Abstract
Introduction
Isoniazid (INH) and rifampicin (RIF), the most common anti-tubercular therapy, causes hepatotoxicity through a multi-step mechanism in certain individuals. The present study was an attempt to evaluate the hepatoprotective effect of coenzyme Q10 against INH + RIF-induced hepatotoxicity in Wistar albino rats.
Methods
Hepatotoxicity was induced by the oral administration of INH + RIF (50 mg/kg b.w. each/day) in normal saline water for 28 days. The hepatoprotective effect of coenzyme Q10 (10 mg/kg b.w./day) was compared with that of the standard drug silymarin (25 mg/kg b.w./day). Animals were sacrificed at the end of the study period, and blood and liver were collected for biochemical, immunological and histological analyses.
Results
Evaluation of biochemical parameters showed that coenzyme Q10 treatment caused significant (P < 0.05) reduction in the elevated levels of serum liver function markers and restored normal levels of total protein, albumin and lipids in INH + RIF-treated rats. Also, it was observed that coenzyme Q10 was able to restore normal levels of enzymic antioxidants, reduced glutathione and lipid peroxidation in the INH + RIF-treated rats. Coenzyme Q10 was found to effectively reduce the extent of liver damage caused due to INH + RIF. In addition, the levels of IL-10 and IL-6 were significantly elevated in the INH + RIF-induced rats treated with CoQ10.
Conclusion
Our study indicates the protective role of coenzyme Q10 in attenuating the hepatotoxic effects of INH + RIF in a rat model and that it could be used as a food supplement during anti-tubercular therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) is a widespread, communicable disease that has been declared as global emergency by the World Health Organisation with nine million new cases and about two million deaths per annum. Some of the side effects of anti-tubercular drugs (ATDs) include hepatotoxicity, gastro-intestinal (GI) intolerance, mild rash or itching, peripheral neuropathy, optic neuritis, fatigue and rarely lupus syndrome. Of these, hepatotoxicity and peripheral neuritis are serious side effects of ATDs which pose the biggest challenge in eradicating this dreadful disease (Vanhoof et al. 2003). Isoniazid (INH) and rifampicin (RIF) are the most common first-line regimen prescribed against TB infection. Although they are most effective in killing almost 99 % of tubercle bacilli within the first 2 months of initiation of therapy, they are both well-known hepatotoxic agents causing severe hepatotoxicity in about 1–2 % of patients. Concomitant administration of these two potent ATDs has shown higher incidence of hepatotoxicity (2.55 %) than that observed in regimens containing one or the other (Steele et al. 1991).
INH is metabolised by the liver primarily by acetylation, and the enzyme responsible for this process is N-acetyl transferase 2 (NAT-2) (Ohkura et al. 2010). Acetyl-isoniazid is then metabolised to form toxic monoacetyl hydrazine (MAH), the much less toxic diacetyl hydrazine (DAH) and certain other minor metabolites of INH (Ellard and Gammon 1976). The microsomal enzymes of the CYP450 superfamily further metabolize the INH intermediates through phase I pathways of drug metabolism. Studies have shown that CYP2E1 plays a major role in the metabolism of INH intermediates (Yue et al. 2004). MAH and its metabolites cause injury to hepatocytes possibly through free radical generation. The reactive oxygen species enhance the extent of lipid peroxidation thereby causing damage to cellular membrane (Attri et al. 2000). The depletion of enzymic and non-enzymic antioxidants leave the hepatocytes unprotected against damage due to ROS. RIF is a potent activator of the pregnane X receptors (PXR) in the liver, thereby inducing CYP450 enzymes (Burk et al. 2004; Rae et al. 2001). Hence, rifampicin potentiates the formation of toxic INH metabolites by enhancing metabolic idiosyncratic reaction in the liver cells. RIF causes conjugated hyperbilirubinemia and rarely hypersensitivity reactions causing hepatocellular damage (Byrne et al. 2002; Grosset and Leventis 1983).
Cytokines play a significant role in regulating immune responses in drug-induced liver injury. The mechanisms underlying the processes of hepatocyte regeneration and repair by regulation of the expression of cytokines are unclear. Studies have shown the protective effects of interleukin-10 (IL-10) in models of hepatic injury (Bourdi et al. 2002; Louis et al. 1997). Aithal et al. (2004) have reported IL-10 polymorphisms in relation to disease susceptibility in patients with diclofenac hepatotoxicity. In addition, the role of IL-6 in hepatocyte regeneration has been proven in a mouse model of acetaminophen-induced hepatotoxicity (James et al. 2003). The role of cytokines in ATD-induced liver injury is unclear.
It has been proven through several studies that compounds with significant antioxidant properties are capable of rendering protection to liver tissues against adverse effects caused by ROS (Dong et al. 2014; Lian et al. 2013). Coenzyme Q10 (CoQ10) is the only endogenously produced lipid-soluble antioxidant with powerful antioxidant properties. It was earlier thought that its only main function was to play the role of electron carrier in the mitochondrial respiratory chain. Later, it was found that this benzoquinone in its reduced form is highly effective in preventing lipid, protein and DNA oxidation (Alam and Rahman 2014). Co-administration of CoQ10 with statins has shown to enhance resistance to oxidative stress (Kettawan et al. 2007). CoQ10 was also found to inhibit dimethyl nitrosamine (DMN)-induced liver fibrogenesis by suppressing transforming growth factor-β1 expression in mice (Choi et al. 2009). The protective role of CoQ10 in various models of oxidative stress is evident from previously published data.
The current study is aimed at investigating the hepatoprotective potential of CoQ10 in attenuating INH + RIF-induced liver injury and also to study the change in the antioxidant status and levels of the cytokines IL-6 and IL-10 on INH and RIF co-treatment.
Materials and methods
Chemicals and reagents
Coenzyme Q10 was obtained from Sigma-Aldrich Co., India. Silymarin capsules were purchased from Quality Pharmaceuticals Ltd., India and INH and RIF from Lupin Ltd., Aurangabad, India. INH and RIF were dissolved in normal saline, while silymarin was dissolved in sterile distilled water. Coenzyme Q10 was dissolved in 0.2 ml corn oil. All the other chemicals and reagents used were of analytical grade and procured from SD Fine Chemicals Pvt. Ltd., Mumbai, India.
Animals
The study was carried out using female Wistar albino rats with a mean weight of 161.11 ± 16.48 g procured from VIT Animal House, VIT University, Vellore, Tamil Nadu, India. The animals were housed at six per cage in a light- and temperature-controlled (24 °C ± 2) room with 12-h dark- light cycles. The animals were acclimatized for a week before the study, and the rats were allowed access to standard pelleted feed (Hindustan Lever Ltd., Mumbai, India) and water ad libitum. Guidelines recommended by the Committee for the Purpose of Supervision and Control of Experiments on Animals (CPSCEA), Government of India, Chennai, Tamil Nadu were followed for the care and maintenance of the animals. The experimental procedure was approved by the institutional ethical committee, VIT University, Vellore, India (VIT/IAEC/10th/March 14th/No. 24).
Isoniazid- and rifampicin-induced hepatotoxicity
Saline suspensions of INH and RIF each at a dosage of 50 mg/kg b.w. were prepared separately and administered (p.o.) to the experimental animals for a duration of 28 days to produce hepatotoxicity.
Experimental design
The animals were treated as follows:
-
Group I
Normal control treated with normal saline
-
Group II
INH and RIF each administered at a dosage of 50 mg/kg b.w./day, p.o.) (Rana et al. 2006)
-
Group III
INH + RIF (50 mg/kg b.w. each/day, p.o.) with concomitant administration of CoQ10 (10 mg/kg b.w./day, p.o.)
-
Group IV
INH + RIF (50 mg/kg b.w. each/day, p.o.) with concomitant administration of silymarin (25 mg/kg b.w./day, p.o.)
-
Group V
CoQ10 (10 mg/kg b.w./day, p.o.)
At the end of the experiment, the animals were fasted overnight and sacrificed after ether anaesthesia. Blood was collected from the trunk, and the livers were procured for further analyses. Whole blood was centrifuged to obtain serum samples which were stored immediately at −70 °C for biochemical analyses. After thorough washing with ice-cold 5 % phosphate-buffered saline (PBS), a portion of the liver was fixed in 10 % formalin for histopathological studies. Liver homogenates were prepared using 5 % PBS (1 g tissue in 5 ml) and used for measuring antioxidant parameters.
Determination of liver index
The body weights of the animals were measured once every 3 days during the study period. After sacrifice, rat liver weights were measured, and liver index was calculated using the formula:
Assessment of liver function
Commercial reagent kits used for the estimation of serum aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), total and direct bilirubin, total protein and albumin, total cholesterol, high-density lipoprotein (HDL) and triglyceride levels were purchased from AutoSpan Diagnostics, India. Gamma glutamyl transferase (GGT) and lactate dehydrogenase (LDH) levels (King 1965) were measured in the serum of the normal and experimental rats.
Assessment of antioxidant status
Assays of antioxidant enzymes like superoxide dismutase (SOD) (Marklund and Marklund 1974), catalase (CAT) (Sinha 1972), glutathione peroxidase (GPx) (Rotruck et al. 1973) and glutathione-S-transferase (G-S-T) (Habig et al. 1974) and total reduced glutathione (reduced GSH) (Moron et al. 1979) and the lipid peroxidation levels (Ohkawa et al. 1979) were determined in the liver tissue homogenates.
Measurement of IL-10 and IL-6 levels
The levels of the cytokines IL-10 and IL-6 in the serum of the experimental animals were measured by ELISA using commercial kits obtained from Sigma-Aldrich, India.
Histopathological analysis
The tissues fixed in 10 % formalin were washed and then dehydrated in descending grades of isopropanol and finally with xylene. The tissues were then embedded in paraffin wax, and sections of 5 μm thickness were cut from the respective blocks. The sections were stained with haematoxylin and eosin (H & E) and examined microscopically for histopathological changes.
Statistical analysis
The data obtained were computed to calculate the mean and standard deviation (S.D). Statistical analysis was carried out by one-way ANOVA followed by Student’s Newman–Keul’s test.
Results
Effect of CoQ10 on liver index in INH + RIF-treated rats
The effect of INH and RIF on liver index in the experimental rats is shown in Fig. 1. The INH + RIF-treated rats showed significant reduction in liver index compared to the normal control. The group co-administered with CoQ10 showed to maintain near-normal liver index which was comparable with that of the silymarin-treated rats.
Effect of CoQ10 on liver function markers in INH + RIF-treated rats
INH + RIF-induced rats showed significant elevation in the levels of liver marker enzymes such as AST, ALT and ALP (Table 1). There was also significant hyperbilirubinaemia in the INH + RIF-induced rats, thereby indicating hepatobiliary dysfunction due to these ATDs. INH + RIF-induced rats also showed significant elevation in the levels of GGT and moderate elevation in the levels of LDH in the serum of the experimental animals (Table 1). There was significant reduction in the levels of total protein and albumin in INH + RIF-induced rats. Concomitant administration of CoQ10 was able to normalize the aforementioned parameters which were comparable with that of the silymarin-treated rats.
Assessment of serum lipid profile in the experimental rats revealed a significant increase in total cholesterol, TGL, LDL and phospholipids in INH + RIF-induced rats (Table 2). On the other hand, there was significant reduction in the levels of HDL. Co-administration of CoQ10 was able to restore near-normal lipid profile in the INH + RIF-treated rats.
Effect of CoQ10 on antioxidant status of INH + RIF-induced rats
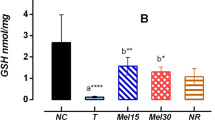
INH and RIF treatment resulted in impaired antioxidant defence system which was evident from the depletion of enzymatic (SOD, CAT, GST and GPx) and non-enzymatic (Reduced GSH) antioxidants and elevated levels of lipid peroxidation (TBARS) (Table 3). CoQ10 treatment was able to restore normal antioxidant status in INH + RIF-induced rats, thereby indicating its potential role as an antioxidant.
Effect of CoQ10 on cytokine levels
The levels of IL-6 and IL-10 were significantly increased in the INH + RIF-treated rats compared to the normal group. Co-administration with CoQ10 showed a decrease in the levels of IL-6 to near normal but augmented the increase in IL-10 levels (Figs. 2 and 3). The effect of CoQ10 on levels of IL-6 and IL-10 was compared to that of the standard hepatoprotective drug silymarin.
Effect of CoQ10 on liver histo-architecture in INH + RIF-induced rats
Microscopic examination of liver sections in the normal control rats (group I) revealed normal morphology of hepatocytes with the central vein (Fig. 4—5a). INH and RIF-treated rats (group II) showed minimal periportal inflammation with microvesicular fatty change in hepatocytes (arrow) along with nuclear pyknosis (double arrow) with lobular architecture being maintained (Fig. 4—5b). Rats co-administered with CoQ10 showed normal liver morphology, while those co-administered with silymarin presented with focal areas showing the pyknotic nuclei of hepatocytes (arrow) (Fig. 4—5c and 5d). CoQ10-alone-treated rats showed normal morphology of hepatocytes (Fig. 4—5e).
Haematoxylin and eosin staining of liver sections: 5a Group I illustrating normal liver histoarchitecture. 5b Group II showing minimal periportal inflammation with microvesicular fatty changes in hepatocytes (arrow) and nuclear pyknosis (double arrow). 5c Group III showing normal hepatocyte morphology. 5d Group IV presenting with focal areas showing pyknotic nuclei of hepatocytes (arrow). 5e Group V illustrating normal liver histology. Total magnification ×400
Discussion
Drug-induced liver injury (DILI) is an adverse effect of current ATDs, which is of major concern. Co-administration of INH and RIF is potentially toxic to the liver due to synergistic effect (Skakun and Shman’ko 1985). INH is metabolised by the CYP450 enzymes, the major toxic metabolite formed being MAH. RIF is a well-known, strong inducer of CYP450 enzymes causing increased metabolic conversion of INH and thereby increased accumulation of its toxic metabolites (Kanebratt et al. 2008). This could be the reason behind the increased incidence of hepatic necrosis in patients administered with INH and RIF as compared to those administered with either of the two drugs. In addition, ATD-induced oxidative stress also plays a major role in the progression of liver injury (Chowdhury et al. 2001).
The present study revealed significantly elevated levels of serum liver functional markers (AST, ALT, ALP, GGT, LDH, total and direct bilirubin), total cholesterol, LDL and triglycerides in INH and RIF treated rats. Also, the levels of serum total protein, albumin and HDL were significantly reduced on the co-administration of INH and RIF. These parameters clearly indicate that INH and RIF co-exposure leaves the hepatocyte membranes unprotected and susceptible to drug-induced peroxidative damage. This might have led to the release of intracellular enzymes into the circulation. Increased activities of serum aminotransferases with concomitant reduction in serum albumin levels are presumptive indicators of acute liver damage (Giannini et al. 2005).
Concurrent supplementation of CoQ10 in INH and RIF-treated rats showed protective effects by reducing the extent of hepatic damage and restoring near-normal levels of antioxidants. ATDs, like many other antibiotics, favour the generation of free radicals resulting in hepatocellular damage. CoQ10, being a powerful antioxidant, might have reduced cellular damage caused due to lipid peroxidation in INH and RIF treated rats. Studies have shown that idiosyncratic drug-induced hepatic injury may be mediated in part by oxidative stress in INH and RIF co-administration (Boelsterli and Lee 2014). This is evident from the depletion of enzymatic and non-enzymatic antioxidants and elevation of lipid peroxidation levels in liver tissue homogenates of INH and RIF-treated rats. In addition, studies have indicated that there is a strong association between hypercholesterolemia and increased generation of ROS (Martinet et al. 2001).
The production of several inflammatory cytokines in DILI has been known to increase the extent of liver tissue damage (Steuerwald et al. 2013). The present study shows elevated levels of IL-6 and IL-10 in INH and RIF-treated rats. IL-6, being a pro-inflammatory cytokine, controls the expression of other cytokines such as TNF-α and IL-10, indicating inflammatory hepatic damage to play a part in ATD-induced hepatotoxicity. A transient increase in IL-6 levels in humans has been shown to induce an anti-inflammatory environment (Steensberg et al. 2003). IL-6 is a well-known mediator of acute phase response, causing transcriptional activation of acute phase plasma proteins (APPs), and it also activates signalling pathways such as JAK/STAT, Ras/MAPK and PI3K. Studies have shown positive feedback mechanisms for IL-6 signalling (Lee et al. 2012). Therefore, IL-6 plays both pro-inflammatory and anti-inflammatory roles. TGF-β- and IL-6-mediated increase in IL-10 has been shown in previous studies (McGeachy et al. 2007). Pachkoria et al. (2008) studied the relevance and outcome of IL-10 polymorphisms in drug-induced liver damage and found that low IL-10 producing haplotype may be associated with worse clinical outcome from DILI.
Conclusion
The current study demonstrates the protective effect of CoQ10 against INH + RIF-induced hepatotoxicity by attenuating oxidative stress and inflammatory damage. This protective effect could be attributed to its ability to restore normal antioxidant levels and reduce the expression of inflammatory cytokines while augmenting the expression of anti-inflammatory cytokines such as IL-10. The efficacy of CoQ10 in reducing the inflammation caused in INH + RIF-induced rats is evidenced by a significant decrease in IL-6 and significant increase in IL-10. The hepatoprotective effect of CoQ10 is further confirmed by histological examination of liver tissues. However, further studies focussing on the mechanistic roles of various pro- and anti-inflammatory cytokines would help unveil immune regulation processes involved in INH + RIF-induced hepatotoxicity.
Abbreviations
- ATDs:
-
Antitubercular drugs
- CoQ10:
-
Coenzyme Q10
- DAH:
-
Diacetyl hydrazine
- DMN:
-
Dimethyl nitrosamine
- INH:
-
Isoniazid
- MAH:
-
Monoacetyl hydrazine
- RIF:
-
Rifampicin
References
Aithal GP, Ramsay L, Daly AK, Sonchit N, Leathart JBS, Alexander G, et al. Hepatic adducts, circulating antibodies, and cytokine polymorphisms in patients with diclofenac hepatotoxicity. Hepatology. 2004;39(5):1430–40.
Alam MA, Rahman MM. Mitochondrial dysfunction in obesity: potential benefit and mechanism of Co-enzyme Q10 supplementation in metabolic syndrome. J Diabetes Metab Disord. 2014;13:60.
Attri S, Rana SV, Vaiphei K, Sodhi CP, Katyal R, Goel RC, et al. Isoniazid- and rifampicin-induced oxidative hepatic injury—protection by N-acetylcysteine. Hum Exp Toxicol. 2000;19(9):517–22.
Boelsterli UA, Lee KK. Mechanisms of isoniazid-induced idiosyncratic liver injury: emerging role of mitochondrial stress. J Gastroenterol Hepatol. 2014;29(4):678–87.
Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, et al. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology. 2002;35(2):289–98.
Burk O, Koch I, Raucy J, Hustert E, Eichelbaum M, Brockmöller J, et al. The induction of cytochrome P450 3A5 (CYP3A5) in the human liver and intestine is mediated by the xenobiotic sensors pregnane X receptor (PXR) and constitutively activated receptor (CAR). J Biol Chem. 2004;279(37):38379–85.
Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, Thompson RJ. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology. 2002;123(5):1649–58.
Choi H-K, Pokharel YR, Lim SC, Han H-K, Ryu CS, Kim SK, et al. Inhibition of liver fibrosis by solubilized coenzyme Q10: role of Nrf2 activation in inhibiting transforming growth factor-β1 expression. Toxicol Appl Pharmacol. 2009;240(3):377–84.
Chowdhury A, Santra A, Kundu S, Mukherjee A, Pandit A, Chaudhuri S, et al. Induction of oxidative stress in antitubercular drug-induced hepatotoxicity. Indian J Gastroenterol. 2001;20(3):97–100.
Dong Y, Huang J, Lin X, Zhang S, Jiao Y, Liang T, et al. Hepatoprotective effects of yulangsan polysaccharide against isoniazid and rifampicin-induced liver injury in mice. J Ethnopharmacol. 2014;152(1):201–6.
Ellard GA, Gammon PT. Pharmacokinetics of isoniazid metabolism in man. J Pharmacokinet Biopharm. 1976;4(2):83–113.
Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. Can Med Assoc J. 2005;172(3):367–79.
Grosset J, Leventis S. Adverse effects of rifampin. Clin Infect Dis. 1983;5(Supplement 3):S440–6.
Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130–9.
James LP, Lamps LW, McCullough S, Hinson JA. Interleukin 6 and hepatocyte regeneration in acetaminophen toxicity in the mouse. Biochem Biophys Res Commun. 2003;309(4):857–63.
Kanebratt KP, Diczfalusy U, Bäckström T, Sparve E, Bredberg E, Böttiger Y, et al. Cytochrome P450 induction by rifampicin in healthy subjects: determination using the karolinska cocktail and the endogenous CYP3A4 marker 4beta-hydroxycholesterol. Clin Pharmacol Ther. 2008;84(5):589–94.
Kettawan A, Takahashi T, Kongkachuichai R, Charoenkiatkul S, Kishi T, Okamoto T. Protective effects of coenzyme Q10 on decreased oxidative stress resistance induced by simvastatin. J Clin Biochem Nutr. 2007;40(3):194–202.
King J. The dehydrogenases or oxidoreductases— lactate dehydrogenase. In: Van D, editor. Practical clinical enzymology. London: Van Nostrand; 1965. p. 93–193.
Lee J, Nakagiri T, Oto T, Harada M, Morii E, Shintani Y, et al. IL-6 amplifier, NF- B-triggered positive feedback for IL-6 signaling, in grafts is involved in allogeneic rejection responses. J Immunol. 2012;189(4):1928–36.
Lian Y, Zhao J, Xu P, Wang Y, Zhao J, Jia L, et al. Protective effects of metallothionein on isoniazid and rifampicin-induced hepatotoxicity in mice. Ho W, editor. PLoS ONE. 2013;8(8):e72058.
Louis H, Le Moine O, Peny M, Quertinmont E, Fokan D, Goldman M, et al. Production and role of interleukin-10 in concanavalin a-induced hepatitis in mice. Hepatology. 1997;25(6):1382–9.
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem FEBS. 1974;47(3):469–74.
Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Oxidative DNA damage and repair in experimental atherosclerosis are reversed by dietary lipid lowering. Circ Res. 2001;88(7):733–9.
McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat Immunol. 2007;8(12):1390–7.
Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582(1):67–78. BBA - Gen Subj.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8.
Ohkura K, Fukino K, Shinohara Y, Hori H. N-Acetyl transferase 2 polymorphisms associated with isoniazid pharmacodynamics: molecular features for ligand interaction. Anticancer Res. 2010;30(8):3177–80.
Pachkoria K, Lucena MI, Crespo E, Ruiz-Cabello F, Lopez-Ortega S, Fernandez MC, et al. Analysis of IL-10, IL-4 and TNF-α polymorphisms in drug-induced liver injury (DILI) and its outcome. J Hepatol. 2008;49(1):107–14.
Rae JM, Johnson MD, Lippman ME, Flockhart DA. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J Pharmacol Exp Ther. 2001;299(3):849–57.
Rana SV, Pal R, Vaiphie K, Singh K. Effect of different oral doses of isoniazid–rifampicin in rats. Mol Cell Biochem. 2006;289(1–2):39–47.
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–90.
Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47(2):389–94.
Skakun NP, Shman’ko VV. Synergistic effect of rifampicin on hepatotoxicity of isoniazid. Antibiot Med Biotechnol. 1985;30(3):185–9. Minist Meditsinskoĭ Promyshlennosti SSSR.
Steele MA, Burk RF, DesPrez RM. Toxic hepatitis with isoniazid and rifampin. A meta-analysis. Chest. 1991;99(2):465–71.
Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285(2):E433–7.
Steuerwald NM, Foureau DM, Norton HJ, Zhou J, Parsons JC, Chalasani N, et al. Profiles of serum cytokines in acute drug-induced liver injury and their prognostic significance. PLoS One. 2013;8(12):e81974.
Vanhoof J, Landewe S, Van Wijngaerden E, Geusens P. High incidence of hepatotoxicity of isoniazid treatment for tuberculosis chemoprophylaxis in patients with rheumatoid arthritis treated with methotrexate or sulfasalazine and anti-tumour necrosis factor inhibitors. Ann Rheum Dis. 2003;62(12):1241–2.
Yue J, Peng R, Yang J, Kong R, Liu J. CYP2E1 mediated isoniazid-induced hepatotoxicity in rats. Acta Pharmacol Sin. 2004;25(5):699–704.
Acknowledgments
The authors would like to thank VIT University for providing the facilities for conducting this study.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baskaran, U.L., Sabina, E.P. The food supplement coenzyme Q10 and suppression of antitubercular drug-induced hepatic injury in rats: the role of antioxidant defence system, anti-inflammatory cytokine IL-10. Cell Biol Toxicol 31, 211–219 (2015). https://doi.org/10.1007/s10565-015-9305-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-015-9305-x