Abstract
Tuberculosis (TB) is a challenging public health issue, particularly in poor and developing countries. Rifampicin (RIF) is one of the most common first-line anti-TB drugs but it is known for its adverse effects on the hepato-renal system. The present study investigated the efficacy of morin hydrate (MH) in protecting hepato-renal damage inflicted by RIF in rats. RIF (50 mg/kg), and a combination of RIF (50 mg/kg) and MH (50 mg/kg) were administered orally for 4 weeks in rats. Silymarin (50 mg/kg) was used as a positive control. Increased levels of serological parameters such as AST, ALT, ALP, LDH, GGT, bilirubin, triglyceride, total cholesterol, urea, uric acid, creatinine, TNF-α, IFN-γ, IL-6 along with the decreased level of IL-10, total protein and albumin were used as markers of hepatic and renal injury. Oxidative damage in the tissues was measured by the increase in lipid peroxidation and decline in GSH, SOD and catalase activities. Histopathology of liver slices was used to study hepatic architecture. Four-week RIF treatment produced altered serological parameters with an increase in pro-inflammatory cytokines in serum suggesting hepatotoxicity and nephrotoxicity. The antioxidant status of the liver and kidney (increased lipid peroxidation and decline in GSH, SOD and catalase) was compromised. Cellular damage and necrosis were observed in liver slices. MH supplementation with RIF improved hepato-renal functions by restoring the serum and tissue markers towards normal values. Histological observations authenticated the results. MH supplementation also reduced the production of pro-inflammatory cytokines. Thus, the results revealed that MH provides protection against RIF-induced hepato-renal injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) is categorized as a pandemic by WHO, infecting one-fourth of the world's population [1]. Globally it is the ninth leading cause of death from a single infectious pathogen, surpassing HIV infection. It is a challenging public health issue, particularly in poor and underdeveloped nations. In the year 2020, WHO reported about 10 million cases of TB infection and 1.5 million TB-related deaths (including 214,000 HIV-positive). Global TB trends indicate a reduction of ~ 2% cases per year, with a 9% decline in cases between the years 2015–2019 [2].
The drugs isoniazid (INH), rifampicin (RIF), pyrazinamide (PYZ), and ethambutol (ETB) are the four primary anti-TB drugs. A standard treatment plan for adult pulmonary TB infection involves two months of intake of INH, RIF, ETB and PYZ, followed by four months of INH and RIF [2]. RIF is now the first-line medicine for the cure of TB, which is still a worldwide severe health issue. The WHO recommends RIF for at least 6 months of TB therapy. RIF binds the β-subunit of DNA-dependent RNA polymerase (RNAP) nearby the RNA–DNA hybrid and inhibits the bacterial RNAP β-subunit, therefore physically inhibiting the elongation of the expanding RNA chain after the addition of 2–3 nucleotides [3]. However, the exact mechanism of transcriptional interference mediated by RIF that eventually results in cell death is unknown.
The liver plays a vital role in drug metabolism and detoxification and hence it is prone to injury because of xenobiotic load. Drug-induced liver injury (DILI) is a severe clinical concern [4]. Similarly, the kidneys are also notable targets of xenobiotics’ toxicity. The drug-triggered renotoxicity is expressed by a diminishing urine concentration, tubular proteinuria, mild glycosuria, slow ammonium elimination, and a waning glomerular filtration rate [5]. Long-term usage of RIF may cause serious side effects, including liver damage, yellow gangrene, hepatomegaly, nephrotoxicity and cholestasis. RIF causes hepatic impairment during the early administration phase, but this symptom vanishes when its intake is discontinued. Furthermore, RIF disturbs bilirubin excretion by producing transient hyperbilirubinemia. Since it causes hepatic damage by cholestasis, it is also associated with typical hepatic lesions arising from hepatocellular alterations together with centrilobular necrosis [6].
Morin hydrate (MH; 2′, 3, 4′, 5, 7-pentahydroxyflavone), a flavonoid, is a yellow-coloured compound found in many herbs, red wine, and fruits [7, 8]. MH possesses antioxidant, neuroprotective, anti-inflammatory, antihypertensive, and anticancer activities [9]. It has been demonstrated to prevent liver damage, renal toxicity, inflammation, and fibrosis in various animal studies [10]. In this study, sub-acute toxicity of RIF in rat liver and kidney was investigated biochemically and histopathologically. Further, the effect of MH supplementation on toxicity alleviation was also studied.
Materials and Methods
Experimental Animals
Healthy albino Wistar rats were maintained in the animal house under standard conditions and fed with a standard diet, and water was given ad libitum.
Experimental Design and Treatment Schedule
After one week of acclimatization, the rats were distributed into six groups (n = 5). All the drugs/compounds (50 mg/kg) were given orally every day for 28 days as follows:
Group-I: Normal control
Group-II: RIF
Group-III: RIF + Silymarin (SLM)
Group-IV: RIF + MH
Group-V: SLM
Group-VI: MH
Measurement of Body Weight and Relative Organ Weight
Rat weight was monitored every day. The relative organ mass was measured as given below:
Relative organ weight (%) = [Organ weight/body weight] × 100.
Blood Sampling
Blood was drawn by heart puncture and serum was separated, and stored at -80 °C for further analysis.
Biochemical Analysis
Assessment of Liver and Kidney Function Markers in Serum
Alanine transaminase (ALT), aspartate transaminase (AST), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), bilirubin, triglycerides, total cholesterol, total protein, albumin, urea, uric acid, and creatinine were assayed in serum using Erba Diagnostics Kits. Absorbance was measured with the help of UV–Visible spectrophotometer (Evolution-201, Thermo scientific).
Determination of Serum Inflammatory Marker
Serum levels of IFN-γ, TNF-α, IL-6 and IL-10 were determined by specific ELISA kits (Krishgen Biosystems, India).
Preparation of Tissue Homogenate
Liver and kidney tissue homogenates (10%) were prepared. The supernatant was used for the assessment of antioxidant enzymes and other analytes [11].
Assessment of Enzymatic and Non-enzymatic Parameters in Tissue Homogenate
Determination of Total Protein
The protein was measured by the method of Lowry et al. [12].
Estimation of Malondialdehyde (MDA)
Lipid peroxidation was measured by the method of Niehaus and Samuelsson [13].
Estimation of the Activities of Antioxidant Enzymes
Superoxide dismutase (SOD) was measured by the method of
Marklund and Marklund [14]. The catalase (CAT) was measured by the method of Beers and Sizer [15].
Estimation of Reduced Glutathione (GSH)
GSH was determined by the method of Ellman et al. [16].
Histological Analysis of Liver and Kidney
The liver and kidney sections of rats were prepared and stained with hematoxylin and eosin [17]. Tissue slices were observed under a light microscope (40\(\times\)).
Statistical Analysis
Experiments were done in triplicate. Results were expressed as mean ± SD. Graphs were plotted using GraphPad Prism software version 5.01. P values (< 0.05) were considered significant.
Results
Alteration in Body Weight and Relative Organ Weight
RIF-treated rats (Group II) lost 22.33% of their body weight over the course of four weeks (Table 1). However, control rats gained approximately 20% of their body weight. There was significantly lesser weight loss in Group IV rats than in Group II rats. The MH and RIF combination had a restorative impact on body weight (10.33% recovery). Similar results were obtained with RIF + SLM treatment (11.33%). Body weight and relative liver and kidney weight were shown to have an inverse relationship. As compared to control rats, RIF treatment caused an increment in relative liver weight (from 2.79% to 4.82%) and relative kidney weight (from 0.30% to 0.54%) whereas MH/SLM treatment had a recuperative effect on relative liver and kidney weight. Thus MH/SLM co-treatment (Group III and IV) resulted in significant recovery (p < 0.05) as compared to Group II rats. The results of Group V and VI were comparable to Group I rats.
Serum Biochemical Analysis
The liver and kidney function indicators are employed to diagnose and treat a wide range of illnesses. After 28 days of RIF treatment, serum levels of ALT, AST, ALP, LDH, GGT, and bilirubin, total cholesterol, triglyceride, creatinine, urea, and uric acid rose significantly, whereas albumin and total protein levels decreased (Table 2; Figs. 1, 2). However, on MH and SLM supplementation the levels of ALT, AST, ALP, LDH, GGT, bilirubin, creatinine, urea, uric acid, total cholesterol, and triglycerides significantly declined with enhancement in total protein and albumin than in RIF-treated rats (p < 0.05). The rats treated with the SLM showed significantly improved serum liver and kidney function parameters (p < 0.05). Thus, the effects of MH therapy were equivalent to that of SLM treatment (Fig. 1).
Effect of morin hydrate on serum total protein (A), albumin (B), total cholesterol (C), and triglycerides (D) in rifampicin-induced toxicity in albino Wistar rats. Each value is expressed in mean ± SD (n = 5). (*) represents a significant difference from the control (p < 0.05). (#) represents a significant difference from group II (p < 0.05). Silymarin (SLM) treated rats were considered as a positive control. Abbreviations: MH-morin hydrate; RIF-rifampicin
Effect of morin hydrate on serum creatinine (A), urea (B) and uric acid (C) in rifampicin-induced toxicity in albino Wistar rats. Each value is expressed in mean ± SD (n = 5). (*) represents a significant difference from the control (p < 0.05). (#) represents a significant difference from group II (p < 0.05). Silymarin (SLM) treated rats were considered as a positive control. Abbreviations: MH-morin hydrate; RIF-rifampicin
Assessment of Antioxidant Enzyme in Tissue Homogenate
The activities of both antioxidant enzymes SOD and CAT in tissue homogenates of Group II rats decreased significantly after four weeks of RIF administration, suggesting toxicity (Table 3). As compared to control rats, activities of SOD (32.37U/mg) and CAT (8.12U/mg) in RIF-treated rat liver declined to 12.16U/mg and 4.22U/mg, respectively. Similarly, in kidney tissue, activities of SOD and CAT also declined from 27.23U/mg to 9.72U/mg and 6.79U/mg to 3.27U/mg, respectively. However, MH supplementation with RIF showed significant restoration of the liver (SOD 23.59U/mg; CAT 6.07U/mg) and kidney enzymes (SOD 22.07U/mg; CAT 4.72U/mg) in tissue homogenate. SLM supplementation also exhibited similar effect on enzyme activities (Table 3).
Assessment of MDA and GSH in Tissue Homogenate
In comparison to the MDA levels in control rats (liver-1.28 nM/mg, and kidney-1.31 nM/mg protein), RIF treatment for four weeks impelled an approximate upsurge in MDA in the liver and kidney tissues with values of 7.03 nM/mg and 7.30 nM/mg protein, respectively (Table 3). MH treatment promoted reduction in MDA levels in liver and kidney tissues (4.80 nM/mg and 5.18 nM/mg protein). However, a greater reduction in MDA was observed with SLM therapy in liver and kidney tissues (3.76 nM/mg and 4.05 nM/mg protein, respectively). However, as compared to the amount of GSH content in control rats (liver-71.60 nM/mg and kidney-71.20 nM/mg protein), the GSH level in group II was considerably lower (37.73 nM/mg and 37.02 nM/mg protein). Furthermore, MH treatment led to an increase in GSH in liver and kidney tissues (55.33 nM/mg and 54.88 nM/mg protein, respectively). Similarly, SLM treatment also enhanced the GSH content in the liver and kidney (63.68 nM/mg and 63.52 nM/mg protein, respectively).
Histopathological Changes in Liver
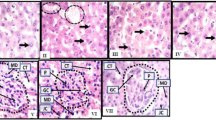
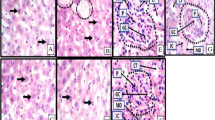
The control rat liver's histo-pathological profile exhibited a standard lobular architecture with a central vein, intact hepatocytes with sinusoidal gaps and uniformly dispersed cytoplasm (Fig. 3A). The RIF-treated group (Fig. 3B) showed morphological changes and fibrosis, as shown by tissue disruption, extensive fibrous septa development, and fibre accumulation coupled with massive hepatocellular degeneration, necrosis, inflammatory cell infiltration, and cytoplasmic vacuolation [18]. Concurrent administration of MH/SLM and RIF accounted for the reasonable improvement (Fig. 3C, D) in hepatic architecture as evidenced by a reduction in liver damage and associated hepatic injuries by suppressing hepatocellular degeneration and necrosis and thus significantly improving liver structure and function. The histological improvement observed in the liver tissue on the combined treatment of RIF and MH corroborates with the biochemical findings.
Serum Inflammatory Marker
Serum levels of TNF-α, IFN-γ and IL-6 increased significantly with a reduction in IL-10 in rats treated with RIF (Fig. 4). MH treatment with RIF reversed the alterations observed in group II rats. The effect of MH on the restoration of inflammatory markers was comparable to that of the standard hepato- renal protective drug SLM which was used as a positive control.
Effect of morin hydrate on serum inflammatory markers IFN-γ (A), TNF-α (B), IL-6 (C), and IL-10 (D) in RIF-induced toxicity in albino Wistar rats. Each value is expressed in mean ± SD (n = 5). (*) represents a significant difference from the control (p < 0.05). (#) represents a significant difference from group II (p < 0.05). Silymarin (SLM) treated rats were considered as a positive control. Abbreviations: MH-morin hydrate; RIF-rifampicin; IFN-γ-interferon gamma; TNF-α-tumor necrosis factor-α; IL-interleukin
Discussion
Hepatic and renal damage is a major concern during TB therapy. Anti-tuberculosis drugs (ATDs) undergo bio-transformation in the liver and kidney producing more harmful products such as free radicals. These free radicals cause oxidative stress which in turn inflicts cell damage and finally culminates in necrosis, or cell death. Oxidative damage has been implicated to be a major mechanism of hepatotoxicity triggered by ATDs [19].
RIF and INH are two of the most important first-line drugs used to treat TB. However, both drugs can cause DILI [20]. RIF inhibits RNAP, a potent inducer of numerous metabolizing enzymes. When RIF is used along with INH, it can increase the metabolism of INH, leading to an augmented buildup of byproducts that can cause liver injury and/or immune reactions [21]. Moreover, RIF can activate the pregnane X receptor (PXR) that regulates the expression of genes involved in drug metabolism. PXR activation can lead to increased production of toxic metabolites, which can further damage the liver [22]. The liver damage caused by existing ATDs is a serious problem that requires urgent attention. New and effective medications are needed to reverse this damage and improve the lives of people with TB [23].
Plant-derived herbal medications are increasingly being used to treat a diversity of clinical ailments including oxidative stress [24, 25]. MH, a plant flavonol, possesses anti-hyperglycemic, anti-inflammatory, anti-diabetic, and hepatoprotective activities [26, 27]. Its antioxidant activity plays a vital role in avoiding organ injury caused by free radicals and thus plays a critical role in cytoprotection against harmful effects of several toxicants [28]. The current work endorses that MH provides protection against RIF-induced hepato- and renotoxicity in rats.
This study found that rats who regularly ingested RIF alone (group II) experienced a significant decrease in body weight with an increase in relative liver weight suggesting its toxic effect (Table 1). RIF intoxication caused an imbalance in hepatic function, as evidenced by significant elevations in serum enzymes. Several reports corroborated these findings [1, 19]. The observed effects are likely due to hepatic injury, as these enzymes are released after cellular damage [1]. Liver histology further substantiated hepatic damage by portraying RBC degeneration in the portal triad and cytoplasmic clearance in disease-control group II rats (Fig. 3). However, rats co-treated with MH showed restorative effects on biochemical and morphological features suggesting the hepatoprotective action of MH (Table 2).
An abnormal increase in bilirubin levels can indicate liver disease or dysfunction. Prolonged RIF intake in rats resulted in significant elevation in serum bilirubin level which may be a consequence of liver damage/obstruction of the bile salt exporter pump by RIF. Suppression of protein synthesis has been linked to the dissociation of ribosomal subunits, an important component in fatty liver disease that can contribute to liver injury [18, 25]. In RIF-intoxicated rats, altered liver function was also signified by a notable diminution in total protein and albumin. Furthermore, supplementation of MH/SLM with RIF resulted in decreased serum bilirubin levels and increased total protein and albumin levels. During the experimental period, regular intake of MH or SLM alone (Group III/IV) did not produce toxicity. These biochemical findings further suggested that MH offers protection against hepatocellular injury caused by RIF. The histopathological analysis validates the biochemical outcomes, as MH and SLM at the studied doses diminished RIF-induced hepatotoxicity. MH had healing effects on cells with a reduction in inflammatory cells, diminished necrosis, and improved hepatocyte regeneration (Fig. 3).
Higher levels of serum kidney function markers viz., creatinine, BUN, and uric acid along with relative kidney weight in RIF-treated rats indicated kidney dysfunction with reduced clearance of these substances. It may be because of acute kidney injury caused by the generation of immune aggregates by anti-RIF antibodies [29]. Reactive oxygen species (ROS) and oxidative stress have also been linked to drug-induced kidney damage [30, 31]. The study showed that MH supplementation significantly lowered serum creatinine, urea, and uric acid levels and was thus effective in preventing renotoxicity by RIF (Fig. 2; Table 1). The restorative effect of MH on kidney function might be attributed to increased glomerular filtration and appropriate tubular reabsorption, thereby reducing metabolite load in blood.
A minor surge in serum levels of total cholesterol and triglycerides was observed in RIF-treated rats, which is suggestive of an altered lipid profile and disturbances in lipid metabolism [32, 33]. Previous studies have shown that treatment with RIF induces the buildup of total cholesterol and triglycerides in the liver due to the inhibition of bile acid secretion [34]. MH treatment for a period of four weeks significantly improved the lipid profile. The exact mechanism by which MH reduces total cholesterol and triglyceride levels is unclear, but it is likely due to the alteration of cholesterol metabolism by the liver or lipoprotein lipase activity [35].
The use of RIF can cause hepato-renal injury, as evidenced by modifications in the cellular antioxidant defence system. RIF is known to induce the CYP450 system, leading to increased RIF metabolism and the production of more toxic metabolites [19]. These toxic metabolites produced by RIF can induce oxidative stress in hepatocytes, leading to hepatocellular injury. This can lead to an accumulation of ROS, depletion of glutathione stores, and hepatocellular injury [36]. MH has been proven to defend against CCl4-induced chemical liver damage by decreasing the oxidative stress response in previous investigations [10, 37].
Augmented lipid peroxidation in RIF-treated rats is shown by elevated MDA in liver and kidney tissues, suggestive of the abundance of ROS [38]. In addition, diminished activity/level of SOD, CAT, and GSH in liver and kidney tissues substantiates augmented oxidative stress in RIF-treated rats signifying compromised redox homeostasis. SOD and CAT are two enzymes that work together to guard cells against damage caused by ROS by converting them into less harmful molecules. The unregulated generation of superoxide anion has the ability to inactivate SOD, which then inactivates CAT [39, 40]. GSH protects liver and kidney tissues from oxidative insult. The supplementation of MH with RIF improved the tissues’ antioxidant status by restoring the levels of antioxidant markers (Table 3). SLM also produced similar results. MH is thought to have inhibited the generation of free radicals, which led to the observed effect in hepato-renal tissue. In the present study, MH administration with RIF was found to somewhat decrease the generation of superoxide anion, as evidenced by the observed elevation in the SOD and CAT activity in the liver and kidney tissues of rats. This suggests that MH can protect the injury to hepatic and renal tissue from oxidative stress by preventing the inactivation of these important antioxidant enzymes and sustaining GSH levels.
Several inflammatory cytokines have been shown to enhance the degree of liver tissue damage in DILI [41]. In the current study, pro-inflammatory cytokines (TNF-α, INF-γ and, IL-6) were found to be higher in RIF-treated rats with lower levels of anti-inflammatory cytokine IL-10, indicating that inflammatory liver damage is involved in ATD-induced hepatotoxicity. In humans, a transitory rise in pro-inflammatory cytokines and a drop in IL-10 have been found to generate an inflammatory milieu (Fig. 4) [42]. Further, pro-inflammatory cytokines are well-known mediators of the acute phase response, activating signalling pathways viz., JAK/STAT, Ras/MAPK, and PI3K and triggering transcriptional activation of acute-phase plasma proteins [43, 44]. MH supplementation in rats decreased the production of inflammatory cytokines and chemokines suggesting an anti-inflammatory action with a return to normalcy. Thus, MH supplementation with RIF is thought to be advantageous for protecting the liver and kidney functions against oxidative damage and inflammation.
Conclusion
RIF is a commonly used anti-tubercular drug (ATD) and its combination with other ATDs is prescribed for long-term treatment of TB. Prolonged intake of RIF evokes harmful effects on the liver and kidney, leading to hepatotoxicity and nephrotoxicity. RIF-induced hepatotoxicity is associated with the induction of metabolizing enzymes, affecting drug metabolism and generating toxic metabolites. The current study emphasized the positive outcome of MH supplementation on the hepato-renal system during RIF-induced toxicity in rats. RIF treatment adversely affected the liver and kidney function markers with increased pro-inflammatory cytokines in serum. The antioxidant status of organs was compromised and aberrations in hepatic sections were visible. The results inferred that oxidative stress resulting from ROS production during RIF metabolism was responsible for cellular damage and necrosis of liver and kidney tissues. MH supplementation improved the liver and kidney functions as evidenced by the restoration of serum biochemical markers towards normal values along with the reduction in organ tissue damage consistent with histological improvements. MH also restored the antioxidant status of the liver and kidney as revealed by the reduction in lipid peroxidation and increment in SOD, CAT and GSH activities in tissue homogenate. Further, MH supplementation also reduced the production of pro-inflammatory cytokines indicating an anti-inflammatory effect. Overall, MH supplementation showed promise in protecting against RIF-induced hepato-renal toxicity by mitigating oxidative stress and reducing inflammation. Further research is needed to understand the underlying mechanisms of MH's protective effects and to explore its possible application in clinical settings.
References
Nwidu LL, Teme RE. Hot aqueous leaf extract of Lasianthera africana (Icacinaceae) attenuates rifampicin-isoniazid-induced hepatotoxicity. J Integrative Med. 2018;16:263–72.
WHO, Global tuberculosis report 2021. https://www.who.int/publications/digital/global-tuberculosis-report-2021/tb-diagnosis-treatment/drug-resistant-treatment
Campbell EA, Korzheva N, Mustaev A, Murakami K, Nair S, Goldfarb A, et al. Structural Mechanism for Rifampicin Inhibition of Bacterial RNA Polymerase. Cell. 2001;104:901–12.
Dubiwak AD, Gerema U, Abdisa D, Tofik E, Reta W. Amelioration of Nephrotoxicity in Mice Induced by Antituberculosis Drugs Using Ensete ventricosum (Welw.) Cheesman Corm Extract. Int J Nephrol. 2022;2022:6941509.
Thuawaini MM, Al-Farhaan MBG, Abbas F, K,. Hepatoprotective and nephroprotective effects of the aqueous extract of turmeric (Curcuma longa) in rifampicin and isoniazid-induced hepatotoxicity and nephrotoxicity in rats. Asian J Pharm Clin Res. 2019;1:293–8.
Kim J-H, Nam W, Kim S, Kwon O, Seung E, Jo J, et al. Mechanism investigation of rifampicin-induced liver injury using comparative Toxico-proteomics in Mice. IJMS. 2017;18:1417.
Lee KM, Lee Y, Chun HJ, Kim AH, Kim JY, Lee JY, et al. Neuroprotective and anti-inflammatory effects of morin in a murine model of Parkinson’s disease: Morin Alleviates Neurotoxicity in PD. J Neurosci Res. 2016;94:865–78.
Caselli A, Cirri P, Santi A, Paoli P. Morin: a promising natural drug. Curr Med Chem. 2016;23:774–91.
Gendy A, Elnagar MR, Soubh A, Al-Mokaddem A, El-Haddad A, El-Sayed MK. Morin alleviates hepatic ischemia/reperfusion-induced mischief: In-vivo and in-silico contribution of Nrf2, TLR4, and NLRP3. Biomed Pharmacother. 2021;138: 111539.
Li X, Yao Q, Huang J, Jin Q, Xu B, Chen F, et al. Morin hydrate inhibits TREM-1/TLR4-Mediated inflammatory response in macrophages and protects against carbon tetrachloride-induced acute liver injury in mice. Front Pharmacol. 2019;10:1089.
Gupta A, Pandey AK. Aceclofenac-induced hepatotoxicity: AN ameliorative effect of Terminalia bellirica fruit and ellagic acid. World J Hepatol. 2020;12:949–64.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75.
Niehaus WG, Samuelsson B. Formation of Malonaldehyde from Phospholipid Arachidonate during Microsomal Lipid Peroxidation. Eur J Biochem. 1968;6:126–30.
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74.
Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–40.
Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colourimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95.
Cardiff RD, Miller CH, Munn RJ. Manual Hematoxylin and Eosin Staining of Mouse Tissue Sections. Cold Spring Harb Protoc. 2014; 2014:pdb.prot073411.
Gupta A, Kumar R, Ganguly R, Singh AK, Rana HK, Pandey AK. Antioxidant, anti-inflammatory and hepatoprotective activities of Terminalia bellirica and its bioactive component ellagic acid against diclofenac induced oxidative stress and hepatotoxicity. Toxicol Rep. 2021;8:44–52.
Chen X, Xu J, Zhang C, Yu T, Wang H, Zhao M, et al. The protective effects of ursodeoxycholic acid on isoniazid plus rifampicin induced liver injury in mice. Eur J Pharmacol. 2011;659:53–60.
Kumar S, Kumar R, Dwivedi A, Pandey AK. In vitro antioxidant, antibacterial, and cytotoxic activity and in vivo effect of Syngonium podophyllum and Eichhornia crassipes leaf extracts on isoniazid induced oxidative stress and hepatic markers. BioMed Res Int. 2014;2014:459452. https://doi.org/10.1155/2014/459452.
Anderson MS, Cote J, Liu Y, Stypinski D, Auger P, Hohnstein A, et al. Effects of Rifampin, a potent inducer of drug-metabolizing enzymes and an inhibitor of OATP1B1/3 transport, on the single dose pharmacokinetics of anacetrapib. J Clin Pharmacol. 2013;53:746–52.
Shehu AI, Li G, Xie W, Ma X. The pregnane X receptor in tuberculosis therapeutics. Expert Opin Drug Metab Toxicol. 2016;12:21–30.
Dong Y, Huang J, Lin X, Zhang S, Jiao Y, Liang T, et al. Hepatoprotective effects of Yulangsan polysaccharide against isoniazid and rifampicin-induced liver injury in mice. J Ethnopharmacol. 2014;152:201–6.
Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Sci World J. 2013;10: 162750.
Ganguly R, Gupta A, Pandey AK. Role of baicalin as a potential therapeutic agent in hepatobiliary and gastrointestinal disorders: a review. World J Gastroenterol. 2022;28(26):3047–62.
Kapoor R, Kakkar P. Protective role of morin, a flavonoid, against high glucose induced oxidative stress mediated apoptosis in primary rat hepatocytes. PLoS ONE. 2012;7:e41663.
Folorunso IM, Lawal AO, Elekofehinti OO, Iwaloye O. Hepatoprotective effect of morin hydrate in type 2 diabetic Wistar rats exposed to diesel exhaust particles. Appl Biochem Biotechnol. 2023. https://doi.org/10.1007/s12010-023-04366-4. Epub ahead of print. PMID: 36708492.
Wu T-W, Zeng LH, Wu J, Fung KP. Morin hydrate is a plant-derived and antioxidant-based hepatoprotector. Life Sci. 1993; 53:PL213–8.
Martin SJ, Sabina EP. Amelioration of anti-tuberculosis drug-induced oxidative stress in kidneys by Spirulina fusiformis in a rat model. Ren Fail. 2016;38:1115–21.
Prince SE, Martin SJ, Lavinya BU, Selvanathan K, Geetha A. Anti-tuberculosis drug-induced oxidative stress in kidneys: role of brahmi as an antioxidant supplement. Pharmacogn Magazani. 2019;15(62):12–6.
Saravana MMG, Ramakrishnan T, Mani V, Achary A. Protective effect of crude sulphated polysaccharide from turbinaria ornata on isoniazid rifampicin induced hepatotoxicity and oxidative stress in the liver, kidney and brain of adult swiss albino rats. Indian J Biochem Biophys. 2018;55(8):237–44.
Jyothi B, Mahalakshmi S, Anitha K. Protective effect of Mirabilis jalapa leaves on anti-tubercular drugs induced hepatotoxicity. Asian J Pharm Clin Res. 2013;6(3):221–4.
Abd El-Reheem AMA, Abdel-Wahhab KG, AbdelWahhab MM, Morsy FA, Soliman SME, Abdel-Tawab MA. Protective effect of some natural extracts against isoniazid-induced hepatotoxicity in adult male rats. Curr Sci Int. 2015;4(3):409–22.
Pal R, Rana SV, Vaiphei K, Singh K. Isoniazid-rifampicin induced lipid changes in rats. Clin Chim Acta. 2008;389:55–60.
Ichikawa T, Liang J, Kitajima S, Koike T, Wang X, Sun H, et al. Macrophage-derived lipoprotein lipase increases aortic atherosclerosis in cholesterol-fed Tg rabbits. Atherosclerosis. 2005;179:87–95.
Fakhruddin S, Alanazi W, Jackson KE. Diabetes-induced reactive oxygen species: mechanism of their generation and role in renal injury. J Diabetes Res. 2017;2017:1–30.
Singaravelu A, Venkatachalam K, Jayaraj RL, Jayabalan P, Nadanam S. Morin treatment for acute ethanol exposure in rats. Biotech Histochem. 2021;96:230–41.
Messarah M, Saoudi M, Boumendjel A, Kadeche L, Boulakoud MS, Feki AE. Green tea extract alleviates arsenic-induced biochemical toxicity and lipid peroxidation in rats. Toxicol Ind Health. 2013;29:349–59.
Sharma UK, Kumar R, Gupta A, Ganguly R, Pandey AK. Renoprotective effect of cinnamaldehyde in food colour-induced toxicity. 3 Biotech. 2018; 8:212.
Ganguly R, Kumar R, Pandey AK. Baicalin provides protection against fluoxetine-induced hepatotoxicity by modulation of oxidative stress and inflammation. World J Hepatol. 2022;14:729–43.
Steuerwald NM, Foureau DM, Norton HJ, Zhou J, Parsons JC, Chalasani N, et al. Profiles of serum cytokines in acute drug-induced liver injury and their prognostic significance. PLoS ONE. 2013;8(12):81974.
Avitsur R, Hunzeker J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain Behav Immun. 2006;20:339–48.
Baskaran UL, Sabina EP. The food supplement coenzyme Q10 and suppression of antitubercular drug-induced hepatic injury in rats: the role of antioxidant defence system, anti-inflammatory cytokine IL-10. Cell Biol Toxicol. 2015;31(4–5):211–9.
Hong DG, Lee S, Kim J, Yang S, Lee M, Ahn J, Lee H, Chang SC, Ha NC, Lee J. Anti-Inflammatory and neuroprotective effects of morin in an MPTP-induced Parkinson’s Disease model. Int J Mol Sci. 2022;23(18):10578.
Acknowledgements
HKR acknowledges UGC, New Delhi for CRET fellowship. AKS acknowledges the CSIR, India for JRF and SRF. All authors also acknowledge UGC-SAP and DST-FIST facilities of the Biochemistry Department, University of Allahabad, Prayagraj, India.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: AKP; Experiments: HKR, AKS; data analysis: HKR, AKS, AKP; first draft: HKR; critically reviewed and supervised: AKP; all authors: read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical Approval
The in-vivo study was performed in accordance with the Guide for the Care and Use of Laboratory Animals as approved by the University of Allahabad, Prayagraj.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rana, H.K., Singh, A.K. & Pandey, A.K. Therapeutic Potential of Morin Hydrate Against Rifampicin Induced Hepato and Renotoxicity in Albino Wistar Rats: Modulation of Organ Function, Oxidative Stress and Inflammatory Response. Ind J Clin Biochem 39, 197–206 (2024). https://doi.org/10.1007/s12291-023-01145-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-023-01145-0