts as a control and shows a clear green shift with decreased red signal. Incubation with 6-HTBN for 1 h (with or without inclusion of the caspase inhibitor QVD) caused no significant change in JC-1 fluorescence demonstrating no decrease in mitochondrial membrane potential.
Nuphar-induced apoptosis does not change the mitochondrial membrane potential. Jurkat wt cells were incubated with 0 or 10 μM 6-HTBN for 1 h or 50 μM CCCP for 5 min as indicated then stained with 2.5 μM JC-1 for 15–30 min. Additionally, some cells were incubated concurrently with 20 μM QVD. Each sample was analyzed by flow cytometry to detect JC1 monomers and aggregates
Discussion
The nuphar alkaloids are of great interest because of their rapid induction of apoptosis. We reiterated this observation in our synthetic program, showing that many nuphar derivatives elicited this effect as long as they retain at least one aminal hydroxyl group (Korotkov et al. 2015; Li et al. 2016, 2017). Those experiments were extended here in an attempt to better understand the mechanism of their action. Many cell lines, both leukemia and carcinoma, underwent apoptosis in less than 1 h. This is the most rapid onset of apoptosis that we are aware of. To date, the only exception we have observed is platelets. Platelets are cytoplasmic fragments derived from megakaryocytes, lacking a nucleus, but retaining mitochondria and other cytoplasmic organelles. Their survival is dependent on BCLX, and apoptosis can be readily observed as either cleavage of gelsolin or externalization of phosphatidylserine (Zhang et al. 2017). Here, we confirmed their sensitivity to the BCLX inhibitor ABT737, but their resistance to 6-HTBN. Why the sensitivity of platelets is so different from cell lines remains a mystery.
The use of genetically modified cells lacking caspase 8 suggests the extrinsic pathway of apoptosis is not involved, while protection by caspase 3 and caspase 9 inhibitors supports an intrinsic mechanism. This contradicts a prior publication suggesting the extrinsic pathway is involved (Matsuda et al. 2006), but we question their evidence as the caspase 9 inhibitor was used at sub-inhibitory concentrations.
Our most unexpected observation was that 6-HTBN still induced apoptosis in the absence of BAX and BAK, the two proteins normally considered essential for the release of cytochrome c and the consequential apoptosis. The mitochondrial membrane potential was also retained suggesting that the mitochondria did not swell and release cytochrome c (Vander Heiden et al. 1997). There is currently no other known mechanism by which cytochrome c might be released from mitochondria.
We have recently identified another compound that has a very similar mechanism of action as 6-HTBN. This compound, A1210477, was developed as an inhibitor of the anti-apoptotic protein MCL1 (Leverson et al. 2015), but at slightly higher concentrations, it induced rapid induction of apoptosis independent of the MCL1 status (Mallick et al. 2019). This apoptosis was also induced in a BAX/BAK–independent manner, and the mitochondrial membrane potential was also retained. Consequently, this acute pathway of apoptosis is not unique to the nuphar alkaloids but may have a much broader applicability. The target for either 6-HTBN or A1210477 remains to be determined, but these results appear to reflect a novel means to induce apoptosis that requires further investigation.
References
Achmatowicz O, Bellen Z. Alkaloids of Nuphar luteum (L) sm. Isolation of alkaloids containing sulphur. Tetrahedron Lett. 1962;3:1121–4.
Bates DJP, Salerni BL, Lowrey CH, Eastman A. Vinblastine sensitizes leukemia cells to cyclin-dependent kinase inhibitors inducing acute cell cycle phase-independent apoptosis. Cancer Biol Therap. 2011;12:314–25.
Ganju N, Eastman A. Zinc inhibits Bax and Bak activation and cytochrome c release induced by chemical inducers of apoptosis but not by death-receptor-initiated pathways. Cell Death Differ. 2003;10:652–61.
Han J, Goldstein LA, Gastman BR, Rabinowitz ZA, Wang G-Q, Fang B, et al. Differential involvement of Bax and Bak in TRAIL-mediated apoptosis of leukemic T cells. Leukemia. 2004;18:16711680.
Jansen DJ, Shenvi RA. Synthesis of (−)-neothiobinupharidine. J Am Chem Soc. 2013;135:1209–12.
Korotkov A, Li H, Chapman C, Povinelli L, MacMillan J, Eastman A, et al. Total synthesis and biological evaluation of both enantiomers of several hydroxylated dimeric nuphar alkaloids. Angew Chem Int Ed Engl. 2015;54:10604–7.
Lacharity JJ, Fournier J, Lu P, Mailyan AK, Herrmann AT, Zakarian A. Total synthesis of unsymmetrically oxidized nuphar thioalkaloids via copper-catalyzed thiolane assembly. J Am Chem Soc. 2017;139:13272–5.
Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J, et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis. 2015;6:e1590.
Li H, Korotkov A, Chapman CW, Eastman A, Wu J. Enantioselective formal synthesis of 11 nuphar alkaloids and discovery of the first known apoptotic monomeric analogs. Angew Chem Int Ed Engl. 2016;55:3509–13.
Li H, Cooke TJ, Korotkov A, Chapman CW, Eastman A. Wu J. Stereoselective synthesis and biological evaluation of C1-epimeric and desmethyl monomeric nuphar analogues. J Org Chem. 2017;82:2648–55.
Mallick DJ, Soderquist RS, Bates D, Eastman A. Confounding off-target effects of BH3 mimetics at commonly used concentrations: MIM1, UMI77 and A1210477. Cell Death Dis. 2019;10:185.
Matsuda H, Yoshida K, Miyagawa K, Nemoto Y, Asao Y, Yoskikawa M. Nuphar alkaloids with immediately apoptosis-inducing activity from Nuphar pumilum and their structural requirements for activity. Bioorg Med Chem Lett. 2006;16:1567–73.
Neron S, Pelletier A, Chevrier MC, Monier G, Lemieux R, Darveau A. Induction of LFA-1 independent human B cell proliferation and differentiation by binding of CD40 with its ligand. Immunol Investig. 1996;25:79–89.
Ozer J, Eisner N, Ostrozhenkova E, Bacher A, Eisenreich W, Benharroch D, et al. Nuphar lutea thioalkaloids inhibit the nuclear factor kappa B pathway, potentiate apoptosis and are synergistic with cisplatin and etoposide. Cancer Biol Ther. 2009;8:1860–8.
Perelman A, Wachtel C, Cohen M, Haupt S, Shapiro H, Tzur A. JC-1: alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012;3:e430.
Single B, Leist M, Nicotera P. Simultaneous release of adenylate kinase and cytochrome c in cell death. Cell Death Differ. 1998;5:1001–3.
Soderquist RS, Bates DJP, Danilov AV, Eastman A. Gossypol overcomes stroma-mediated resistance to the BCL-2 inhibitor ABT-737 in chronic lymphocytic leukemia. Leukemia. 2013;27:2262–4.
Soderquist RS, Danilov AV, Eastman A. Gossypol increases expression of the pro-apoptotic protein through a novel mechanism involving phospholipase A2, cytoplasmic calcium and endoplasmic reticulum stress. J Biol Chem. 2014;289:16190–9.
Tada N, Jansen DJ, Mower MP, Blewett MM, Umotoy JC, Cravatt BF, et al. Synthesis and sulfur electrophilicity of the Nuphar thiaspirane pharmacophore. ACS Cent Sci. 2016;2:401–8.
Vander Heiden MG, Chandel NS, Williamson EK, Thompson CB. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–37.
Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2017;14:943–51.
Funding
This research was supported by grant RSG-13-011-01-CDD from the American Cancer Society to JW and AE, a pilot grant from the COBRE Institute for Biomolecular Targeting (P20GM113132), and an NCI Cancer Center Support Grant 5P30 CA023108 to the Norris Cotton Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mallick, D.J., Korotkov, A., Li, H. et al. Nuphar alkaloids induce very rapid apoptosis through a novel caspase-dependent but BAX/BAK-independent pathway. Cell Biol Toxicol 35, 435–443 (2019). https://doi.org/10.1007/s10565-019-09469-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-019-09469-5