Abstract
The potential mammalian hepatotoxicity of nanomaterials was explored in dose-response and structure-activity studies in human hepatic HepG2 cells exposed to between 10 and 1000 μg/ml of five different CeO2, three SiO2, and one TiO2-based particles for 3 days. Various biochemical parameters were then evaluated to study cytotoxicity, cell growth, hepatic function, and oxidative stress. Few indications of cytotoxicity were observed between 10 and 30 μg/ml. In the 100 to 300 μg/ml exposure range, a moderate degree of cytotoxicity was often observed. At 1000 μg/ml exposures, all but TiO2 showed a high degree of cytotoxicity. Cytotoxicity per se did not seem to fully explain the observed patterns of biochemical parameters. Four nanomaterials (all three SiO2) decreased glucose 6-phosphate dehydrogenase activity with some significant decreases observed at 30 μg/ml. In the range of 100 to 1000 μg/ml, the activities of glutathione reductase (by all three SiO2) and glutathione peroxidase were decreased by some nanomaterials. Decreased glutathione concentration was also found after exposure to four nanomaterials (all three nano SiO2 particles). In this study, the more responsive and informative assays were glucose 6-phosphate dehydrogenase, glutathione reductase, superoxide dismutase, lactate dehydrogenase, and aspartate transaminase. In this study, there were six factors that contribute to oxidative stress observed in nanomaterials exposed to hepatocytes (decreased glutathione content, reduced glucose 6-phosphate dehydrogenase, glutathione reductase, glutathione peroxidase, superoxide dismutase, and increased catalase activities). With respect to structure-activity, nanomaterials of SiO2 were more effective than CeO2 in reducing glutathione content, glucose 6-phosphate dehydrogenase, glutathione reductase, and superoxide dismutase activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is difficult to evaluate nanomaterials to determine their degree and type of toxicity and to subsequently make science-based decisions (Holsapple et al. 2005; Warheit et al. 2007; Walker and Bucher 2009). For nanomaterials, a major determinant of their biological action may be their surface properties, particularly the ability to donate or accept electrons (Thompson and Yates 2006), to release or absorb oxygen (Merrifield et al. 2013) and to generate free radicals such as reactive oxygen species (ROS) (Nel et al. 2006, Khan et al. 2015).
Thus, oxidative stress has frequently been hypothesized as a major possible mode of action of nanomaterials (Nel et al. 2009; Yokel et al. 2014; Shvedova et al. 2012). A second major theory of nanomaterial toxicity is the inflammation theory (Nel et al. 2006; Park and Park 2009). Oxidative stress and inflammation can be closely related in many ways.
Good reviews of CeO2 nanomaterials are available stressing various aspects such as redox and physical-chemical properties (Grulke et al. 2014), fuel additives, and toxicology (Cassee et al. 2011) and in vivo and in vitro inhalation exposures (Demokritou et al. 2013). For TiO2 nanomaterials, in vitro toxicology (Iavicoli et al. 2011) and inhalation toxicology (Shi et al. 2013) have been recently reviewed. After i.p. administration of a SiO2 nanomaterial to mice, increased oxidative stress, inflammation, and DNA damage parameters were observed in several mouse organs including the liver (Nemmar et al. 2016).
This biochemical study is part of a large, coordinated US Environmental Protection Agency study of metal oxide nanomaterials composed of CeO2, SiO2, and TiO2 for systemic toxicity in several organs including the liver. Because study parameters have been selected to evaluate cell growth, cytotoxicity, hepatic function, and oxidative stress, comparisons can be made to determine which parameters respond to low exposure concentrations, which parameters are the most responsive, and to what degree the observed effects are driven by cytotoxicity. Other completed studies in this series include in vitro immuno spin-trapping effects (oxidative stress) (Kitchin et al. 2011), proteomics effects, (Ge et al. 2011) genomics studies in HepG2 cells (Thai et al. 2015a, b, 2016), and metabolomics (Kitchin et al. 2014).
The central purpose of this study was to further investigate the potential hepatotoxicity of CeO2 containing nanoparticles. Thus, nano CeO2 particles W4, X5, Y6 and Z7 were selected (see Table 1 for particle descriptions). CeO2 Q was selected as a larger, not nano sized, CeO2 particle which had been well studied by a European group (Geraets et al. 2012). Nano SiO2 particles K1 and N2 were selected in an attempt to study thin coatings of nano CeO2 on a SiO2 base particle (J0)). Finally, TiO2 T8141 was included as an additional control of a (a) not CeO2 and (b) not nano sized but still a metal oxide particle. The four major purposes of this present study were (a) dose-response, (b) structure-activity, (c) better connecting the physical-chemical characterization information to their toxic biochemical effects, and (d) develop a nanomaterial-toxicity database useful for structure-activity and dose-response modeling. Many of the study parameters were related to oxidative stress (e.g., superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GRD), glucose 6-phosphate dehydrogenase (G6PDH), gamma glutamyltranspeptidase (GGT), reduced glutathione concentration (GSH), and thioredoxin reductase (THRR)). Two other parameters were related to cell growth (microalbulmin (MIA) and protein concentration). Cytotoxicity-related parameters were done by a variety of methods (cytotoxicity by dyes and by visual criteria using a microscope), released enzymes subsequent to membrane damage and toxicity (percentage of total lactate dehydrogenase (%LDH), alanine aminotransferase (%ALT), and aspartate transaminase (%AST). The half-life of LDH enzyme that has been released from cells into the surrounding medium is approximately 9 h (information from Promega Technical Bulletin TB163 at www.promega.com/protocols/CytoTox 96® Non-Radioactive Cytotoxicity Assay (Product G1780)). In circulating human blood, the plasma half-life of AST is 17 ± 5 h while the half-life for ALT is 47 ± 10 h (Price and Alberti 1979). Hepatic function was assessed by measuring the alkaline phosphatase (ALP) activity and the concentration of total bilirubin (T BIL) and triglycerides (TRIG).
In respect to structure-activity issues, we tried to determine if the studied CeO2, SiO2, and TiO2 nanomaterials are similar toxicologically or if they have quite different biological properties. Specifically, we studied the differences in biochemical effects of these nine metal oxide nanomaterials ranging in dry primary particle size of 8 to 214 nm. These nine particles (Table 1) also differed in other physical-chemical characteristics (e.g., specific surface area/porosity, primary and agglomerated particle size and particle shape, as well as oxygen, electron, and metal vacancies or excesses on the surfaces).
These biochemical parameters were chosen to (a) evaluate the type and degree of possible cellular toxicity and to (b) evaluate the oxidative stress theory of nanomaterial-induced toxicity. The resulting data is interpreted in terms of possible mode of action (free radical attack, glutathione depletion, and oxidative stress), dose-response and structure-activity relationship.
Methods
Chemicals and related items
The chemicals and suppliers used in this study were as follows: bovine serum albumin and dimethyl sulfoxide (Sigma of St. Louis, MO, USA), fetal bovine serum, phosphate buffered saline (PBS), Dulbecco’s PBS (DPBS), glutamate, sodium pyruvate, penicillin/streptomycin (Invitrogen of Carlsbad, CA, USA), and corn oil (Food Lion of Durham, NC, USA). The nanomaterials sources (Nano-oxides, Aldrich, Alfa Aesar, Sigma, Sigma Aldrich and US Research Nanomaterials) and the available physical-chemical characterization are presented in Table 1.
Nanomaterials, their dispersion via ultrasound, and their characterization
The nine nanomaterials used in this study (Table 1) were primarily selected to explore the biochemical and metabolomics effects of different CeO2 nanomaterials. Atomic layer deposition (170 or 350 cycles (for SiO2 K1 and SiO2 N2 respectively) of 250 °C for 40 min) was used in the attempt to put a thin coat of CeO2 (particles SiO2 K1 and SiO2 N2) on top of a base SiO2 particle (J0). This CeO2 coating endeavor was not successful. The TiO2 T8141 particle was included in this study as a non-CeO2 and non-nano control particle.
All CeO2 and SiO2 particles have been well characterized by either Dr. Eric Grulke’s group at the University of Kentucky or in Geraets et al.’s study (Geraets et al. 2012) (Table 1). Nanomaterial physical-chemical characterization was done by a variety of techniques for primary particle size, range of particle size, surface area, % purity, and crystal form by either their manufacturer or by an independent party (University of Kentucky, Chemical & Engineering Department) under a US EPA contract. Other physical-chemical characterization data available from the University of Kentucky studies on our nanomaterials includes elemental analysis by TEM/EDX, primary and agglomerated particle size, crystal structure by XRD, and particle shape and morphology by TEM and SEM. This detailed nanomaterial physical-chemical characterization information for nanomaterials W4, X5, Y6, Z7, J0, K1, and N2 will be published elsewhere (Hancock et al. in preparation). Of these nine particles, seven have dry sizes in the nano range, while two have larger dry sizes (CeO2 Q and TiO2 T8141) (Table 1).
In the text of this paper, the primary particle size presented is either from the University of Kentucky or Geraets et al.’s (2012) study (OECD) (Geraets et al. 2012) and not from the vendors. The physical-chemical characterization of CeO2 Q has been already published (Geraets et al. 2012). By TEM, the primary dry particle sizes of the seven nanomaterials ranged from 5 to 50 nm. For CeO2 Q size, estimates were > 500 nm by TEM and < 615 nm by SEM (Table 1). The surface area estimates of these nine particles ranged from 3.73 to 137.4 m2 per gram (Table 1).
For dispersion, measured amounts of bovine serum albumin solution (200 mg/ml in deionized water), sonicated and filter sterilized corn oil (0.01% (v/v) in PBS), and PBS were added to the dry nanomaterials in a glass vial. The general nanomaterial coating recipe of Dale Porter (Porter et al. 2008) was followed in that the mass ratio of the albumin to the nanomaterial was 0.6/1 and the mass ratio of the albumin to the corn oil was 60/1.
The recipe for the preparation of SiO2 “J0” was 30.15 mg of nanomaterial “J0,” 18.09-mg bovine serum albumin, 3.28-ml of 0.01% corn oil, and 6.05 ml of PBS. Sonication occurred at a nanomaterial concentration of 3.20 mg/ml and 9.42 ml of volume in three tubes. Sonication was done for two 10-min cycles of 13 s on, 7-s off with a total typical power of about 132 watts and 159,351 joules with a S-4000 Misonix Ultrasonic Liquid Processor with a 2.5-in. cup horn (part #431-A, Farmingdale, NY). Excess unbound albumin and corn oil were removed by centrifugation of the nanomaterials (9300×g for 5 min) and then resuspending them in cell culture media without any sonication of the culture media.
After nanomaterial dispersion, the degree of agglomeration was determined by dynamic light scattering at 35 °C with a Malvern Model Zen3600 Zetasizer. Refractive index values used were 2.33 for CeO2, 1.544 for SiO2 and 2.488 for TiO2. Size and zeta potential determinations were performed both just after sonication and 3 days later at the end of cell culture.
Cell culture methods
Human hepatocellular carcinoma cells (HepG2, ATCC cat# HB-8065) were obtained and expanded through passage seven using Eagle’s minimum essential medium (Basal Medium Eagle (BME) containing 2-mM GlutaMAX™, 1-mM sodium pyruvate, and 10% fetal bovine serum (all from InVitroGen)) and then frozen in liquid nitrogen. Cells were subsequently carefully thawed and expanded before experimentation between passage 10 and 15. Cell cultures were maintained in a humidified incubator at 37 °C and 95% air/5% CO2 during the study. Cells were plated at a density of 30,000 cells/cm2 in 60-mm dishes (Corning) for 48 h prior to nanomaterial exposure.
Working stock dispersions of each nano material were prepared in cell culture media at 1.0 mg/ml and diluted as needed using cell culture media. Individual dishes were dosed with 200 μl/cm2 of the appropriate nano material dilution. Two separate cultures were done for the purposes of (a) cytotoxicity via the dyes MTS and alamar blue and (b) the three release enzymes (LDH, AST, and ALT) and the biochemical parameters (e.g., G6PDH and SOD).
Cultures were then incubated for 72 h prior to harvesting. At 72 h, the media was vacuum aspirated and the dishes rinsed with warm DPBS. The DPBS was removed, cells were scraped free of the dish, and then the cells were collected in 1 ml of warm DPBS by micropipette and transferred into a labeled 15-ml tube. The cells were then centrifuged at 10×g for 5 min. The supernatant was removed via vacuum aspiration and the cellular pellet was placed on dry ice before transfer to − 80 °C freezer for storage prior to all biochemical analysis. For enzyme release samples, the cells were taken up in PBS rather than DPBS.
Cytotoxicity assays and kits
Determining cytotoxicity in nanomaterial research can be a major challenge (Monteiro-Riviere et al. 2009). Briefly, nanomaterials may interfere with common cytotoxicity assays by scattering light, absorbing light, fluorescence and precursor dyes, and/or product dyes adsorption onto the nanomaterial surface. Many common cytotoxicity assay kits (MTT (3-[4,5-dimethyl-2-thiazol]-2,5-diphenyl-2H-tetrazolium bromide, CAS 298-93-1, Sigma-Aldrich, St Louis, MO), MTS (4-[5-[3-(carboxymethoxy)phenyl]-3-(4,5-dimethyl-1,3-thiazol-2-yl)tetrazol-3-ium-2-yl]benzenesulfonate, CAS 138169-43-4, Promega, Madison, WI), alamar blue (resazurin, CAS 62758-13-8, Cell Titer-Blue, Promega, Madison, WI), ATP (CAS 34369-07-8, CellTiter-Glo® Luminescent Cell Viability Assay, Promega, Madison, WI), and simple visual examination of the cells) have been used by our laboratory seeking to avoid or minimize interferences from the study nanomaterials themselves. After 3 days of nanomaterial treatment, cytotoxicity assays based on MTT, MTS, and alamar blue were performed using commercial kits. Cytotoxicity assay results were always checked with each other and with visual assessment of the cells to ensure that the cytotoxicity assays were working well. Based on microscopic examination of the cultured cells at 20X with a Zeiss inverted microscope, they were classified into the categories of healthy, normal cells, or cells displaying slight toxicity, moderate toxicity, or a high degree of toxicity. A PerkinElmer 1420 Multilabel Counter Victor3V was used as the plate reader for all cytotoxicity assays.
Biochemical assays via Konelab Arena 30
Media was removed and the cultured cells were rinsed with 2 ml of warm DPBS. Then 500 μl of cold PBS was added and the cells removed by scraping. Harvested cells were subjected to five cycles of freezing on dry ice and thawing as a method of cellular disruption. The disrupted cells were then spun at 1500×g for 5 min, the supernatant was transferred to new microfuge tube, and the samples were frozen. All samples were maintained at − 80 °C until processed. The Konelab Arena 30 clinical chemistry instrument (Thermo Scientific) depends on absorption of visible light and was used to determine many enzyme activities (GRD, THRR, SOD, GPx, G6PDH, GGT, CAT, and ALP) and biochemical concentrations (TRIG, T BIL, MIA, GSH, and protein) via commercial kits. The protein assay is based on coomassie blue binding.
For GSH assay, media were decanted and then the cells rinsed with 2000 μl of warm DPBS. Five hundred microliters of cold PBS is added and the cells were removed by scraping. The cells were spun down at 100×g for 10 min and the supernatant was removed. One hundred microliters of a solution of 270-mM trichloroacetic acid and 6.6-mM Na4EDTA was added to the cell pellet. The cell containing the tube was vortexed for 1 min and then spun at 10,000 rpm for 5 min at room temperature. The supernatant was used for the determination of GSH concentration.
LDH enzyme activities do not tolerate freezing and thawing, so LDH assays were done on the day of cell harvesting without freezing. %LDH values are corrected for culture media activity of LDH. The half-life of LDH enzyme that has been released from cells into the surrounding medium is approximately 9 h (information from Promega Technical Bulletin TB163 at www.promega.com/protocols/ CytoTox 96® Non-Radioactive Cytotoxicity Assay (Product G1780)). LDH, AST, and ALT determinations were done from both cells and the culture media. For cellular enzyme determinations of LDH, AST, and ALT, 760 μl of 1% Triton X-100 was added to each culture well and then incubated at 37 °C for 5 min. Supernatants were spun at 1500×g for 5 min and the supernatants stored at 2 to 8 °C (for cellular LDH) or frozen at − 80 °C until processed (for cellular AST and ALT).
Study design
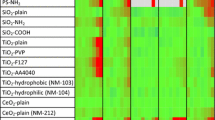
This study was done to determine the biochemical effects of CeO2, SiO2, and TiO2-based particles (dose range 10 to 1000 μg/ml) on enzyme activities in HepG2 cells. The number of samples per group is usually 6 but sometimes is as low as 3 (e.g., for TiO2 T8141 treatments, all experimental N are tabulated in Supplementary Table 1). The major comparisons in this study are between different nanomaterials (structure-activity relationship (SAR), different exposure concentrations (dose-response), and degree of responsiveness of the multiple experimental parameters (Tables 2 and 3)).
Statistical analysis
All data were normalized to protein concentration with the exception of the enzyme release data (%LDH, %AST, and %ALT), GSH, and the protein content itself. All numerical data were analyzed using mixed-effects models in SAS PROC MIXED (SAS v. 9.3 (SAS Institute, Cary, NC)). The data met the assumptions of parametric statistical tests (normal distribution and homogenous variances), and therefore, the data were not transformed. Restricted maximum likelihood estimation was used to estimate the model parameters. A model of Y = dose effects plus an error term with time as a random variable was used to minimize the impact of day effects of measurement. P values were adjusted for multiplicity of testing by a Tukey multiple-comparison test (Table 3). In supplementary Figs. 1 to 10, the displayed standard error of the mean error bars includes both the variation within days and the variation between different experimental days (day effects). Results were considered statistically significant at the P value of < .05. The degree of statistical significance presented in this study is both the common P < .05 and additionally the P < .010 and P < .001 levels. For quantitative comparisons, experimental results are presented as mean, standard deviation, standard error of the mean (SEM), and N in Supplementary Table 1 (e.g., for %LDH, %ALT, %AST, G6PDH, GRD, GPx, and SOD).
Results
Nanomaterial characterization
In this series of nine particles, the range of zeta potentials in cell culture media was − 6.1 to − 13.1 mV (data not shown). In PBS, TiO2 T8141 gave the lowest zeta potential recorded − 16.5 mV. Thus, in cell culture media, the measured zeta potential for all nine particles were in the range where monodispersed nanomaterials are energetically unfavored under the colloidal DLVO (Derjaguin, Landau, Verwey and Overbeek) theory (Mishchuk 2011). Thus, it is not surprising that attractive van der Waals forces were larger than electrostatic repulsion forces and the nanomaterials agglomerated in a cell culture media containing 10% FBS.
Generally, the CeO2 particles showed the smallest hydrodynamic diameters by dynamic light scattering with values ranging from 102 to 597 nm for CeO2 X5, Y6, and Q. For nano CeO2 W4, wet sizes ranged from 116 to 994 nm while nano CeO2 Z7 sizes ranged from 297 to 1326 nm (data not shown). The three SiO2 based particles agglomerated much more in cell culture media giving a size range from about 170 to 2320 nm in the concentration range of 10 to 1000 μg/ml of SiO2 J0, SiO2 K1, and SiO2 N2. TiO2 T8141 gave wet sizes between 505 nm and 897 nm.
As the SiO2 gave the highest wet sizes of any of the nanomaterials we tested, more DLS sizing data is provided for one of the SiO2 series—uncoated JO. All the zeta potentials were unremarkable for JO ranging from a low of − 12.5 to − 9.9 mV in cell culture media. In PBS, 100 μg/ml of J0 had a zeta potential of − 14.6 on day 1 and − 16.1 mV on day 3 compared to − 6.8 (day 1) and − 7.8 mV (day 3) for the PBS solvent alone. The baseline DLS size values recorded for the cell culture media alone were 20 and 175 nm on day 1 and day 3, respectively. On day 1 of cell culture, the mean DLS wet sizes were 172, 739, 1290, and 1744 nm at 30, 100, 300, and 1000 μg/ml of JO, respectively. On day 3 of cell culture, the mean DLS wet sizes were 201, 196, 234, and 2321 nm at 30, 100, 300, and 1000 μg/ml of JO respectively. Thus, for JO, there was clear concentration-dependent agglomeration occurring during cell culture.
Cytotoxicity and released enzymes
Table 2 presents the overall patterns in the dose-dependent degree of cytotoxicity observed after exposures to nine metal oxide particles. At 10 μg/ml, no treatment showed any cytotoxicity using our multiple parameter cytotoxicity system outlined at the bottom of Table 2. However, at 1000 μg/ml, all treatments except TiO2 T8141 (not a nano-sized particle with a low surface area (9.7 m2/g)) (by the Brunauer, Emmett, Teller method (BET)) were graded as high in cytotoxicity degree. The most cytotoxic particles were nano CeO2 Z7, nano SiO2 J0, and nano SiO2 K1 all of which also showed a high degree of cytotoxicity in the dose range of 100 to 300 μg/ml. All the nano CeO2 and nano SiO2 particles showed at least a low or medium degree of cytotoxicity in the dose range of 30 to 300 μg/ml. For exposures to these particles, %LDH and %AST were the major drivers in determining the cytotoxicity rating of these CeO2, SiO2, and TiO2 particles.
With respect to cytotoxicity, a clear overall dose-response pattern was seen (Table 2). No signs of cytotoxicity were seen at 10 μg/ml of any of the nine particles. A low degree of cytotoxicity was seen at 30 to 300 μg/ml, depending on the particle. A medium degree of cytotoxicity was observed at 30 to 1000 μg/ml doses. Finally, at 1000 μg/ml, high degrees of cytotoxicity were seen for everything except TiO2 T8141. The doses required to cause high degrees of cytotoxicity in our HepG2 cells ranged from a low of 100 μg/ml (for nano CeO2 Z7 and nano SiO2 J0) to 1000 μg/ml (the highest concentration used) (for nano CeO2 W4, nano CeO2 X5, nano CeO2 Y6, CeO2 Q, and nano SiO2 N2).
%LDH released
In the data and discussion sections of this paper, the quantitative data itself is primarily presented in Supplementary Table 1 and Supplementary Figs. 1–10. The direction of the biological effects and degree of statistical significance achieved are presented in Table 3. Table 3 allows one to see the pattern of biological responses in respect to dose-response, treatment chemical, and biological parameter. For example, all nine metal oxide treatments were capable of increasing the %LDH released. The dose at which initial membrane dysfunction or damage occurs with LDH release varies but all nine compounds are showing major %LDH effects (all P < .01 and more elevated than 2.4-fold) at 1000 μg/ml (Table 3). Among the CeO2 particles, nano CeO2 Z7 and CeO2 Q appeared to be the most potent cytotoxins to HepG2 cells, with nano CeO2 X5 as the least potent. Nano SiO2 K1 was active at increasing %LDH at much lower doses than either nano SiO2 J0 or nano SiO2 N2. The larger TiO2 particle had a lower degree of %LDH release (Table 3). Graphical representation of all the %LDH released to the media data are provided in Supplementary Fig. 1.
%AST released
Compared to %LDH release, generally HepG2 cells showed the same or less degree of response with %AST (Table 3). In two cases, nano CeO2 Y6 and TiO2 T8141, no significant %AST effects were observed even at 1000 μg/ml. Nano CeO2 Z7 again was the strongest CeO2 particle giving P < .001 responses at 100, 300, and 1000 μg/ml. The parameter %AST was increased by several doses of nano SiO2 J0 and nano SiO2 K1 (at 100, 300, and 1000 μg/ml). Graphical representation of all the %AST released to the media data are provided in Supplementary Fig. 2.
%ALT released
As seen before with other metal oxide nanoparticles (Kitchin et al. 2016), %ALT was much less responsive to nanomaterial exposure than was either %LDH or %AST, the two other cytotoxicity release enzymes. Only three significant findings were observed for %ALT (nano SiO2 K1 at 300 and 1000 μg/ml and nano SiO2 N2 at 1000 μg/ml) (Table 3). %ALT was nonresponsive for all other seven treatments.
Graphical representation of all the %ALT released to the media data is provided in Supplementary Fig. 3.
Biochemical assays via Konelab Arena 30
Parameters related to hepatotoxicity—ALP, MIA, T BIL, TRIG, and protein
No significant effects were seen in either ALP or TRIG for any of the nine particle treatments (Table 3 presents all hepatotoxicity related data). In respect to MIA, only one significant effect was observed, a decrease in MIA following 1000 μg/ml nano SiO2 N2. Nano CeO2 Y6 (100, 300, and 1000 μg/ml) reduced total bilirubin concentration. In contrast, at 1000 μg/ml nano SiO2 J0 increased T BIL, a significant effect in the opposite direction. There were three significant increases in protein concentration found at 300 μg/ml and 1000 μg/ml with nano CeO2 Y6 and 30 μg/ml with nano SiO2 N2.
Graphical representations of all the T BIL and protein data are provided in Supplementary Figs. 4 and 5, respectively.
Parameters related to oxidative stress—CAT, GGT, GPx, SOD, THRR, GSH, GRD, and G6PDH
Catalase enzyme activity was significantly increased by only nano CeO2 Z7 at 1000 μg/ml (Table 3 presents all oxidative stress related data). Three significant increases were observed with GGT, nano CeO2 W4 at 30 μg/ml, nano SiO2 N2 at 1000 μg/ml, and TiO2 T8141 at 300 μg/ml. Nano CeO2Y6 (at 100, 300, and 1000 μg/ml) was the only one of the nine particle treatments that decreased GPx activity (supplementary Fig. 6). Nano SiO2 J0 and nano SiO2 N2 caused reductions in SOD in fairly low (10 and 30 μg/ml for nano SiO2 N2) or more intermediate dose range (100 μg/ml of nano SiO2 N2, 100 and 300 μg/ml of nano SiO2 J0) (Supplementary Fig. 7). Unexpectedly at the highest dose of 1000 μg/ml, no SOD decreases were observed for any of the nine particulate treatments. The sole significant finding with THRR was a reduction at 100 μg/ml of nano SiO2 N2.
Nano CeO2 Z7, nano SiO2 J0, nano SiO2 K1, and nano SiO2 N2 all significantly decreased GSH concentration at 1000 μg/ml (Supplementary Fig. 8). Nano CeO2 Z7 also decreased GSH concentration at 300 μg/ml. Only nano SiO2 (J0, K1, and N2) particles caused significant decreases in GRD activity. These GRD reductions were observed in the dose range of 30 to 1000 μg/ml. G6PDH enzyme activity was the most responsive hepatic enzyme of this study. For two particles (nano CeO2 W4 at 10 and 30 μg/ml and also TiO2 T8141 at 10 μg/ml), significant increases were observed at low exposures. However, the much more common G6PDH biochemical response was decreases in the 30 to 1000 μg/ml range observed with CeO2 Q, nano SiO2 J0, nano SiO2 K1, and nano SiO2 N2. In addition, there were observed G6PDH activity decreases (even though the individual P values ranged only between .15 and .05, thus not reaching the common standard for statistical significance of P < .05) in the dose range of 100 to 1000 μg/ml for nano CeO2 Y6, nano CeO2 Z7, and CeO2 Q, (decreases were seen in six out of eight cases). Graphical representations of all the GRD and G6PDH data are provided in Supplementary Figs. 9 and 10, respectively. Out of all these parameters, GRD and G6PDH stand out as the most responsive.
Discussion
Dose-response
In Table 3, some “statistically significant” responses (P < .05 or even lower) may be spurious as they appear to occur randomly in the data set. These putative “random effects” were not confirmed by similar statistically significant findings at higher doses (possible examples of increased GGT by nano CeO2 W4 at 30 μg/ml, increased G6PDH at 10 and 30 μg/ml by nano CeO2 W4, decreased G6PDH at 30 and 100 μg/ml of CeO2 Q, increased G6PDH at 10 of TiO2 T8141, decreased SOD by 100 and 300 μg/ml of nano SiO2 J0, decreased THRR by 100 μg/ml of nano SiO2 N2, and increased protein in 30 μg/ml of nano SiO2 N2). Other than these possibly random effects, the overall biological responses (particularly with %LDH and %AST) generally appeared fairly monotonic (always increasing with dose). However, the dose-response slope was not high in many cases in the upper dose region. Thus, there was a degree of “upper asymptote” character to some of the dose-response curves. Several examples of biochemical parameters that showed somewhat of an upper asymptote in the quantitative degree of change are shown in Supplementary Table 1. For example, this pattern can be observed with G6PDH by nano SiO2 J0 and nano SiO2 N2, GPx by nano CeO2 Y6, GRD by nano SiO2 J0 and nano SiO2 N2, and T BIL by nano CeO2 Y6.
It is well known that CeO2, SiO2, and TiO2 are highly insoluble in water and do not contribute high concentrations of soluble and potentially toxic metal ions to cause biological effects. This low degree of solubility and ionization of CeO2, SiO2, and TiO2 may be a reason that the slope of dose-response curves for CeO2, SiO2, and TiO2 is so flat in the upper dose region.
In comparing the responses between this study’s five nano CeO2 particles and our group’s prior study (Kitchin et al. 2016) with four other nano CeO2 particles, some conclusions are possible. First, in respect to oxidative stress-related parameters, the current group of nano CeO2 particles showed fewer statistically significant effects in regard to G6PDH, GRD, GPx, and SOD. Second, this same pattern of fewer effects was observed with respect to both HepG2 protein and MIA with the current nano CeO2 particles showing fewer statistically significant effects. Why the current nano CeO2 particles are less biologically active than the prior CeO2 set (Kitchin et al. 2016) is not known. It may be that the current nano CeO2 particle surfaces are less active in generating ROS species.
Structure-activity
The most cytotoxic nano CeO2 was Z7 (by cytotoxicity grading and %AST release); nano CeO2 Z7 also had the largest wet size of these CeO2 particles (1325-nm peak on day 1 of cell culture). These two observations may not be causally related. With respect to G6PDH, GRD, GPx, SOD, MIA, and HepG2 protein, the low number of significant responses seen in the present study was noteworthy when compared with more active CeO2 particles in a prior study (Kitchin et al. 2016).
Attempts to coat SiO2 particles with CeO2 by atomic layer deposition were unsuccessful as determined by multiple chemical assays for Ce concentration by multiple groups. By ICP-OES analysis, the ratio of the coated (K1 or N2)/uncoated (J0) concentrations for several other metals was quite large—for Mn over 80-fold, for Cu over 90-fold, and for Zn over 10-fold. Thus, particles K1 and N2 were inadvertently coated with Mn, Cu, and Zn during the atomic layer deposition procedure. After the atomic layer deposition procedure, the three nano particles appeared to be somewhat different in some of their biological effects. Examples include cytotoxicity grades which were higher for SiO2 J0, intermediate for SiO2 K1, and lowest for SiO2 N2. It was quite surprising that %ALT which often is an unresponsive parameter showed significant changes with both nano SiO2 K1 and nano SiO2 N2, the two “coated” SiO2 particles, but for not for any other of the seven particles tested. For both G6PDH, GRD, and SOD, nano SiO2 J0 and nano SiO2 N2 showed significant reductions in enzyme activity, while nano SiO2 K1 showed fewer effects.
In respect to G6PDH, GRD, and SOD, there was a similarity of effect (all decreases) between the nano SiO2 particles of this study and by the prior nano CeO2 particles (Kitchin et al. 2016). Some metal oxide nanomaterial surfaces may generate ROS and/or RNS free radicals and decrease cellular glutathione concentrations which has been observed in other published studies as well as our own HepG2 studies. For example, GSH concentration decreases have been observed after exposure to CeO2 (Lin et al. 2006) (Monteiller et al. 2007; Kitchin et al. 2014) or SiO2 particles (Ramesh et al. 2013; Polimeni et al. 2008). In murine alveolar macrophages (MH-S) exposed in vitro to 1–10-μm diameter quartz particles, decreases were observed for G6PDH, the pentose phosphate pathway and GSH while increases were seen for thiobarbituric acid reactive substances (Polimeni et al. 2008). In human lung cells (A549), CeO2 nanomaterial exposure produced decreases in GSH and alpha-tocopherol concentration and increases in malondialdehyde and 2′,7′-dichlorofluorescin diacetate fluorescence (Lin et al. 2006).
In human lung cells (A549) exposed to SiO2 nanomaterials, decreases were observed with SOD and GPx activity (Yu et al. 2015). However, increases were seen in respect to malondialdehyde, 2′,7′-dichlorofluorescin diacetate fluorescence and DNA damage by the comet assay. Viewed collectively, our structure-activity data and the published redox-related data of others suggest that some but not all CeO2 and SiO2 surfaces may generate ROS and deplete GSH concentration directly as well as perturb the cellular G6PDH, GR,D and SOD antioxidant defence enzyme systems. Depending on the material and the biological system, nanomaterials are known to have prooxidant, antioxidant, or neither overall biological effect (Merrifield et al. 2013). In our studies, G6PDH, GRD, and SOD are the best biomarkers or functional assays, for oxidative stress (this study and (Kitchin et al. 2016)).
Glutathione, oxidative stress, and literature review
All nine particles tested in the concentration range of 10 to 100 μg/ml did not significantly decrease HepG2 GSH concentration. Above this dose level (at both 300 and 1000 μg/ml), only one CeO2-based nanomaterial (Z7) decreased GSH concentrations. However, at 1000 μg/ml, all three nano SiO2 (J0, K1, and N2) reduced GSH concentration at a high P < .001 level of significance. In our present study, the particle exposures that reduced GSH often caused other biochemical effects as well—decreased activities of G6PDH, GRD, and SOD. It is difficult to know if all four biochemical effects are coming from the same cause or if GSH depletion itself is driving the observed decreases in the activities of G6PDH and GRD.
With respect to published nano CeO2 studies, decreased GPx was found in three different biological systems—in the rat liver (Tseng et al. 2012), human hepatoma (SMMC-7721) cells (Cheng et al. 2013), and rat brain (Hardas et al. 2012). These three GPx studies agree with our observation of nano CeO2 Y6 causing GPx decreases in HepG2 cells (Table 3).
With respect to nano SiO2 studies, GSH depletion has been observed by two investigators in human lung A549 cells (Akhtar et al. 2010; Yu et al. 2015) and also in carp liver (Stanca et al. 2013). In human lung A549 cells treated with SiO2 nanoparticles, decreased GRD was observed (Akhtar et al. 2010). Glutathione concentration decreases are a well-known effect of many metal oxide nanomaterial exposures (Kumar et al. 2011; Kitchin et al. 2014). Following the administration of Si/SiO2 nanoparticles, decreased carp liver G6PDH has been observed (Stanca et al. 2013). Finally, after nano SiO2 exposures to human lung A549 cells, decreased SOD was found (Yu et al. 2015). Overall, several of our observations of the health effects presented in Table 3 (decreased GSH, GPx, GRD, G6PDH, and SOD) are in agreement with observations of others in different biological systems.
Does “cytotoxicity” explain the observed biochemical findings?
The major problems with the interpretation that cytotoxicity causes biochemical effects are that none of the responsive cytotoxic parameters (%LDH, %AST, or combined interpretations of cytotoxicity parameters as a whole) (Table 2) matched the responses of the biological parameters well (Table 3). For example, in 16 out of 18 cases, doses of 300 μg/ml or higher caused medium or high degrees of cytotoxicity (Table 2). However, with the exception of GSH, in all 12 other cases, there was not a medium or higher degree of biological response seen throughout these two higher dose groups (e.g., GPx, SOD, GGT, and protein). At the usually cytotoxic dose of 1000 μg/ml, four of the eight tested particles did indeed significantly deplete GSH concentration (mostly nano SiO2 particles) but four CeO2 particles did not cause GSH depletion at any dose. Thus, overall, the correlation between observed HepG2 cytotoxicity and other biochemical effects was poor. In prior HepG2 hepatotoxicity data sets, there appeared to be a higher degree of possible correlation between cytotoxicity and other biochemical effects (Kitchin et al. 2016).
Physical-chemical characterization to biological effects connection
Extensive physical-chemical characterization on eight of the studied nanomaterials is available from two sources (Geraets et al. 2012, Hancock et al. in preparation) and also summarized in Table 1. In decreasing order, the BET surface area (in m2/g) of the nine studied nanomaterials was 137.4 (SiO2 J0), 128.8 (SiO2 K1), 120.5 (SiO2 N2), 57.0 (CeO2 Z7), 52.8 (CeO2 W4), 40.3 (CeO2 Y6), 20.8 (CeO2 X5), 9.7 (TiO2 T8141), and 3.73 (CeO2 Q) m2/g (Table 1). The larger surface area of the nano SiO2 particles gives an obvious potential cause of the higher degree of activity of these nano SiO2 particles observed for GSH, G6PDH, GRD, and SOD. If surface area is the largest cause of effects however, it is difficult to understand why nano SiO2 K1 has so few effects (3), and nano SiO2 J0 (11) and SiO2 N2 (16) have so many effects. All of nano SiO2 K1-induced effects (G6PDH, GRD, and GSH) were also duplicated by nano SiO2 J0 and nano SiO2 N2. Relative to the number of significant biochemical effects found at the P < .05 level or lower, the order was 16 (SiO2 N2), 11 (SiO2 J0), 8 (CeO2 Y6), 3 (CeO2 W4, CeO2 Z7, and SiO2 K1), 2 (CeO2 Q and TiO2 T8141), and 0 (CeO2 X5). The company Nano-oxides of Salt Lake, Utah, was the source of both nano CeO2 W4 (surface area of 52.8 and three noted significant effects) and nano CeO2 X5 (surface area of 20.8, zero noted significant effects) (Table 1). So, in this case with CeO2, larger surface area (W4 more than X5) correlates with more significant effects. However, the biological effects are probably being determined by many physical-chemical factors; only one of which is surface area.
Utility of this experimental data set to modeling
This large data set should contribute to an even larger potentially useful data set for modelers with respect to dose-response, structure-activity, and linking physical-chemical characteristics with in vitro hepatic effects and other more “big data” orientated uses. In the future, this data from the current and other related hepatotoxicity studies should become available in an EPA knowledge base called “NaKnowBase” that can be used by multiple people in many different institutions for different purposes. From the point of view of modeling, there are two negative aspects to consider. First, the current group of nano CeO2 particles was considerably less active than the first group of studied nano CeO2 particles (Kitchin et al. 2016). Second, with the three nano SiO2-based particles, attempts to coat them with CeO2 failed and we were not able to demonstrate any CeO2-dependent effects with particles K1 and N2 versus J0. As atomic layer deposition placed three new metal contaminants (Mn, Cu, and Zn) on the surface of particles K1 and N2, any differences in the biological properties of the coated particles versus J0 can be due to these three factors. This makes the SiO2 J0 versus SiO2 K1 versus SiO2 N2 SAR comparison quite problematic.
Summary and conclusions
With CeO2, SiO2, & TiO2 particle exposures to HepG2 cells for 3 days, our major findings are:
-
a)
decreased GSH was found after exposure to four nanomaterials (all three nano SiO2 particles),
-
b)
reduced G6PDH activity was observed following many exposures (six out of nine nanomaterials). Decreases in the activity of this enzyme could deplete NADPH and GSH concentrations and lead to oxidative stress (Xu et al. 2010),
-
c)
decreases in SOD and GRD were also observed with exposure to several SiO2 nanomaterials; these biological effects will also contribute to oxidative stress,
-
d)
nano SiO2 was more active than nano CeO2 in respect to decreasing GSH content and G6PDH, GRD, and SOD enzyme activities,
-
e)
cytotoxicity per se did not correlate or explain well the patterns of biological responses observed. The pattern of cytotoxicity degree observed (Table 2) does not match well the number of significant effects found (Table 3) and
-
f)
in this study, the more responsive and informative assays were G6PDH, GRD, SOD, %LDH, and %AST.
References
Akhtar MJ, Ahamed M, Kumar S, Siddiqui H, Patil G, Ashquin M, et al. Nanotoxicity of pure silica mediated through oxidant generation rather than glutathione depletion in human lung epithelial cells. Toxicology. 2010;276(2):95–102.
Cassee FR, van Balen EC, Singh C, Green D, Muijser H, Weinstein J, et al. Exposure, health and ecological effects review of engineered nanoscale cerium and cerium oxide associated with its use as a fuel additive. Crit Rev Toxicol. 2011;41(3):213–29.
Cheng G, Guo W, Han L, Chen E, Kong L, Wang L, et al. Cerium oxide nanoparticles induce cytotoxicity in human hepatoma SMMC-7721 cells via oxidative stress and the activation of MAPK signaling pathways. Toxicol in Vitro. 2013;27(3):1082–8.
Demokritou P, Gass S, Pyrgiotakis G, Cohen JM, Goldsmith W, McKinney W, et al. An in vivo and in vitro toxicological characterisation of realistic nanoscale CeO(2) inhalation exposures. Nanotoxicology. 2013;7(8):1338–50.
Ge Y, Bruno M, Wallace K, Winnik W, Prasad RY. Proteome profiling reveals potential toxicity and detoxification pathways following exposure of BEAS-2B cells to engineered nanoparticle titanium dioxide. Proteomics. 2011;11(12):2406–22.
Geraets L, Oomen AG, Schroeter JD, Coleman VA, Cassee FR. Tissue distribution of inhaled micro- and nano-sized cerium oxide particles in rats: results from a 28-day exposure study. Toxicol Sci. 2012;127(2):463–73.
Grulke E, Reed K, Beck M, Huang XY, Cormack A, Seal S. Nanoceria: factors affecting its pro- and anti- oxidant properties. Environ Sci Nano. 2014;1:429–44.
Hardas S, Sultana SR, Warrier G, Dan M, Florence RL, Wu P, et al. Rat brain pro-oxidant effects of peripherally administered 5 nm ceria 30 days after exposure. Neurotoxicology. 2012;33(5):1147–55.
Holsapple MP, Farland WH, Landry TD, Monteiro-Riviere NA, Carter JM, Walker NJ, et al. Research strategies for safety evaluation of nanomaterials, part II: toxicological and safety evaluation of nanomaterials, current challenges and data needs. Toxicol Sci. 2005;88(1):12–7.
Iavicoli I, Leso V, Fontana L, Bergamaschi A. Toxicological effects of titanium dioxide nanoparticles: a review of in vitro mammalian studies. Eur Rev Med Pharmacol Sci. 2011;15(5):481–508.
Khan MM, Farooq S, Al-Mayouf AA. Metal oxides as photocatalysts. J Saudi Chem Soc. 2015;19:462–4.
Kitchin KT, Prasad RY, Wallace KA. Oxidative stress studies of six TiO(2) and two CeO(2) nanomaterials: immuno-spin trapping results with DNA. Nanotoxicology. 2011;5(4):546–56.
Kitchin KT, Grulke EA, Robinette BL, Castellon BT. Metabolomic effects in HepG2 cells exposed to four TiO2 and two CeO2 nanomaterials. Environ Sci Nano. 2014;1:466–77.
Kitchin KT, Robinette BL, Richards J, Coates NH, Castellon BT. Biochemical effects in HepG2 cells exposed to six TiO2 and four CeO2 nanomaterials. J Nanosci Nanotechnol. 2016;16(9):9505–34.
Kumar A, Pandey AK, Singh SS, Shanker R, Dhawan A. Engineered ZnO and TiO(2) nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic Biol Med. 2011;51(10):1872–81.
Lin W, Huang YW, Zhou XD, Ma Y. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int J Toxicol. 2006;25(6):451–7.
Merrifield RC, Wang ZW, Palmer RE, Lead JR. Synthesis and characterization of polyvinylpyrrolidone coated cerium oxide nanoparticles. Environ Sci Technol. 2013;47(21):12426–33.
Mishchuk NA. The model of hydrophobic attraction in the framework of classical DLVO forces. Adv Colloid Interf Sci. 2011;168(1–2):149–66.
Monteiller C, Tran L, MacNee W, Faux S, Jones A, Miller B, et al. The pro-inflammatory effects of low-toxicity low-solubility particles, nanoparticles and fine particles, on epithelial cells in vitro: the role of surface area. Occup Environ Med. 2007;64(9):609–15.
Monteiro-Riviere NA, Inman AO, Zhang LW. Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line. Toxicol Appl Pharmacol. 2009;234(2):222–35.
Nel AE, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–7.
Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–57.
Nemmar A, Yuvaraju P, Beegam S, Yasin J, Kazzam EE, Ali BH. Oxidative stress, inflammation, and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles. Int J Nanomedicine. 2016;11:919–28.
Park EJ, Park K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol Lett. 2009;184(1):18–25.
Polimeni M, Gazzano E, Ghiazza M, Fenoglio I, Bosia A, Fubini B, et al. Quartz inhibits glucose 6-phosphate dehydrogenase in murine alveolar macrophages. Chem Res Toxicol. 2008;21(4):888–94.
Porter D, Shiram K, Wolfarth M, Jefferson A, Schwegler-Berry D, Andrew M, et al. A biocompatible medium for nanoparticle dispersion. Nanotoxicology. 2008;2(3):144–54.
Price C, Alberti K. Biochemical assessment of liver function. In: Wright RM, Alberti K, Karran S, Millward-Sadler G, editors. Liver and Biliary Disease-Pathophysiology, Diagnosis, Management. London: W. B. Saunders; 1979. p. 381–416.
Ramesh R, Kavitha P, Kanipandian N, Arun S, Thirumurugan R, Subramanian P. Alteration of antioxidant enzymes and impairment of DNA in the SiO2 nanoparticles exposed zebra fish (Danio rerio). Environ Monit Assess. 2013;185(7):5873–81.
Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. https://doi.org/10.1186/1743-8977-10-15.
Shvedova AA, Pietroiusti A, Fadeel B, Kagan VE. Mechanisms of carbon nanotube-induced toxicity: focus on oxidative stress. Toxicol Appl Pharmacol. 2012;261(2):121–33.
Stanca L, Petrache SN, Serban AI, Staicu AC, Sima C, Munteanu MC, et al. Interaction of silicon-based quantum dots with gibel carp liver: oxidative and structural modifications. Nanoscale Res Lett. 2013;8(1):254. https://doi.org/10.1186/1556-276X-8-254.
Thai SF, Wallace KA, Jones CP, Ren H, Castellon BT, Crooks J, et al. Differential genomic effects on signaling pathways by two different CeO2 nanoparticles in HepG2 cells. J Nanosci Nanotechnol. 2015a;15(12):9925–37.
Thai SF, Wallace KA, Jones CP, Ren H, Grulke EA, Castellon BT, et al. Differential genomic effects of six different TiO2 nanomaterials on human liver HepG2 cells. J Biochem Mol Toxicol. 2015b;30(7):331–41.
Thompson TL, Yates JT Jr. Surface science studies of the photoactivation of TiO2--new photochemical processes. Chem Rev. 2006;106(10):4428–53.
Tseng MT, Lu X, Duan X, Hardas SS, Sultana R, Wu P, et al. Alteration of hepatic structure and oxidative stress induced by intravenous nanoceria. Toxicol Appl Pharmacol. 2012;260(2):173–82.
Walker NJ, Bucher JR. A 21st century paradigm for evaluating the health hazards of nanoscale materials? Toxicol Sci. 2009;110(2):251–4.
Warheit DB, Borm PJ, Hennes C, Lademann J. Testing strategies to establish the safety of nanomaterials: conclusions of an ECETOC workshop. Inhal Toxicol. 2007;19(8):631–43.
Xu Y, Zhang Z, Hu J, Stillman IE, Leopold JA, Handy DE, et al. Glucose-6-phosphate dehydrogenase-deficient mice have increased renal oxidative stress and increased albuminuria. FASEB J. 2010;24(2):609–16.
Yokel RA, Hussain S, Garantziotis S, Demokritou P, Castranova V, Cassee FR. The Yin: an adverse health perspective of nanoceria: uptake, distribution, accumulation, and mechanisms of its toxicity. Environ Sci Nano. 2014;1(5):406–28.
Yu Y, Duan J, Li Y, Jin M, Li C, Wang Y, et al. Combined toxicity of amorphous silica nanoparticles and methylmercury to human lung epithelial cells. Ecotoxicol Environ Saf. 2015;112:144–52. https://doi.org/10.1096/fj.09-135731.
Acknowledgments
We are grateful for the participation of many individuals in this study. Particularly, we thank Drs. Carl Blackman, Michael F. Hughes, and Urmila Kodavanti for reviewing this manuscript as part of EPA clearance procedures. Dr. Xinhua Liang of Missouri University of Science and Technology performed the atomic layer deposition which produced nano SiO2 K1 and SiO2 N2.
Funding
US EPA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Disclaimer
The information in this document has been funded wholly by the U. S. Environmental Protection Agency. It has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents necessarily reflect the views of the Agency, nor does mention of trade names or commercial products that constitute endorsement or recommendation for use.
Electronic supplementary material
Supplementary Table 1
(DOCX 94 kb)
Supplementary Figure 1
% LDH released following metal oxide treatment of HepG2 cells (DOCX 155 kb)
Supplementary Figure 2
% AST released following metal oxide treatment of HepG2 cells (DOCX 165 kb)
Supplementary Figure 3
% ALT released following metal oxide treatment of HepG2 cells (DOCX 215 kb)
Supplementary Figure 4
Effects of nine metal oxides on T BIL concentration (DOCX 172 kb)
Supplementary Figure 5
Effects of nine metal oxides on HepG2 protein content (DOCX 201 kb)
Supplementary Figure 6
Effects of nine metal oxides on GPx activity (DOCX 178 kb)
Supplementary Figure 7
Effects of nine metal oxides on SOD activity (DOCX 199 kb)
Supplementary Figure 8
Effects of nine metal oxides on GSH concentration (DOCX 133 kb)
Supplementary Figure 9
Effects of nine metal oxides on GRD activity (DOCX 204 kb)
Supplementary Figure 10
Effects of nine metal oxides on G6PDH activity (DOCX 189 kb)
Rights and permissions
About this article
Cite this article
Kitchin, K.T., Richards, J.A., Robinette, B.L. et al. Biochemical effects of some CeO2, SiO2, and TiO2 nanomaterials in HepG2 cells. Cell Biol Toxicol 35, 129–145 (2019). https://doi.org/10.1007/s10565-018-9445-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-018-9445-x