Abstract
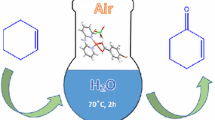
Copper pyrithione was used for the first time as the catalyst for oxidation secondary and primary benzyl alcohols to furnish corresponding carbonyl compounds in high yields of up to 98%. This type of reactions can be carried out in mild conditions, using molecular oxygen or air as the oxidant, and exhibiting a wide substrate scopes and selectivity.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Aromatic aldehydes and ketones are versatile synthetic intermediates for various C–C/O bond formation reactions, which are widely used in the preparation of natural products, biologically active molecules, and polymer materials [1]. Although several methodologies have been established for the synthesis of aromatic aldehydes and ketones, aerobic selective oxidation of primary or secondary alcohols to the corresponding carbonyl derivatives is one of the most effective protocols [2,3,4,5,6]. Traditional oxidation procedures for these transformations often involve the use of stoichiometric amount of oxidants such as Mn salts [7], Cr salts [8] and hypervalent iodines [9, 10], which usually cause the side reactions and over-oxidation of alcohols to carblic acids, as well as the formation of harmful gases and toxic heavy metal salts. Therefore, from the economic and environment viewpoint, development of cleaner catalytic oxidation systems for this reaction is highly desirable. In comparison to these conventional inorganic oxidants, molecular oxygen or air is an ideal oxidant because of its non-toxic, natural abundance, low cost and eco-friendly benign, and it has been successfully employed for various oxygenated chemicals synthesis.[11,12,13,14] However, due to the low reactivity and thermodynamically-stable of oxygen, the oxidation processes with oxygen or air generally have to rely on the presence of transition-metal catalysts, such as Pd [15, 16], Ru [17,18,19], Au [20, 21] or other noble metals. In recent years, significant efforts have been made to achieve economic and environmental friendly reaction conditions, including the use of cheaper and earth-abundant metal (e.g. Cu [4, 22,23,24,25,26,27,28,29] or Ni [30, 31]), heterogeneous catalysts [32,33,34], or photocatalysts [35,36,37] and so on. Among the mentioned copper-based catalysts, (ligand)Cu/TEMPO (2,2,6,6-tetramethylpiperidinyl-1-l, and its derivatives)/base systems are proved to be efficient and selective for the oxidation of alcohols to aldehydes or ketones utilization of O2 or air as the oxidant under mild conditions [23,24,25,26,27,28,29], [38,39,40,41,42,43,44,45,46,47,48], which have emerged as some of the most versatile bench scale methods for alcohol oxidation. Nevertheless, there is still a need to further develop a generally efficient catalytic system for this class of reactions.
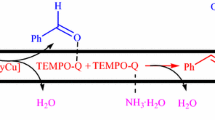
Copper pyrithione (bis(1-hydr-1H-pyridine-2-thionato-O,S)copper, CuPT), as an inexpensive, low toxicity and stable bivalent copper complex (Scheme 1), is often used as antifungal and antimicrobial agents in aquatic applications [49]. Very recently, we found that CuPT could act as an effective catalyst and/or coupled partner for selective C-N/C-S coupling reactions [50]. In order to further explore the scope of CuPT to other types of organic reactions, we herein report CuPT as a novel and high active catalyst for oxidation of secondary benzyl alcohols (without TEMPO) and primary benzyl alcohols (with TEMPO) to ketones and aldehydes under air or O2 atmosphere.
2 Results and Discussion
Our preliminary research began with diphenylmethanol (1a) as the model substrate for the oxidative reaction catalyzed by CuPT with air as the sole oxidant in DMSO at 70 °C for 30 min, and the results were illustrated in Table 1. Interestingly, the oxidation efficiency was found to be obviously dependent on the nature of inorganic base (entries 1–6). The bases with strong basicity demonstrated higher yields than the weak ones, and KOH was proved to be the best one with 97% yield (entry 6). No oxidated product was observed in the absence of base (entry 7). To our delight, decreasing the amount of CuPT and KOH to 5 mol% and 25 mol%, respectively, the yield of diphenylmethanone (2a) still remained constant at 97%. However, further decreasing the loading of CuPT and KOH all resulted in lower yields (entries 8–12). In different solvents other than DMSO, catalytic activities of the reactions were decreased significantly (entries 13–15). Fortunately, when the reaction temperature was reduced to 25 °C, a 97% yield was also obtained with only prolonging the reaction time to 40 min (entries 16–18). The reaction was investigated under Ar and no desired product was formed, indicating that air was the oxidant source (entry 19). The reaction yields were suppressed with other simple copper salts as the catalysts, such as CuSO4 and Cu(OAc)2 (entries 20 and 21). Finally, we obtained an optimal reaction conditions as follows: 5 mol% of CuPT and 25 mol% of KOH in DMSO under air at 25 °C.

With the optimized conditions in hand, the scope of secondary benzyl alcohols oxidation reactions was explored (Table 2). The results showed that the electronic effect of the substituents had slight effect on the reactivity. For example, the aromatic ring bearing electron-rich groups, such as Me, OMe and NH2 (2a-2f and 2m, 88–98% yields), gave the corresponding oxidative products in relatively higher yields than the cases containing electron-deficient groups, such as F or Cl (2g-2l, 2n and 2o, 49–90% yields). Notably, sterically 2-substituted substrate did not hamper the reaction. For example, excellent yields of 98% and 92% could be obtained when oxidation of 2-methyl or 2-amino diphenylmethanols (2d and 2e). Because of incomplete conversion, lower yields for 1-aryl-ethan-1-ols were observed (2p-2r). The results might be due to their less reactivity than diarylmethanols, and more catalyst amount was needed. Moreover, 1,2,3,4-tetrahydronaphthalen-1-ol (2s), 2,3-dihydro-1H-inden- 1-ol (2t) and 9H-fluoren-9-ol derivatives (2u-2w) were also well-tolerated and produced the desired oxidation products in 43–91% yield. Gram-scale reaction was carried out with 10 mmol scale to generate the product in 98% yield (2a, 1.78 g), indicating the practical application of the present method.
The oxidation time on the product concentration of 2b and 2j were tested under the optimal conditions, and the results were shown in Fig. 1. Most of the raw materials were converted to the corresponding oxidation products within 1 h, and only a little improvement of the yield with further prolonging the reaction time to 4 h. These results suggested that the present oxidation proceeded rapidly under the established conditions.
The success of the above oxidation of secondary benzyl alcohols prompted us to investigate the feasibility of primary benzyl alcohols under the same condition. Unfortunately, only 41% yield of 4-bromo benzaldehyde was obtained with a 53% of carblic acid byproduct from over-oxidation. Therefore, we chose 2-naphthalenemethanol (3a) as a model substrate to optimize the oxidation reaction for primary benzyl alcohol (Table 3). First, we attempted to examine the effect of bases under the standard conditions for secondary benzyl alcohols. As we can see, higher yields were observed for KOH, NaOH and Cs2CO3 than K2CO3 and K3PO4 (entries 1–5). However, K2CO3 was the recommended base as its weaker basicity and no over-oxidation 2-naphthoic acid byproduct formation. Slight improvement in the transformation of 3a into 4a was obtained by elevating the reaction temperature (entries 6–8). Replacing air with O2 balloon provided a significant increased yield of 4a (62%, entry 9). Considering the high catalytic efficiency of TEMPO in copper-catalyzed alcohol oxidation reaction, the reaction was then carried out with the addition of 5 mol% of TEMPO as the promoter in combination with O2 balloon. As expected, the oxidation reaction proceeded to complete conversion of 3a and gave a 95% yield of 4a, whereas the yield reduced to 55% under air (entries 10 and 11). Lowering results were observed by decreasing the amount of CuPT or TEMPO (entries 12–14). Prolonging the reaction time to 12 h, the reaction was also worked well to give a yield of 96% even dropped the temperature to 40 °C (entries 15 and 16). Other simple copper salts still gave depressed yields similar with oxidation of secondary alcohols (entries 17 and 18). Finally, in the oxidation reaction of 2-naphthalenemethanol to 2-naphthaldehyde, the highest yield of 96% was gained when 5 mol% of CuPT as well as 5 mol% of TEMPO were used as the catalysts in DMSO at 40 °C for 12 h under O2 atmosphere (entry 16).

As indicated in Table 4, various type of primary benzyl alcohols bearing electron-donating, electron-withdrawing, or electron-neutral groups at the para-, meta-, or ortho- positions of the aromatic ring could smoothly be converted to the corresponding aldehydes in 65–98% yield (4a–4n). It is clear that the reactions of electron-donating substituent substrates are faster and more efficient than the electron-withdrawing ones. The oxidation of cinnamaldehyde was occurred well in 94% yield and the conjugated C = C bond did not influence the activity under the optimized conditions, showing the high selectivity of the present catalytic system (4o). Notably, ferrocenemethanol was also a suitable case for this reaction, affording the desired ferrocenecarboxaldehyde (4p) in 64% yield, which highlighted that this oxidation is a useful approach for producing metal containing aldehyde. Moreover, heteroaromatic alcohol reacted excellently under our conditions to furnish the aldehyde in good yield (4q). We are pleased to find that treatment of double-oxidation of 1,4-benzenedimethanol proceeded without any difficulty to give 4r in 80% yield. Compared to the absence of TEMPO, higher yield for 9H-fluoren-9-ol was delivered with the addition of TEMPO (2u, 85% vs 77%). In addition to these, the reaction could also be scaled to 10 mmol without loss of efficiency (91% yield).
We further attempted to investigate the scope of this reaction to some drug-like molecules 5 and 6a-6c. However, all these substrates failed under the two optimized reaction conditions (Scheme 2).
Based on related reports [23, 51,52,53,54], a proposed mechanism for CuPT-catalyzed oxidation reaction was shown in Scheme 3. First, one ligand from the initial CuPT was cleaved and formed OH-copper complex A in alkaline media. Then, the intermolecular elimination was occurred between OH ligand and alcohol to generate copper-alkoxide complex B with a loss of one water molecule. Under the TEMPO-free conditions, carbonyl-copper(I) π complex C was formed via H-atom abstraction and one-electron transfer reaction of B. Oxidation reaction of C with O2 produced ketones and D, which was easily reacted with water and regenerate A. In the presence of TEMPO, it preferred to coordinate with B to form E, followed by β-hydrogen transfer to TEMPO led to complex F. Intramolecular one-electron transfer provided aldehyde, TEMPOH and Cu(I) species G. Finally, A and TEMPO were all regenerated by the aerobic oxidation with O2.
3 Conclusion
In summary, we have for the first time established a very simple and efficient protocol for CuPT-catalyzed oxidation of secondary and primary benzyl alcohols with air or O2 as the oxidant under mild conditions. In the process, ketones were obtained in good to excellent yields in the presence of CuPT-KOH catalytic system, while CuPT-TEMPO-K2CO3 was more suitable for selective oxidation of primary benzyl alcohols to aldehydes. The low-cost, broad substrate tolerance with green oxidant will make it attractive both in lab research and industrial applications.
4 Experimental
4.1 Materials and Instruments
Unless otherwise stated, CuPT and other reagents were purchased from Adamas and Energy-Chemical, and used without further purification. Column chromatography and thin-layer chromatography were performed with silica gel (200–300 mesh) and GF254 plates purchased from Qingdao Haiyang Chemical Co. Ltd. 1H NMR and 13C NMR were recorded on a Bruker Avance III HD 400 instrument using TMS as the internal standard and DMSO-d6 or CDCl3 as the solvent.
4.2 General Procedure for CuPT-Catalyzed Oxidation of Secondary Benzyl Alcohols
To a 10 mL reaction tube was added secondary alcohol (0.5 mmol), CuPT (0.025 mmol, 5 mol%), KOH (0.125 mmol, 25 mol%) and DMSO (1 mL). The reaction tube was stirred at 25 °C in an open air for the mentioned time in Table 2. The reaction solution was diluted with 10 mL saturated brine, and then extracted with ethyl acetate (3 × 10 mL). Combined the organic phases, washed with saturated brine for twice, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (petroleum ether-ethyl acetate) to afford the target compound 2a-2w in Table 2.
4.3 General Procedure for CuPT- Catalyzed Oxidation of Primary Benzyl Alcohols
To a 10 mL reaction tube was added benzyl alcohol (0.5 mmol), CuPT (0.025 mmol, 5 mol%), K2CO3 (0.125 mmol, 25 mol%), TEMPO (0.025 mmol, 5 mol%) and DMSO (1 mL). The reaction tube was stirred at 40 °C under O2 balloon for the mentioned time in Table 4. The reaction solution was diluted with 10 mL saturated brine, and then extracted with ethyl acetate (3 × 10 mL). Combine the organic phases, washed with saturated brine for twice, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (petroleum ether-ethyl acetate) to afford the target compound 4a-4r and 2u in Table 4.
4.4 General Procedure for Time-Concentration Experiments
Five parallel experiments were conducted for 2b and 2j under CuPT-KOH catalytic system condition, respectively. The reactions were stopped after 20 min, 40 min, 1 h, 2 h and 4 h, and then the reactions mixture were purified to afford the target products in corresponding yields. The time-concentration curves were next drawn as shown in Fig. 1.
References
Denmark SE, Fu J (2003) Catalytic enantioselective addition of allylic organometallic reagents to aldehydes and ketones. Chem Rev 103(8):2763–2794
Hudlicky M (1990) Oxidations in organic chemistry. American Chemical Society, Washington
Sheldon RA, Arends IWCE, ten Brink G-J et al (2002) Green, catalytic oxidations of alcohols. Acc Chem Res 35(9):774–781
Allen SE, Walvoord RR, Padilla-Salinas R et al (2013) Aerobic copper-catalyzed organic reactions. Chem Rev 113(8):6234–6458
Liu K-J, Duan Z-H, Zeng X-L et al (2019) Clean oxidation of (hetero)benzylic Csp3–H bonds with molecular oxygen. ACS Sustain Chem Eng 7(12):10293–10298
Liu K-J, Jiang S, Lu L-H et al (2018) Bis(methpropyl) ether-promoted oxidation of aromatic alcohols into aromatic carblic acids and aromatic ketones with O2 under metal- and base-free conditions. Green Chem 20(13):3038–3043
Bachkvall JE (2004) Modern oxidation reactions. Wiley-VCH, Weinheim
Cainelli G, Cardillo G (1984) Chromium oxidants in organic chemistry. Springer-Verlag, Berlin
Dess DB, Martin JC (1983) Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J Org Chem 48(22):4155–4156
Ren Q-G, Chen S-Y, Zhou X-T et al (2010) Highly efficient controllable oxidation of alcohols to aldehydes and acids with sodium periodate catalyzed by water-soluble metalloporphyrins as biomimetic catalyst. Bioorg Med Chem 18(23):8144–8149
Liu K-J, Wang Z, Lu L-H et al (2021) Synergistic cooperative effect of CF3SO2Na and bis(2-butethyl)ether towards selective genation of sulfides with molecular oxygen under visible-light irradiation. Green Chem 23(1):496–500
Liu K-J, Deng J-H, Yang J et al (2020) Selective oxidation of (hetero)sulfides with molecular oxygen under clean conditions. Green Chem 22(2):433–438
Xie L-Y, Bai Y-S, Xu X-Q et al (2020) Visible-light-induced decarblative acylation of quinoxalin-2(1H)-ones with α-oxo carblic acids under metal-, strong oxidant- and external photocatalyst-free conditions. Green Chem 22(5):1720–1725
Wu Y, Chen J-Y, Ning J et al (2021) Electrochemical multicomponent synthesis of 4-selanylpyrazoles under catalyst- and chemical-oxidant-free conditions. Green Chem 23(11):3950–3954
Chen G-J, Wang J-S, Jin F-Z et al (2016) Pd@Cu(II)-MOF-catalyzed aerobic oxidation of benzylic alcohols in air with high conversion and selectivity. Inorg Chem 55(6):3058–3064
Weerachawanasak P, Hutchings GJ, Edwards JK et al (2015) Surface functionalized TiO2 supported Pd catalysts for solvent-free selective oxidation of benzyl alcohol. Catal Today 250:218–225
Musawir M, Davey PN, Kelly G et al (2003) Highly efficient liquid-phase oxidation of primary alcohols to aldehydes with oxygen catalysed by Ru–Co oxide. Chem Commun 12:1414–1415
Htet Y, Tennyson AG (2016) Catalytic radical reduction in aqueous solution by a ruthenium hydride intermediate. Angew Chem Int Ed 55(30):8556–8560
Ray R, Chandra S, Maiti D, Lahiri GK (2016) Simple and efficient ruthenium-catalyzed oxidation of primary alcohols with molecular oxygen. Chem Eur J 22(26):8814–8822
Ide MS, Davis RJ (2014) The important role of hydroxyl on oxidation catalysis by gold nanoparticles. Acc Chem Res 47(3):825–833
Zhu J, Wang PC, Lu M (2014) Selective oxidation of benzyl alcohol under solvent-free condition with gold nanoparticles encapsulated in metal-organic framework. Appl Catal A Gen 477:125–131
Wu J, Liu Y, Ma X et al (2016) Highly selective copper-catalyzed oxidation of benzyl alcohols to aromatic aldehydes in water at room temperature. Appl Organomet Chem 30(7):577–580
Figiel PJ, Sibaouih A, Ahmad JU et al (2009) Aerobic oxidation of benzylic alcohols in water by 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)/copper(II) 2-N-arylpyrrolecarbaldimino complexes. Adv Synth Catal 351(16):2625–2632
Ansari IA, Gree R (2002) TEMPO-catalyzed aerobic oxidation of alcohols to aldehydes and ketones in ionic liquid [bmim][PF6]. Org Lett 4(9):1507–1509
Mei Q, Liu H, Yang Y et al (2018) Base-free aerobic oxidation of alcohols over copper-based complex under ambient condition. ACS Sustain Chem Eng 6(2):2362–2369
Iron MA, Szpilman AM (2017) Mechanism of the Copper/TEMPO-catalyzed aerobic oxidation of alcohols. Chem Eur J 23(6):1368–1378
Hossain MM, Shyu S-G (2010) Efficient and selective aerobic alcohol oxidation catalyzed by copper(II)/2,2,6,6,-tetramethylpiperidine-1-oxyl at room temperature. Adv Synth Catal 352(17):3061–3068
Kumpulainen ETT, Koskinen AMP (2009) Catalytic activity dependency on catalyst components in aerobic copper–TEMPO oxidation. Chem Eur J 15(41):10901–10911
Figiel PJ, Leskelä M, Repo T (2007) TEMPO-copper(II) diimine-catalysed oxidation of benzylic alcohols in aqueous media. Adv Synth Catal 349(7):1173–1179
Weiss CJ, Das P, Miller DL et al (2014) Catalytic oxidation of alcohol via nickel phosphine complexes with pendant amines. ACS Catal 4(9):2951–2958
Chakraborty S, Piszel PE, Brennessel WW et al (2015) A single nickel catalyst for the acceptorless dehydrogenation of alcohols and hydrogenation of carbonyl compounds. Organometallics 34(21):5203–5206
Adil SF, Assal ME, Shaik MR et al (2020) Efficient aerial oxidation of different types of alcohols using ZnO nanoparticle–MnCO3-graphene oxide composites. Appl Organomet Chem 34(8):e5718
Khoshyan A, Pourtahmasb M, Feizpour F et al (2019) Aerobic Mo72V30 nanocluster-catalysed heterogeneous one-pot tandem synthesis of benzimidazoles. Appl Organomet Chem 33(2):e4638
Reddy PL, Arundhathi R, Tripathi M et al (2017) Solvent-free oxidative synthesis of 2-substituted benzimidazoles by immobilized cobalt oxide nanoparticles on alumina/silica support. ChemistrySelect 2(13):3889–3895
Yan P, Wang R, Wu S et al (2008) Photo oxidation of alcohols in water under solar light. Catal Commun 9(3):406–408
Yang X-J, Zheng Y-W, Zheng L-Q et al (2019) Visible light-catalytic dehydrogenation of benzylic alcohols to carbonyl compounds by using an eosin Y and nickel–thiolate complex dual catalyst system. Green Chem 21(6):1401–1405
Schilling W, Riemer D, Zhang Y et al (2018) Metal-free catalyst for visible-light-induced oxidation of unactivated alcohols using air/oxygen as an oxidant. ACS Catal 8(6):5425–5430
Hoover JM, Stahl SS (2011) Highly practical copper(I)/TEMPO catalyst system for chemoselective aerobic oxidation of primary alcohols. J Am Chem Soc 133(42):16901–16910
Nishii T, Ouchi T, Matsuda A et al (2012) Modified Markó’s aerobic oxidation of alcohols under atmospheric pressure with air or molecular oxygen at room temperature. Tetrahedron Lett 53(44):5880–5882
Ryan MC, Whitmire LD, McCann SD et al (2019) Copper/TEMPO redox redux: analysis of PCET oxidation of TEMPOH by copper(II) and the reaction of TEMPO with copper(I). Inorg Chem 58(15):10194–10200
Walroth RC, Miles KC, Lukens JT et al (2017) Electronic structural analysis of copper(II)-TEMPO/ABNO complexes provides evidence for copper(I)-oxoammonium character. J Am Chem Soc 139(38):13507–13517
Steves JE, Stahl SS (2015) Stable TEMPO and ABNO catalyst solutions for user-friendly (bpy)Cu/nitroxyl-catalyzed aerobic alcohol oxidation. J Org Chem 80(21):11184–11188
Steves JE, Preger Y, Martinelli JR et al (2015) Process development of CuI/ABNO/NMI-catalyzed aerobic alcohol oxidation. Org Process Res Dev 19(11):1548–1553
Ryland BL, Stahl SS (2014) Practical aerobic oxidations of alcohols and amines with homogeneous copper/TEMPO and related catalyst systems. Angew Chem Int Ed 53(34):8824–8838
Steves JE, Stahl SS (2013) Copper(I)/ABNO-catalyzed aerobic alcohol oxidation: alleviating steric and electronic constraints of Cu/TEMPO catalyst systems. J Am Chem Soc 135(42):15742–15745
Hoover JM, Ryland BL, Stahl SS (2013) Copper/TEMPO-catalyzed aerobic alcohol oxidation: mechanistic assessment of different catalyst systems. ACS Catal 3(11):2599–2605
Hill NJ, Hoover JM, Stahl SS (2013) Aerobic alcohol oxidation using a copper(I)/TEMPO catalyst system: a green, catalytic oxidation reaction for the undergraduate organic chemistry laboratory. J Chem Educ 90(1):102–105
Hoover JM, Steves JE, Stahl SS (2012) Copper(I)/TEMPO-catalyzed aerobic oxidation of primary alcohols to aldehydes with ambient air. Nature Protoc 7(6):1161–1166
Almond KM, Trombetta LD (2016) The effects of copper pyrithione, an antifouling agent, on developing zebrafish embryos. Ecotoxicology 25(2):389–398
Song B, Cao N, Zhang J et al (2021) Copper pyrithione (CuPT)-catalyzed/mediated amination and thioarylation of (hetero)aryl halides: a competition. Mol Catal 516:111981
Gamez P, Arends IWCE, Sheldon RA et al (2004) Room temperature aerobic copper–catalysed selective oxidation of primary alcohols to aldehydes. Adv Synth Catal 346(7):805–811
Gamez P, Arends IWCE, Reedijk J et al (2003) Copper(II)-catalysed aerobic oxidation of primary alcohols to aldehydes. Chem Commun 19:2414–2415
Wang Y, DuBois JL, Hedman B et al (1998) Catalytic galactose oxidase models: biomimetic Cu(II)-phenoxyl-radical reactivity. Science 279(5350):537–540
Markó István E, Giles Paul R, Tsukazaki M et al (1996) Copper-catalyzed oxidation of alcohols to aldehydes and ketones: an efficient, aerobic alternative. Science 274(5295):2044–2046
Acknowledgements
This research was funded by the National Natural Science Foundation of China (NSFC, No. 21868032) and Natural Science Foundation of Hunan Province (2021JJ30290).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, Q., Song, B., Sun, N. et al. Copper Pyrithione (CuPT)-Catalyzed Oxidation of Secondary and Primary Benzyl Alcohols with Molecular oxygen or Air Under Mild Conditions. Catal Lett 153, 2665–2673 (2023). https://doi.org/10.1007/s10562-022-04172-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04172-3