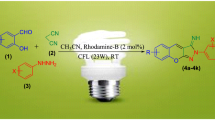
Abstract
Herein, an apposite and straightforward methodology has been established for the synthesis of 4,5-dihydro-1H-pyrazole and its derivatives by using Chalcones and Hydrazines as substrates in EtOH under 20W CFL irradiation at room temperature. The distinctive trait of this protocol is the utilization of an efficient, green, highly water soluble organophotoredox catalyst “Rhodamine B”, a simple red xanthene dye, to create C–N bonds via formation of free radicals and eventually terminating with intramolecular cyclization. Short reaction time, environmentally benign approach, cost effectiveness, feasibility and adaptability with respect to a wide range of substrates and good to excellent yield of the product supplements the synthesis.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
For last few decades, the scientific communities have together augmented their voice about the impact of our acts on the environment, putting pressure on the authorities and looking for explications to address the issue. Amongst them, chemistry has received foremost attention, as many conventional chemical procedures are not sustainable over long periods of time, with disastrous outcomes for the environment and human well-being. Within this frame of reference, the concept ‘Green Chemistry’ is slowly but continuously attaining popularity [1, 2].
Visible-light photoredox catalysis has been experiencing a resurgence as a consequence of the rising interest in renewable energy and green chemistry [3,4,5]. On considering recent environmental concerns which necessitates the development of sustainable methodologies over the conventional practices, visible light procedures are a judicious choice to explore the full possibility of this protocol. In the last few years, visible light triggered transformations have captured a notable attention from scientists and organic chemists in the area of environmentally benign synthesis with various advantages like eco-sustainability, ubiquity, operational feasibility, mild reaction conditions, non-hazardous nature and thus have emerged as a valuable approach for a myriad of organic synthesis [6, 7].
Although, some organic compounds do not undergo the required reaction due to their incapability to absorb visible light. To overcome this constraint, chemists use transition metals, nanoparticles and organic dyes as photo catalysts [8,9,10,11]. In this regard, a highly water soluble and simple red xanthene dye, Rhodamine-B (RB), is a fascinating alternative and is being widely used as an organophotoredox catalyst for organic interconversions [12,13,14].
Nitrogen containing compounds symbolize an indispensable category of compounds because of their relevance to biological and medicinal significance. Within this context, a five membered nitrogen containing heterocycle, 4,5-dihydro-1H-pyrazole exhibits various biological and pharmaceutical properties like being anti-cancer, anti-convulsant, anti-depressant, anti-melanoma, anti-microbial and anti-infective activities [15,16,17,18,19,20], etc. (Fig. 1).
Up to now, several synthetic routes for 4,5-dihydro-1H-pyrazoles have been developed, but these procedures are linked with numerous disadvantages like utilization of large quantities of reagents or catalysts, long reaction time, use of inorganic bases, very high temperature, etc. [21,22,23,24,25]. Henceforth, the scheming of an easy and proficient method for direct formation of nitrogen comprising heterocycle, 4,5-dihydro-1H-pyrazole, posed as an alluring and a challenging pursuit for us. In order to expand our continuing efforts on environmentally benign synthesis [26,27,28,29,30,31] and the huge biological attributes of 4,5-dihydro-1H-pyrazoles, herein we have developed an easy, green, visible light driven, two component protocol for the synthesis of 4,5-dihydro-1H-pyrazole and its derivatives by using Rhodamine B as organophotoredox catalyst and Chalcones (1) and Hydrazines (2) as reactants in EtOH under 20W CFL irradiation at room temperature (Scheme 1).
2 Results and Discussion
Our initial efforts focused on the exploration of optimized reaction conditions by electing chalcone (1a) and phenylhydrazine (2b) as model substrates. The results have been abridged in (Table 1 and 2).
To begin with, we took chalcone (1a) and phenylhydrazine (2b) in toluene using 5 mol% of Rhodamine B under CFL (20 W) irradiation and found that only 25% product was formed (Table 1, entry 1). Subsequently, we have carried out this reaction with other solvents like DMF, DMSO, CH3CN, DCM, MeOH and EtOH (Table 1, entries 2–7). Fascinatingly, it was observed that EtOH is a perfect solvent for the present methodology which afforded 85% yield of the product within 1 h (Table 1, entry 7). In an effort to further upsurge the efficacy of this reaction, we performed a number of test reactions in presence of different photocatalysts and varied sources of visible light (Table 2). We discovered that 10 mol% Rhodamine-B afforded 90% yield of the product in 1 h (Table 2, entry 5). Our next focus consisted of deciding on the appropriate photocatalyst loading (Table 2, entries 4–6). Multiple trials led us to deduce that 10 mol% Rhodamine-B is the most favorable for this protocol (Table 2, entry 5). Our next venture was to examine the varying effects of employing different intensities of visible light source (CFL) on the present procedure (Table 2, entries 5 and 7–10). In order to achieve this goal, we carried out a series of reactions by using 18 W, 20 W, 22 W CFLs, sunlight and white LED as a source of visible light and observed that 20 W CFL is the most appropriate for the present protocol (Table 2, entry 5). Further, to get an insight into the mechanism of proposed synthetic route, we used a radical scavenger “TEMPO” [(2,2,6,6-Tetramethyl-piperidin-1-yl)oxyl] and established that the reaction was inhibited which directs towards the participation of radicals in the reaction [32] (Table 2, entry 12).
Eventually, we succeeded in establishing the finest reaction conditions for this procedure with model substrates and succeeded; Rhodamine-B (10 mol%) catalyzed, visible-light-facilitated synthetic route for 4,5-dihydro-1H-pyrazoles in EtOH under 20 W CFL irradiation with 90% optimal yield of the product in 1 h.
As soon as the optimal reaction conditions were deciphered, we were enthused about the evaluation of the possibilities and restrictions of this reaction pathway with various derivatives of chalcone (1) and hydrazine (2). To our delectation, reactants containing electron withdrawing group or electron donating group, both were well abided by and provided substantial yield of the product (Scheme 2).
2.1 Gram Scale Synthesis
We have also executed the above experimentation on a large scale to corroborate the practicability of the proposed transformation. For this, we took EtOH in a round bottom flask and added Chalcone (1a, 10 mmol, 2.0826 g), phenylhydrazine (2b, 10 mmol) in presence of Rhodamine B (10 mol%) under 20 W CFL irradiation at room temperature and product 4,5-dihydro-1H-pyrazole (3e) was formed within 1 h in 90% yield by employing common laboratory glasswares (Scheme 3).
2.2 Mechanism
On the basis of above experimental results and literature studies, [13, 14, 26, 27, 29, 33] a proposed mechanism has been discussed thoroughly in scheme 4. In presence of light, on photo-absorption, Rhodamine B goes to its excited state (RB*) and a single electron transfer (SET) takes place which converts chalcone (1) and phenyl hydrazine (2) into their corresponding radical-anion (A) and radical-cation (B), respectively. Simultaneously, Rhodamine B radical-cation and Rhodamine B radical-anion that are formed in the first step get quenched by oxygen and this completes the photocatalytic cycle of Rhodamine B. [13, 14] Thereafter, coupling of the radical-anion (A) and radical-cation (B) takes place and results into intermediate (C). Now, the homolytic cleavage of N–H bond and C=C bond of intermediate (C) occurs which results into intermediate (D). Eventually, the biradical coupling of intermediate (D) followed by dehydration results into the desired product (3).
3 Conclusion
In conclusion, we have efficaciously developed a visible-light-triggered, eco-efficient and facile synthetic route for 4,5-dihydro-1H-pyrazoles using Rhodamine B as a photoredox catalyst and Chalcones (1), Hydrazines (2) as substrates in EtOH under 20W CFL irradiation at room temperature. Rhodamine B is a water soluble, cost effective and easily obtainable organic dye and has proven to be the most effective photocatalyst for this procedure. To the best of our knowledge, this is the first visible-light-driven synthetic route starting from Chalcones (1) and Hydrazines (2) which exhibits hallmarks like being straightforward, simplistic and green to synthesize a series of 4,5-dihydro-1H-pyrazoles and its derivatives with good to excellent yields of the product in short reaction time. Practicability, mild reaction conditions, broad substrate scope, utilization of CFL and low-priced organic dye unveils this method as more interesting and performable with respect to the aforementioned reported methods. Besides, it is an effortless and environmentally benign method which can be profitable for chemical and therapeutical industries.
References
Kulshreshtha A (2015) Soc Issues Environ Probl 3:1
Ivankovic A, Dronjic A, Bevanda AM, Talic S (2017) IJSGE 6:39
Lang X, Zhaob J, Xiaodong C (2016) Chem Soc Rev 45:3026–30386
Xu G, Xu P (2021) Chem Commun 57:12914–12935
Chen J, Hu X, Lu L, Xiao W (2016) Chem Soc Rev 45:2044–2056
Dai C, Liu B (2020) Energy Environ Sci 13:24–52
Uygur M, Danelzik T, Mancheño OG (2019) Chem Commun 55:2980–2983
Srivastava V, Singh PP (2017) RSC Adv 7:31377–31392
Peng Y, Feng CT, Li YQ, Chen FX, Xu K (2019) Org Biomol Chem 17:6570–6573
Saravanan R, Gupta VK, Narayanan V, Stephen A (2014) J Taiwan Inst Chem Eng 45:1910–1917
Helm MPV, Klemm B, Eelkema R (2019) Nat Rev Chem 3:491–508
Zhang JJ, Cheng YB, Duan XH (2017) Chin J Chem 35:311–315
Xie LY, Hu JL, Song YX, Jia GK, Lin YW, He JY, Cao Z, He WM (2019) ACS Sustain Chem Eng 7:19993–19999
Xie LY, Chen YL, Qin L, Wen Y, Xie JW, Tan JX, Huang Y, Cao Z, He WM (2019) Org Chem Front 6:3950–3955
He H, Xu N, Zhang H, Chen B, Hu Z, Guo K, Chun J, Cao S, Zhu Y (2021) RSC Adv 11:17340–17345
Aboul-Enein MN, El-Azzouny AA, Attia MI, Maklad YA, Amin KM, Abdel-Rehim M, El Behairy MF (2012) J Med Chem 47:360–369
Li QS, Lv XH, Zhang YB, Dong JJ, Zhou WP, Yang Y, Zhu HL (2012) Bioorg Med Chem Lett 22:6596–6601
Goodell JR, Puig-Basagoiti F, Forshey BM, Shi PY, Ferguson DM (2006) J Med Chem 49:2127–2137
Agrawal M, Sonar PK, Saraf SK (2011) Med Chem Res 21:3376–3381
Sivakumar PM, Seenivasan SP, Kumar V, Doble M (2010) Chem Lett 20:3169–3172
Bhat P, Shridhar G, Ladage S, Ravishankar L (2017) J Chem Sci 129:1441–1448
Gadekar SP, Pawar GT, Magar RR, Lande MK (2020) Polycycl Aromat Compd 40:126–134
Pise AS, Jadhav SD, Burungale AS, Devkate SS, Gawade RB (2018) Asian J Chem 30:894–896
Babiola AS, Pothiappan V, Subburethinam R (2018) RSC Adv 8:30071–30075
Jaiswal D, Tiwari J, Singh S, Sharma AK, Singh J, Singh J (2018) Curr Organocatalysis 5:229–238
Mishra A, Srivastava M, Rai P, Yadav S, Tripathi BP, Singh J, Singh J (2016) RSC Adv 6:49164–49172
Mishra A, Jaiswal A, Sharma AK, Pandey YK, Singh J, Singh J (2023). Catal Lett. https://doi.org/10.1007/s10562-023-04279-1
Mishra A, Rai P, Singh J, Singh J (2018) ChemistrySelect 3:8408–8414
Mishra A, Rai P, Srivastava M, Tripathi BP, Yadav S, Singh J, Singh J (2017) Catal Lett 147:2600–2611
Tripathi BP, Mishra A, Rai P, Pandey YK, Srivastava M, Yadav S, Singh J, Singh J (2017) New J Chem 41:11148–11154
Sharma AK, Jaiswal A, Mishra A, Jaiswal D, Singh S, Singh J, Singh J (2020) New J Chem 44:13350–13356
Yadav AK, Yadav LDS (2015) Org Biomol Chem 13:2606–2611
Bu MJ, Lu GP, Cai C (2016) Catal Sci Technol 6:413–416
Acknowledgements
The authors are thankful to SAIF, Punjab University, Chandigarh, India and SAIF, CDRI, Lucknow, India for providing spectral data. Prof. Jagdamba Singh acknowledges the financial support from UGC, New Delhi in the form of BSR Faculty Fellowship (No. F.18-1/2011 (BSR)).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishra, A., Pandey, Y.K., Sharma, A.K. et al. A Rhodamine-B Catalyzed Visible-Light-Mediated Benign Synthetic Route for 4,5-Dihydro-1H-pyrazoles. Catal Lett 154, 4169–4175 (2024). https://doi.org/10.1007/s10562-024-04591-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-024-04591-4