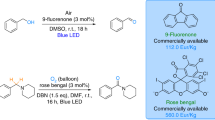
Abstract
A novel photocatalytic cyclization reaction was developed for the synthesis of quinazolinones from o-aminobenzamides and in-situ generated aldehydes from alcohols using 9-fluorenone as the photocatalyst through a "one-pot" process. Furthermore, alcohols are perfect alternatives to aldehydes due to some unique advantages, such as being green, less toxic, available, and economical. The present protocol showed good tolerance for various substrates and could afford a range of quinazolinones (29 examples) up to 91% under ambient conditions.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
N-containing heteroaromatic compounds are important structures found in natural products, pharmaceutically important molecules, and organic functional materials [1,2,3]. Among them, quinazolinone and its derivatives have received considerable attention in recent years, because they exhibit significant pharmacological and biological activities, such as antibacterial, antineoplastic, anti-in ammatory, anticonvulsant, antimalarial, anti-asthmatic, anti-Alzheimer, and anticancer (Scheme 1) [4,5,6,7]. In addition, quinazolinones play an important role in organic reactions due to their excellent characteristics. Hence, the excellent characteristics of quinazolinone derivatives have promoted extensive studies of their synthesis, and a number of synthetic methods have been developed over the past few decades [8,9,10,11,12]. Despite these significant advances, the most classical and general approaches still utilized o-aminobenzamides and aldehydes as the substrates to form quinazolinones followed by the oxidation of the resulting aminal intermediates in the laboratory and industry [13,14,15,16]. Most of these processes generally require the use of stoichiometric of non-renewable oxidants (KMnO4 [17], DDQ [18], and CuCl2 [19]) and metal catalysts [20,21,22]. However, aldehydes were required as the reagents to generate quinazolinones with good yields in the above reactions, which were significantly toxic and sensitive to unavoidably occurring self-aldol side reactions. Therefore, the development of greener and more harmless material instead of aldehydes for the synthesis of quinazolinones is highly desirable. As a class of compounds, alcohols are perfect alternatives to aldehydes because they are greener, more available, more economical, more stable, and less toxic than aldehydes, which are smoothly oxidized to aldehydes followed by the condensation with o-aminobenzamides forming quinazolinones. In recent years, various metal catalysts or oxidants (such as Ir [23], Pd [24], Ru [25], Mn [26], Fe [27], Cu [28], DMSO [29], TBHP [30]) have been used to prepare quinazolinones (Scheme 2). Although these protocols have certain disadvantages, these condensations require the use of metal catalysts or stoichiometric quantities of toxic oxidants. Therefore, it is imperative to develop a more practical, green, and efficient approach to constructing quinazolinones.
Recently, visible-light catalysis has attracted widespread research interest in light degradation [31, 32] and organic synthesis owing to the inherent green, mild, and character of light [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. Many kinds of organic transformations have already been accomplished with good to excellent yields under ambient conditions by using transition-metal catalysts, such as Aza-Henry reaction, oxidative addition, and cross-coupling reaction [48,49,50,51]. However, compared to transition metal catalysts, organic dyes, and small organic molecules, as photosensitizers, have recently aroused growing interest due to their high efficiency of visible-light absorption, enhanced stability, and easy modification for visible light catalysis [52,53,54,55,56,57]. Advantageously, these organic molecules show unique reactivity and unparalleled selectivity in organic reactions, and the structures of these molecules can be easily optimized for obtaining the desired products. Especially, 9-fluorenone, as a commercially available and cheap metal-free photocatalyst, can activate O2 molecule to transform it into reactive oxygen species (ROS) such as superoxide anion radical, hydrogen peroxide, singlet oxygen and hydroxy radical which are the key oxidants in many organic reactions [58,59,60].
Based on all this information and our own interest to explore metal-free catalysis, we develop an efficient, highly atom economical, and environmentally friendly one-pot strategy for constructing quinazolinones from alcohols with o-aminobenzamides in good yields using 9-fluorenone as the photocatalyst by irradiation of visible light under ambient conditions.
2 Experiment
2.1 Materials
All reagent-grade chemicals were obtained from commercial suppliers and were used as received unless otherwise noted (Table S1, Support Information). DMSO (anhydrous, ≥ 99.9%) and CH3CN (anhydrous, ≥ 99.9%) were purchased from Sigma Aldrich.
2.2 General Procedure for the Synthesis of Quinazolinones Using Alcohol
O-aminobenzamides (0.2 mmol), alcohols (0.24 mmol), 9-fluorenone (0.01 mmol, 5 mol %), p-TsOH (0.02 mmol, 10 mol %), CH3CN (1.8 mL), and DMSO (0.2 mL) were added to a 10 mL flat quartz glass jar and placed in a photocatalytic parallel reactor. The container was placed under the 10 W blue LEDs lamp at room temperature for 16 h. After completion of the reaction, 100 mL of distilled water is added to the mixture. Then, the mixture was extracted with ethyl acetate (50 mL×3), dried over anhydrous sodium sulfate, filtered, and the solvent was rotary evaporated to obtain a crude product. The produce was obtained by column chromatography on silica gel and was identified by NMR analyses. All analytical data of the known compounds are consistent with those reported in the literatures.
2.3 Gram-Scale Synthesis of 3aa
O-aminobenzamides (8 mmol), benzyl alcohol (9.6 mmol), 9-fluorenone (5 mol%), p-TsOH (10 mol%), CH3CN (9 mL), and DMSO (1 mL) were added into a 25 mL flat quartz glass jar with a stirrer under 10 W blue LEDs at room temperature for 16 h. After completion, 500 mL of distilled water was added to the mixture. The mixture was extracted with ethyl acetate (100 mL×3), dried over anhydrous sodium sulfate, and filtered. The mixture was concentrated in vacuo and purified by flash column chromatography with hexanes/ethyl acetate to afford the product 3aa.
2.4 Product Analysis
Melting points of all products were measured on an RY-1 micro melt apparatus. Proton nuclear magnetic resonance (1H NMR) spectra were recorded using a 400 spectrometer at 400 MHz. Chemical shifts in 1H NMR spectra are reported in parts per million (ppm) on the scale from an internal standard of DMSO-d6 (2.50 ppm). Coupling constant J values are reported in hertz (Hz), and the corresponding representation of splitting mode is as follows: s, singlet; d, doublet; t, triplet; m, multiplet; b, broad. Carbon-13 nuclear magnetic resonance (13C NMR) spectra were recorded at 100 MHz using a 400 spectrometer. Chemical shifts are reported in delta (δ) units and the ppm from the center of the peak of DMSO-d6 (39.520 ppm). 13C NMR spectra were routinely run with broadband decoupling.
3 Results and Discussions
3.1 Optimization of Reaction Conditions
To optimize the reaction conditions, we initially chose o-aminobenzamide (1a) to react with benzyl alcohol (2a) as a model reaction.To achieve a green reaction outcome, we chose a series of organic dyes (eosin Y, fluorescein, pyrenedione, and 9-fluorenone as the photocatalysts (2 mol%) to give the target product under 10 W blue LEDs in the air within 10 h under ambient conditions. During these preliminary tests, 9-fluorenone showed reasonable activity in the preparation of quinazolinone (3aa) in the presence of 10 mol% p-TsOH (Table 1, entries 1–4). It was found that the efficiency of the reaction was reduced markedly in the absence of p-TsOH (Table 1, entry 5). Next, we examined different solvents. The use of other nonpolar and polar aprotic solvents such as toluene, THF, and DMF resulted in the formation of the desired product in ≤ 7% yields (Table 1, entries 6–8). According to previous research, the lifetime of the excited state of 9-fluorenone could be increased with an additional stabilizing effect of DMSO [61]. To our delight, a further improved yield (52%) was achieved in the mixture of CH3CN and DMSO (Table 1, entry 9). In addition, the results showed that the catalyst dosage and extending reaction time were advantageous to improve the yield of 3aa (Table 1, entries 10–12). In addition, the effect of the dosage of 9-fluorenone on the yield of the target product 3aa and the methods of improving photocatalytic activity were further studied [31, 32, 62,63,64,65,66]. In summary, the reaction works best using 5 mol% 9-fluorenone and 10 mol% p-TsOH in the mixed solvent of CH3CN and DMSO for 16 h under air and blue LEDs at room temperature (Table 1, entry 11).

3.2 Synthesis of Quinazolinone.
After optimization of the reaction conditions and finding the best photocatalyst, we become interested in exploring the scope of the reactions and the results are listed in Table 2. Benzyl alcohols bearing one or two electron-donating groups, such as methyl (2b, 2c, and 2d), isopropyl (2e), methoxy (2f and 2g), dimethoxy (2h), and methyl-enedioxy (2i) were converted to the corresponding products (3ab–3ai) in 87–89% yields. In the case of halide-substituted benzyl alcohol, the corresponding desired products (3aj–3am) were obtained in good to excellent yields. No cleavage of halogen atoms was observed when halide-substituted alcohols were utilized. In addition, 4- hydroxybenzyl alcohol (2n) and methyl 4-(hydroxymethyl)benzoate (2o) were also suitable substrates, which gave the corresponding products 3an and 3ao in 89% and 85% yields, respectively. In this case, benzyl alcohols with a strong electron-withdrawing group, such as cyano (2p), trifluoromethyl (2q), and trifluoromethoxy(2r) were used as substrates, the desired products 3ap–3ar could be obtained in 84–88% yields. Also, heteroatom-containing and fused-ring primary alcohols (2s and 2t) reacted with 1a to provide the corresponding products (3as and 3at) in 80% and 87% yields. Finally, aliphatic alcohols such as cyclohexanemethanol (2u), 1-octanol (2v), and 1-butanol (2w) were tested successively, and the desired products 3au–3aw were obtained in 32–45% yields. Unfortunately, when ethanol (2x) was chosen as the substrate, the target product was not obtained.

Encouraged by the above results, we then extended this method to different o-aminobenzamides (1). As can be seen in Table 3, the electronic properties of the substituents on the phenyl group of o-aminobenzamides did not have a significant impact on the reaction outcome, and the desired products (3ba–3ea) could be obtained in 81–85% yields. To our delight, the N-substituted o-aminobenzamides 1f and 1 g could also react with 2a to satisfactorily generate the desired products 3fa and 3ga in 85% and 78% yields, respectively.

Further, to test the utility of this photochemical strategy, an 8 mmol scale-up reaction was conducted under optimized reaction conditions (Scheme 3). To our delight, the reaction of o-aminobenzamide (1a) and benzyl alcohol (2a) was also performed well under blue LEDs, giving the product 3aa in 62% yield (1.10 g). Therefore, the catalytic system works well for the synthesis of quinazolinones on a gram scale.
3.3 Controlled Experiment
Through the UV–visible diffuse reflectance spectra (UV–vis DRS), the absorption profiles of the 9-fluorenone have wide absorption in the visible light region [82, 83] (Fig. S3, Supporting Information). Next, the recyclability of the optimized catalyst (9-fluorenone) for the model reaction was investigated (Fig. S4, Support Information). After each recycling, the catalyst was recovered by column chromatography, and applied to the next cycle. The activity of the catalyst decreased a little during the five cycles.
To shed light on the mechanism of this visible-light-induced reaction for the preparation of quinazolinones from primary alcohols and o-aminobenzamides, several control experiments were conducted (Scheme 4). When various amounts of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) as radical trapping the agent was added to the photocatalytic reaction under an identical reaction conditions and the yield of (3aa) significantly decreased upon increasing the amount of TEMPO, implying that the radical the pathway may be involved (Scheme 4a). The reaction of o-aminobenzamide 1a and benzyl alcohol 2a, in the absence of light or O2, was performed under optimal conditions, with no product (3aa) was observed, suggesting the essentiality of light and O2 in such a transformation (Scheme 4b and c). Equally unsurprisingly, when 1a was treated with 2a without photocatalyst, nearly no product was observed for the standard reaction (Scheme 4d). In addition, the reaction of intermediate 4 could proceed smoothly to furnish the desired product 3aa in 97% yield under the optimized reaction conditions, which strongly demonstrated the formation of intermediate 4 in the reaction. Moreover, we carried out a set of electron paramagnetic resonance (EPR) experiments. The ESR experiments verified that both superoxide radical anion and singlet oxygen radical were included in the reaction (Fig. S6, Support Information). These experimental results clearly revealed that the route was involved in the reaction.
3.4 Reaction Mechanism
On the basis of the experimental results and literature references [84, 85], a possible mechanism for the photocatalytic reaction of the condensation of o-aminobenzamide (1a) with benzyl alcohol (2a) to quinazolinone (3aa) is suggested in Scheme 5. Initially, 9-fluorenone* was produced by 9-fluorenone under visible light conditions. Benzyl alcohol was converted to activated benzyl alcohol with the action of 9-fluorenone* via single electron transfer (SET) producing 9-fluorenone*−. Accompanied by the oxidation of 9-fluorenone*− under O2 in the air, 9-fluorenone was generated by releasing singlet oxygen radical and superoxide radical anion. Next, the activated benzyl alcohol reacted with the superoxide radical anion to generate peroxide radical and further abstraction of one more hydrogen atom by the peroxide radical generated benzaldehyde (A) and H2O2. Then, H2O2 was reacted with DMSO to generate dimethyl sulfone and H2O. Intermediate 4 was formed by the condensation between o-aminobenzamide (1a) and benzaldehyde (A) through a stepwise acid-promoted cyclization in the presence of p-TsOH. Intermediate C was generated through single electron transfer (SET) from the reaction of 9-fluorenone* and 4. The superoxide radical anion from O2 was reacted with C to produce intermediate D and the peroxide radical. Finally, the peroxide radical abstracted one hydrogen atom from D to give the target product (3aa) by producing H2O2, which was transformed to dimethyl sulfone in the presence of DMSO.
4 Conclusions
In conclusion, we have developed a novel and efficient method for the synthesis of quinazolinones from o-aminobenzamides and primary alcohols using 9-fluorenone as the cheap and high active photocatalyst under irradiation of visible light. Base on this approach, various multi-substituted quinazolinones were easily synthesized in good yields under mild reaction conditions. Moreover, it could achieve the gram-scale transformation in a satisfactory yield, which might indicate that this strategy has more applications in the future. Further study on the synthesis of other heterocyclic structures by photoredox catalysis is currently underway in our laboratory.
References
Tietze LF, Modi A (2000) Med Res Rev 20(4):304–322
Godula K, Sames D (2006) Science 312(5770):67–72
Belenkii LI, Gramenitskaya VN, Evdokimenkova YB (2011). In: Katritzky AR (ed) Advances in Heterocyclic Chemistry. Academic press, Cambridge
Cao SL, Feng YP, Jiang YY, Liu SY, Ding GY, Li RT (2005) Bioorg Med Chem Lett 15(7):1915–1917
Kumar D, Jacob MR, Reynolds MB, Kerwin SM (2002) Bioorg Med Chem 10(12):3997–4004
Rida SM, Ashour FA, El-Hawash SAM, Badr H, Shalaby MA (2005) Eur J Med Chem 40(9):949–959
Murty MSR, Ram KR, Rao RV, Yadav JSU, Murty SN, Kumar KP (2011) Med Chem Res 20(5):626–636
Horton DA, Bourne GT, Smythe ML (2003) Chem Rev 103(3):893–930
Pawar OB, Chavan FR, Sakate SS, Shinde ND (2010) Chinese J Chem 28(1):69–71
Zhao D, Wang T, Li JX (2014) Chem Commun 50(49):6471–6474
Tavakoli-Hoseini N, Davoodnia AA (2011) Chinese J Chem 29(8):1685–1688
Mohammadi AA, Sadat-Hossini SS (2011) Chinese J Chem 29(9):1982–1984
Hioki H, Matsushita K, Nakamura S, Horiuchi H, Kubo M, Harada K, Fukuyama YJ (2008) J Comb Chem 10(5):620–623
Cheng R, Guo T, Daisy ZN, Du YF, Zhao K (2013) Synthesis 45(21):998–3006
Chen JX, Su WK, Wu HY, Liu MC, Jin C (2007) Green Chem 9(9):972–975
Zhan D, Li T, Wei H, Weng W, Ghandi K, Zeng QA (2013) RSC Adv 3(24):9325–9329
Roy AD, Subramanian A, Roy R (2006) J Org Chem 71(1):382–385
Rachakonda S, Pratap PS, Rao MVB (2012) Synthesis 44(13):2065–2069
Abdel-Jalil RJ, Voelter W, Saeed M (2004) Tetrahedron Lett 45(17):3475–3476
Li F, Lu L, Ma J (2015) Org Chem Front 2(12):1589–1597
Gong W, Chen X, Jiang H, Chu DD, Cui Y, Liu Y (2019) J Am Chem Soc 141(18):7498–7508
Prakash M, Jayakumar S, Kesavan V (2013) Synthesis 45(16):2265–2272
Zhou J, Fang J (2011) J Org Chem 76(19):7730–7736
Hikawa H, Ino Y, Suzuki H, Yokoyama Y (2012) J Org Chem 77(16):7046–7051
Watson JAA, Maxwell C, Williams JMJ (2012) Org Biomol Chem 10(2):240–243
Zhang Z, Wang M, Zhang C, Zhang Z, Lu J, Wang F (2015) Chem Commun 51(44):9205–9207
Hu Y, Chen L, Li B (2016) RSC Adv 6(69):65196–65204
Das S, Sinha S, Samanta D, Chakraborty G, Brandao P, Paul ND (2019) J Org Chem 84(16):10160–10171
Ge W, Zhu X, Wei Y (2013) RSC Adv 3(27):10817–10822
Xie Z, Lan J, Zhu H, Lei G, Jiang G, Le Z (2021) Chinese Chem Lett 32(4):1427–1431
Hamad H, Bailon-Garcia E, Maldonado-Hodar FJ, Perez-Cadenas AF, Carrasco-Marin F, Morales-Torres S (2019) Appl Catal B: Environ 241:385–392
Hamad H, Bailón-García E, Morales-Torres S, Carrasco-Marín F, Pérez-Cadenas AF, Maldonado-Hódar FJ (2020) Nanomaterials Basel. 10(4):729
Qi MY, Conte M, Anpo M, Tang ZR, Xu YJ (2021) Chem Rev 121(21):13051–13085
Narayanam JMR, Stephenson CRJ (2011) Chem Soc Rev 40(1):102–113
Zhu C, Yue H, Chu L, Rueping M (2020) Chem Sci 11(16):4051–4064
Ou W, Zou R, Han M, Yu L, Su C (2020) Chinese Chem Lett 31(7):1899–1902
Zhou R, Goh YY, Liu H, Tao H, Li L, Wu J (2017) Angew Chem Int Ed 56(52):16621–16625
Han C, Tang ZR, Liu J, Jin S, Xu YJ (2019) Chem Sci 10(12):3514–3522
Lina Q, Lia YH, Qi MY, Li JY, Tang ZR, Anpo M, Yamada YMA, Xu YJ (2020) Appl Catal B-Environ 271:118946
Yang MQ, Xu YJ (2013) Chem Chem Phys 15(44):19102–19118
Li YH, Zhang F, Chen Y, Li JY, Xu YJ (2020) Green Chem 22(1):163–169
Zhou R, Ma L, Yang X, Cao J (2021) Org Chem Front 8(3):426–444
Qi MY, Conte M, Tang ZR, Xu YJ (2022) ACS Nano 16(10):17444–17453
Li JY, Li YH, Qi MY, Lin Q, Tang ZR, Xu YJ (2020) ACS Catal 10(11):6262–6280
Li YH, Qi MY, Tang ZR, Xu YJ (2022) J Phys Chem C 126(4):1872–1880
Qi MY, Li YH, Anpo M, Tang ZR, Xu YJ (2020) ACS Catal 10(23):14327–14335
Yang MQ, Xu YJ (2013) Phys Chem Chem Phys 15(44):19102–19118
Chan AY, Perry IB, Bissonnette NB, Buksh BF, Edwards GA, Frye LI, Garry OL, Lavagnino MN, Li BX, Liang YF, Mao E, Millet A, Oakley JV, Reed NL, Sakai HA, Seath CP, MacMillan DWC (2021) Chem Rev 122(2):485–1542
Lang X, Ma W, Chen C, Ji H, Zhao J (2014) Acc Chem Res 47(2):355–363
Chen YZ, Wang ZU, Lu HJ, Yu SH, Jiang HL (2017) J Am Chem Soc 139(5):2035–2044
Zhao QQ, Hu XQ, Yang MN, Chen JR, Xiao WJ (2016) Chem Commun 52(86):12749–12752
Meng C, Yang K, Fu XZ, Yuan RS (2015) ACS Catal 5(6):3760–3766
Zhou R, Liu R, Zhang K, Han L, Zhang H, Gao W, Li R (2017) Chem Commun 53(51):6860–6863
Ravelli D, Fagnoni M, Albini A (2013) Chem Soc Rev 42(1):97–113
Romero NA, Nicewicz DA (2016) Chem Rev 116(17):10075–10166
Srivastava V, Singh PP (2017) RSC Adv 7(50):31377–31392
Peng JB, Qi X, Wu XF (2016) Chemsuschem 9(17):2279–2283
Wang YF, Xu WG, Sun B, Yu QQ, Li TJ, Zhang FL (2019) J Org Chem 84(20):13104–13111
Xia JB, Zhu Z, Chen C (2013) J Am Chem Soc 135(46):17494–17500
Okamoto H, Yamaji M, Gohda S, Kubozono Y, Komura N, Sato K, Sugino H, Satake K (2011) Org Lett 13(10):2758–2761
Margrey KA, Nicewicz DA (2016) Acc Chem Res 49(9):1997–2006
Ali El-Remaily MA, Hamad HA, Soliman AM, Elhady OM (2021) Appl Organomet Chem 35(7):e6238
Fathy M, Hamad H (2016) RSC adv 6(9):7310–7316
Hamad H, Abd El-latif M, Kashyout AEH, Sadik W, Feteha M (2015) Process Saf Environ 98:390–398
Hamad H, Castelo-Quibén J, Morales-Torres S, Carrasco-Marín F, Pérez-Cadenas AF, Maldonado-Hódar FJ (2018) Materials 11(9):1766
Hamad H, Bailon-Garcia E, Morales-Torres S, Carrasco-Marin F, Perez-Cadenas AF, Maldonado-Hodar FJ (2018) J Environ Chem Eng 6(4):5032–5041
Jiang X, Tang T, Wang JM, Chen Z, Zhu YM, Ji SJ (2014) J Org Chem 79(11):5082–5087
Xu W, Jin Y, Liu H, Jiang Y, Fu AH (2011) Org Lett 13(6):1274–1277
Pieterse L, Van der Walt MM, Terre’Blanche G (2020) Bioorg Med Chem Lett 30(16):127274
Balaji S, Balamurugan G, Ramesh R, Semeril D (2021) Organometallics 40(6):725–734
Heravi M, Montazeri N, Rahimzadeh M, Bakavoli M, Ghassemzadeh M (2004) Pol Jo Chem 78:2101–2103
Sylvain L, Marine H, Julien G, Isabelle SA, Laurent B, Christophe H, Vincent L, Corinne F, Thierry B (2015) Org Lett 17(7):1700–1703
Yao D, Jiang J, Zhang H, Huang Y, Huang J, Wang J (2021) Bioorg Med Chem Lett 47:128204
Abdullaha M, Mohammed S, Ali M, Kumar A, Vishwakarma RA, Bharate SB (2019) J Org Chem 84(9):5129–5140
Hao S, Yang J, Liu P, Xu J, Yang C, Li F (2021) Org Lett 23(7):2553–2558
Parua S, Das S, Sikari R, Sinha S, Paul ND (2017) J Org Chem 82(14):7165–7175
Ma B, Wang Y, Peng J, Zhu Q (2011) J Org Chem 76(15):6362–6366
Lee S, Sim J, Jo H, Viji M, Srinu L, Lee K, Lee H, Manjunatha V, Jung JK (2019) Org Biomole Chem 17(35):8067–8070
Feng Y, Li Y, Cheng G, Wang L, Cui X (2015) J Org Chem 80(14):7099–7107
Fang J, Zhou J (2012) Org Biomol Chem 10(12):2389–2391
Hikawa H, Nakayama T, Takahashi M, Kikkawa S, Azumaya I (2021) Adv Synth Catal 363(16):4075–4084
Hamad H, Elsenety MM, Sadik W, El-Demerdash AG, Nashed A, Mostafa A, Elyamny S (2022) Sci Rep-UK 12(1):1–20
Ali El-Remaily MA, Kamel MS, Halim SA, Shokr EK, Abdel-Ghany H, Hamad H (2023) Mater Chem Phys 293:126972
Ghosh I, Mukhopadhyay A, Koner AL, Samanta S, Nau WM, Moorthy JN (2014) Chem Chem Phys 16(31):16436–16445
Bains AK, Ankit Y, Adhikari D (2021) Org Lett 23(6):2019–2023
Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (22005179), and Natural Science Foundation of Shandong Province (ZR2020QB113, ZR2020MB018 and, ZR2021QB049).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Ren, J., Xiao, Q. et al. Photocatalytic One-Pot Synthesis of Quinazolinone Under Ambient Conditions. Catal Lett 153, 3771–3782 (2023). https://doi.org/10.1007/s10562-022-04266-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04266-y