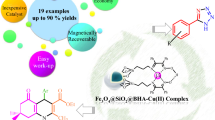
Abstract
Two magnetic nano catalysts of nickel and copper, Fe3O4@SiO2@DOP-BenPyr-M(II), (M=Ni and Cu) have been synthesized. These catalysts were applied as recoverable, efficient and new heterogeneous catalysts for the high yielding and room temperature one-pot procedure of selective oxidation of sulfides to sulfoxides and oxidative coupling of thiols to disulfides. In addition, the catalytic activity of Fe3O4@SiO2@DOP-BenPyr-Ni(II) was investigated as heterogeneous nanocatalyst for synthesis of 2,3-dihydroquinazolin-4(1H)-ones, 5-substituted 1H-tetrazoles and polyhydroquinolines. The synthesized catalysts were characterized by FT-IR, TGA, XRD, VSM, EDX, ICP and SEM techniques. These catalysts were recovered by an external magnet and reused several times without significant loss of catalytic efficiency.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Magnetic nanoparticles have attracted much attention from a wide range of applications as catalyst in many organic reactions because of their high surface area, the active sites and facile separation. Also magnetic nanoparticles have been extensively reported in various fields [1,2,3,4], including biosensor and therapeutic applications [5], organic transformation such as cross-coupling reactions and esterification [6], medicine [7], hydrogenation of carbonyl compounds and alkynes in the petrochemical and food industries [8] and etc. Among the various magnetic nanoparticles, the direct use of magnetic Fe3O4 nanoparticles are developed and disputably the most extensively under investigation because of facilitate their separation from the reaction mixture with an external magnet and remain unchanged after reaction [9,10,11]. To protect and also prevent affect redox reactions of the magnetic core with supplemental component, a silica shell was first coated on the magnetic core (SMNP), then the molecular catalysts grafting on the silica surface by the binding sites (Si–OH units) [1, 12]. Also control of properties of nanocatalysts such as size, shape, morphology, and dispersity can be achieved [13]. Therefore, developments of catalysts that are stable and recoverable seem very attractive [14].
There upon the new occasion by application of magnetic nanoparticles as catalyst can be obtained to the synthesis of heterocyclic compounds such as 2,3-dihydroquinazolin-4(1H)-ones, 5-substituted 1H-tetrazoles and polyhydroquinolines. Often heterocyclic structures owning potential biological and pharmaceutical activities. Among them, quinazolinone derivatives possess useful properties like antitumor, anticancer [15], diuretic, herbicidal agents [16] and etc. The common procedure for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones is the condensation reaction of anthranilamide with various aldehydes under a variety of catalysts [17, 18], such as MNPs-TEDETA tribromide [19], Cu-CNTs [20], silica-bonded n-propylsulfamic acid [21], H3BO3-MCM-41 [22] and etc.
As well as, polyhydroquinoline that include a six membered heterocyclic ring [19] possess vasodilator, hepatoprotective, antiatherosclerotic, bronchodilator, antitumor, geroprotective, and antidiabetic activities [23]. This compound synthesis from dimedone, arylaldehydes, ethyl acetoacetate and ammonium acetate and catalyzed by various catalyst such as Fe3O4-SA-PPCA nanoparticles [24], MNPs-TEDETA tribromide [19], sulfonic acid functionalized SBA-15 [25] and etc.
Tetrazoles are valuable group of heterocycles that can use in the multiple fields including antibiotic activity, biological activity, anti-allergic herbicides, complexing ability toward various metals, information recording system and etc. Also those were prepared by cycloadditions between nitriles and azide anion [26,27,28]. Several pathways have been reported for the synthesis of 5-substituted 1H-tetrazoles such as Cu-MCM-41 nanoparticles [29], salen complex of Cu(II) supported on superparamagnetic Fe3O4@ SiO2 nanoparticles [30] and etc.
Synthesis of sulfoxides is in demand, because of this compound are a common merit found in therapeutic agents including antibacterial, anti-ulcer, antihypertensive, cardiotonic [31, 32] and antifungal [33]. Also, these compounds have widespread utilization as polymer materials, and ligands in asymmetric catalysis [34]. Also the selective oxidation of thiols and synthesis of corresponding disulfides has gained significant importance due to many applications of these products, such as: biological processes, chemical industry [35], as useful reagents in organic synthesis, in sulphenylation of enolates and other anions, essential moieties of biological active compounds for peptides and protein stabilization [36], vulcanizing agents for rubber [37]. Overoxidation of disulfides leading to other products such as sulfinates, sulfoxides, sulfonates and sulfonic acids. Overcome to this problem and selective oxidation of thiols has attractive attention. Because of the selective oxidation have many benefits such as low cost and energy, minimal waste, short reaction time and less workup [38].
Therefore, design of a catalytic system that has not the problem of overoxidation is necessary.
Magnetic nanoparticles (MNPs) due to mentioned benefits have applied as a support for synthesis of new heterogeneous catalyst. In this light we are synthesized Fe3O4@SiO2@DOP-BenPyr-M(II) (M=Ni and Cu) and applied them as catalysts for the oxidation of sulfides to sulfoxides and oxidative coupling of thiols to disulfides and also synthesis of 2,3-dihydroquinazolin-4(1H)-ones, 5-substituted 1H-tetrazoles and polyhydroquinolines by the Ni catalyst.
2 Experimental
2.1 Materials and Physical Measurements
All reagents and solvents used in this work were purchased from Aldrich or Merck chemical companies. All the melting points were listed by open capillary method and are uncorrected. The synthesized catalysts were recognized by X-ray diffraction (XRD, GBC-Difftech MMA), thermogravimetric analysis (TGA, PerkinElmer Pyris Diamond, UK), Fourier transform infrared spectroscopy (FT-IR, Bruker, Germany, VRTEX 70), scanning electron microscopy (SEM, FESEM-TESCAN MIRA3), energy dispersive X-ray (EDX, FESEM-TESCAN MIRA3) and vibrating sample magnetometer (VSM, MDKFD).
2.2 Synthesis of catalyst
For the synthesis of magnetic Fe3O4 nanoparticles, the mixture of FeCl3·6H2O (5.858 g) and FeCl2·4H2O (2.221 g) in 100 mL deionized water was stirred until the salts dissolved completely. Then, 10 mL of 30% aqueous ammonia was added in to the reaction mixture under N2 atmosphere about 30 min at 80 °C under vigorous mechanical stirring. Finally, the black precipitate of MNPs was collected and washed with doubly distilled water [19]. Then for the coating of a layer of silica on the surface of the Fe3O4 nanoparticles, magnetic Fe3O4 nanoparticles (1 g) were dispersed in 10 mL deionized water and 50 mL absolute ethanol, and sonicated for 30 min. After sonication of MNPs, 5.39 g PEG, 10 mL NH3 and 2 mL tetraethylorthosilicate (TEOS) was added to the reaction mixture and stirred at room temperature for 38 h. Finally, the product (Fe3O4@SiO2) was isolated by external magnet, washed with deionized water and ethanol and dried at room temperature. The resultant product in the previous step (Fe3O4@SiO2, 1 g) was dispersed in 25 mL absolute ethanol using an ultrasonic bath for 30 min. Then 1.5 g of 4-(2-aminoethyl) benzene-1,2-diol (dopamine) was added to the reaction mixture and stirred at room temperature for 24 h. The solid product was separated by an external magnet and washed with ethanol to remove unreacted species and dried at room temperature.
Then, 1 g of Fe3O4@SiO2@DOP was dispersed in 25 mL absolute ethanol using an ultrasonic bath for 30 min. Then 2-benzoyl pyridine (2 mmol) and acetic acid (0.3 mL) were added to mixture reaction and stirred in reflux condition for 48 h. The solid product washed with ethanol and dried to obtain the corresponding Fe3O4@SiO2@DOP-BenPyr.
In the final step, for the preparation of the catalysts, Fe3O4@SiO2@DOP-BenPyr (1 g) was dispersed in 25 mL absolute ethanol, and sonicated for 30 min. For the synthesis of Fe3O4@SiO2@DOP-BenPyr-Ni(II), Ni(NO3)2·6H2O (2 mmol) was added to the solution and stirred for 48 h in the reflux condition. Also for the preparation of Fe3O4@SiO2@DOP-BenPyr-Cu(II), the Cu salt (Cu(NO3)2·3H2O, 3 mmol) was applied. In the end of the procedure, the resultant solid was separated with an external magnet, washed with ethanol and dried at room temperature.
2.3 General Procedure for the Oxidation of Sulfides
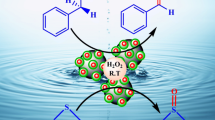
A mixture of sulfide (1 mmol) and H2O2 30% (0.4 mL) was stirred in the presence of catalyst [Fe3O4@SiO2@DOP-BenPyr-M(II) (M=Ni or Cu)] (0.01 g) at room temperature under solvent-free conditions. After completion of the reaction (monitored by TLC), the catalyst was separated by an external magnet, then CH2Cl2 (4 × 5 mL) was added and the mixture was washed with water (20 mL) and decanted. The organic solvent was dried over anhydrous Na2SO4, then, the mixture was filtered and solvent was evaporated to produce sulfoxides.
2.4 General Procedure for the Oxidative Coupling of Thiols
In the typical procedure for oxidative coupling of thiols, 0.01 g Fe3O4@SiO2@DOP-BenPyr-M(II) (M=Ni or Cu) as catalyst was added to a mixture of thiol (1 mmol), 0.4 mL H2O2 and PEG and stirred at room temperature. The progress was monitored by TLC. After the completion of the reaction, the catalyst was separated by an external magnet, then product extracted with CH2Cl2 (4 × 5 mL). Anhydrous Na2SO4 was used for drying of the organic layer, then, the mixture was filtered and solvent was evaporated to obtain the disulfide product.
2.5 General Procedure for the Preparation of 2,3-Dihydroquinazolin-4(1H)-ones
A mixture of 2-aminobenzamide (1.2 mmol), aldehydes (1 mmol) and catalyst (Fe3O4@SiO2@DOP-BenPyr-Ni(II) 0.03 g) was stirred under solvent-free condition at 110 °C for the appropriate time. After completion of the reaction (TLC monitoring), the reaction mixture was cooled down to room temperature and the crude product extracted by ethanol. At the end, catalyst was separated using an external magnet, washed with ethanol. The filtrate was evaporated to remove solvent, and the crude solid product was recrystallized with ethanol to afford pure 2,3-dihydroquinazolin-4(1H)-ones in 90–98% yields.
2.6 General Procedure for the Preparation of Polyhydroquinoline Derivatives
A mixture of aldehyde (1 mmol), dimedone (1 mmol), ethyl acetoacetate (1 mmol) and ammonium acetate (1.2 mmol) was added to 0.03 g catalyst [Fe3O4@SiO2@DOP-BenPyr-Ni(II)]. Then the reaction mixture was stirred at 100 °C under solvent-free condition and the progress of the reaction was monitored by TLC. The resulting solid product was dissolved in the ethanol. Then, the catalyst was separated by an external magnet and washed with ethanol. Also the solvent was evaporated and the product was recrystallized with ethanol for further purification, and the pure polyhydroquinoline derivatives were obtained in good to excellent yields (90–98%).
2.7 General Procedure for Preparation of 5-Substituted 1H-Tetrazoles
A mixture of sodium azide (1.1 mmol) and nitrile (1 mmol) in the presence of 0.05 g of catalyst [Fe3O4@SiO2@DOP-BenPyr-Ni(II)], was stirred at 120 °C in PEG-400. After completion of the reaction (observed by TLC), the reaction mixture was cooled down, and catalyst was isolated by an external magnet, then 10 mL HCl (4N) and 20 mL H2O was added to the residue solution. The products extracted with ethyl acetate (20 mL). The organic solvent was dried over anhydrous sodium sulfate, and concentrated to give the crude solid product. The precipitate was crystallized in a mixture of water and ethanol, and the pure 5-substituted 1H-tetrazoles were obtained in good to excellent yields (70–94%).
3 Result and Discussion
3.1 Preparation of Fe3O4@SiO2@DOP-BenPyr-M(II) (M=Ni and Cu)
In this paper we report novel and recoverable magnetite nanocatalysts for oxidation of sulfides to sulfoxides, oxidative coupling of thiols to disulfides, synthesis of 2,3-dihydroquinazolin-4(1H)-ones, 5-substituted 1H-tetrazoles and polyhydroquinolines. These catalysts were synthesised by the concise route outlined in Scheme 1. In order to prepare Fe3O4@SiO2@DOP-BenPyr-M(II) (M=Ni and Cu), initially magnetic nano particles of Fe3O4 has been synthesised by a mixture of FeCl3·6H2O, FeCl2·4H2O in aqueous ammonia under N2 atmosphere at 80 °C, then was coated with TEOS. In the next step, the resultant product was reacted with dopamine to afford MNPs@SiO2@DOP. The MNPs@SiO2@DOP-BenPyr was prepared using reaction of MNPs@SiO2@DOP with 2-benzoyl pyridine. In this step, the NH2 group of dopamine reacted with carbonyl group of 2-benzoyl pyridine. Finally, for the synthesis of MNPs@SiO2@DOP-BenPyr-Ni(II), the Ni(NO3)2·6H2O was applied. Also for synthesis of MNPs@SiO2@DOP-BenPyr-Cu(II), the Cu(NO3)2·3H2O was applied.
4 Catalyst Characterization
4.1 X-ray Diffraction (XRD)
After synthesis of the two nanocatalyst, we were characterized their by XRD, FT-IR, SEM, EDX, TGA, ICP and VSM techniques. Figure 1 indicated the XRD pattern of Fe3O4, Fe3O4@SiO2@DOP-BenPyr-Ni(II) and Fe3O4@SiO2@DOP-BenPyr-Cu(II). This pattern indicated that the surface modification of the Fe3O4 nanoparticles does not lead to their phase change and the XRD patterns of two catalysts are in accord with standard XRD pattern of Fe3O4.
4.2 FT-IR Spectroscopy
Figure 2 shows FT-IR spectra for the Fe3O4@SiO2, Fe3O4@SiO2@DOP, Fe3O4@SiO2@DOP-BenPyr, Fe3O4@SiO2@DOP-BenPyr-Ni(II) and Fe3O4@SiO2@DOP-BenPyr-Cu(II). The FT-IR spectra of Fe3O4@SiO2 show the peak at 584 cm−1 contributed to the Fe–O vibration. The peaks appeared at 1094 and 802 cm−1 corresponding to the asymmetric and symmetric stretching vibration of Si–O–Si bond respectively. Also the bands indicted at 3440 and 1624 cm−1 are, respectively, assigned to the O–H bonds which are attached to the surface iron atoms and deforming vibrations of adsorbed water. Figure 2b, is contributed to Fe3O4@SiO2@DOP. The presence of the linked dopamine groups are confirmed by C–H symmetric stretching vibrations that appear at 2830–2905 cm−1 and also, the N–H stretching vibration modes as a broad band that appear at 3403 cm−1. This curve also shows the peaks at 1442 and 1620 cm−1 each of them contributed to stretching vibration of C–N and bending of NH respectively. The spectrum of Fe3O4@SiO2@DOP-BenPyr (Fig. 2c) indicates peak at 1623 cm−1 is assigned to the stretching of the C=N bond in the imine. As shown in Fig. 2d, FT-IR spectra for Fe3O4@SiO2@DOP-BenPyr-Ni(II) indicate several peaks such as: Fe–O vibration, asymmetric and symmetric stretching vibration of Si–O–Si, stretching of the C=N bond and C–H stretching that each of the bonds show peaks at 588, 803–1094, 1626 and 2882–2986 cm−1. Coordination of the nitrogen with the metal of nickel leads to shift the C=N bond. Eventually, we investigate the FT-IR spectra of Fe3O4@SiO2@DOP-BenPyr-Cu(II). In this spectrum the adsorption band appears at 590 cm−1, assign to Fe–O vibrations. Also, asymmetric and symmetric stretching vibration of Si–O–Si clear at 802 and 1097 cm−1. The peak appears at around 2873–2982 cm−1 corresponds to the aliphatic C–H stretching. In this spectrum, stretching of the C=N bond is shifted to 1627 cm−1 which is due to coordination of the nitrogen with the metal.
4.3 Scanning Electron Microscopy (SEM)
The surface morphology and particle sizes of the synthesized catalysts were determined using SEM. The SEM images of Fe3O4@SiO2@DOP-BenPyr-Ni(II) (Fig. 3a) and Fe3O4@SiO2@DOP-BenPyr-Cu(II) (Fig. 3b) showed that the catalysts were made up of uniform-sized particle with a size less than 8 and 13 nm respectively. Also the shapes of them are spherical with uniform size, and showed good dispersity.
4.4 Energy Dispersive X-ray (EDX)
For the confirmation of the kinds of elements present in synthesized catalysts, the energy dispersive X-ray (EDX) spectrum employed. As shown in Fig. 4a, the EDX spectrum of Fe3O4@SiO2@DOP-BenPyr-Ni(II) indicates the several elements such as: C, N, O, Si, Fe and Ni. Also the EDX spectrum of Fe3O4@SiO2@DOP-BenPyr-Cu(II), shows the elements of C, N, O, Si, Fe and Cu (Fig. 4b).
4.5 Thermo Gravimetric Analysis (TGA)
The thermo gravimetric analysis (TGA) curve can be showed the mass loss of the organic materials as they decompose upon heating. As shown in Fig. 5, for the synthesized catalysts, two weight loss steps were observed. The first mass loss at temperatures below 200 °C is contributed to the removal of physically adsorbed surface hydroxyl groups and solvent. The second mass loss contributed to the thermal decomposition of organic groups grafted to Fe3O4. The weight loss of 6% observed for Fe3O4@SiO2@DOP-BenPyr-Ni(II) between 225 and 430 °C. Also the mass loss of 5% observed for Fe3O4@SiO2@DOP-BenPyr-Cu(II) between 240 and 440 °C. These results were confirmed that the organic moieties have been supported on the surface of Fe3O4 nanoparticles.
4.6 Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-OES)
For the investigation of metal content in two synthesized catalysts, we were applied inductively coupled plasma atomic emission spectroscopy (ICP-OES). According to the ICP-OES analysis, the exact amount of Cu in the catalyst was obtained to be 0.0115 mol g−1. Also the Ni amount in the Fe3O4@SiO2@DOP-BenPyr-Ni(II) was obtained to be 0.0153 mol g−1. In order to shown the analysis of leaching in catalyst, the amount of Ni in recycled catalyst in reaction of 4-chlorobenzaldehyde with dimedone, ethylacetoacetate and ammonium acetate was determined by ICP that was found to be 0.0145 mol g−1 (0.4% leaching). These results from ICP were showed that leaching of nickel during the reaction is insignificant, so the catalyst can be recovered and reused several times.
4.7 Vibrating Sample Magnetometry (VSM)
Figure 6 indicated the vibrating sample magnetometer (VSM) analysis for Fe3O4, Fe3O4@SiO2@DOP-BenPyr-Ni(II) and Fe3O4@SiO2@DOP-BenPyr-Cu(II). This analysis is employed to measure the magnetic properties of catalysts. This is clear that the bare MNPs showed the higher magnetic value in comparison with functionalized Fe3O4, this result is due to the coated silica and the layer that grafted to support.
5 Catalytic Studies
After characterization of Fe3O4@SiO2@DOP-BenPyr-Ni(II) and Fe3O4@SiO2@DOP-BenPyr-Cu(II), their catalytic activity was investigated in some organic reactions.
First, oxidation of sulfides was examined. In this light, initially the reaction conditions were optimized by reaction of methyl phenyl sulfide (1 mmol), 30% H2O2 and Fe3O4@SiO2@DOP-BenPyr-M(II) (M=Ni or Cu) as a model reaction in the presence of different organic solvents. As shown in Table 1, solvent free condition is the best choice. Then effect of catalysts on the synthesis of sulfoxides was studied by varying amount of them. The results show that 0.01 g of Fe3O4@SiO2@DOP-BenPyr-M(II) (M=Ni or Cu) was found to be the most effective (Table 1, entry 4).
The optimal conditions for the oxidation of sulfides obtained (Table 1, entry 4) and we explored the scope to study the oxidation of other sulfides. Finally, a wide range of aromatic and aliphatic sulfides were applied and successfully converted to the corresponding sulfoxides (Scheme 2). The results are indicated in Table 2.
In second part of our search, we have focused on the utility of these catalysts for oxidation of thiols to corresponding disulfides. Initially, to optimize the reaction conditions, a mixture of 4-methylthiophenol (1 mmol), H2O2 and Fe3O4@SiO2@DOP-BenPyr-M(II) (M=Ni or Cu) as catalyst at room temperature applied as a model reaction in the presence of several organic solvents. As shown in Table 3, entry 4, PEG showed better results in terms of the reaction yield and rate. Then the effect of various amount of catalysts investigated and the best results were obtained in 0.01 g of Fe3O4@SiO2@DOP-BenPyr-M(II) (M=Ni or Cu) (Table 3, entry 4).
Then oxidative couplings of other thiols were tested (Scheme 3). Results indicated that disulfides produced in high yields (Table 4).
Proposed mechanism for oxidation of sulfides and oxidative coupling of thiols in the presence of Fe3O4@SiO2@DOP-BenPyr-M(II) (M=Ni or Cu) indicated in Scheme 4. At first, the reaction of H2O2 with synthesized catalyst leads to the synthesis of intermediate A that converted to active oxidant B. Then, nucleophilic reaction of the sulfide or thiol with intermediate B occurs to obtain the cations of C and D. Finally, these cations converted to corresponding products [43].
In the next section of work, synthesis of 2,3-dihydroquinazolin-4(1H)-ones, polyhydroquinolines and 5-substituted 1H-tetrazoles in the presence of Fe3O4@SiO2@DOP-BenPyr-Ni(II) was investigated.
Initially, the prepared catalyst has been employed for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones (Scheme 5). The optimized reaction conditions were applied to the reaction between benzaldehyde and 2-aminobenzamide for synthesis of 2-(phenyl)-2,3-dihydroquinazolin-4(1H)-one (Table 5). In this light, various amounts of catalyst [Fe3O4@SiO2@DOP-BenPyr-Ni(II)] and different solvent were screened at different temperatures. It is obvious that the best result is obtained when using the 0.03 g catalyst under solvent-free condition at 110 °C for 1 h (Table 5, entry 3).
After the optimization of the reaction condition, 2,3-dihydroquinazolin-4(1H)-one derivatives (3a–3j) have been synthesized using the optimum conditions and the products were obtained in high yields. The turnover frequency (TOF) is reported in h–1 and showed the efficiency of the catalyst. The results of these studies are summarized in Table 6.
The proposed mechanism for synthesis of 2,3-dihydroquinazolin-4(1H)-one showed in Scheme 6. Based on this scheme, the 2-aminobezamide reacted with the activated aldehyde and the intermediate E produced. Then dehydration of intermediate E occurred to formed imine intermediate F. Finally, the intramolecular cyclization of imine intermediate F produced the final product [44].
Then, in order to found the optimum conditions for synthesis of polyhydroquinolines the reaction between 4-chlorobenzaldehyde, dimedone and ethyl acetoacetate was used as a model reaction in the presence of various amounts of catalyst, ammonium acetate and different solvents and temperatures (Table 7). It was observed that in the presence of 0.03 g catalyst and 1.2 mmol ammonium acetate, the best results were obtained in solvent-free condition at 100 °C after 120 min (Table 7, entry 3).
After optimization the reaction, we assessed the reaction of different electron-withdrawing and electron-donating substituted aldehyde (Scheme 7). As shown in Table 8 the wide range of polyhydroquinoline derivatives (4a–4j) were obtained in excellent yields (90–98%).
As shown in Scheme 8, the proposed mechanism for synthesis of polyhydroquinolines was indicated. Knoevenagel condensation between aldehydes and active methylene compounds produced the α,β-unsaturated compound. Then, the Michael-type addition of the resultant intermediates occur to form the final product [44].
In this part, for synthesis of 5-substituted 1H-tetrazoles, we chose phenyl cyanide to react with sodium azide in the presence of various amounts of Fe3O4@SiO2@DOP-BenPyr-Ni(II) as a model reaction (Table 9). We examined the effects of various solvents such as DMSO, EtOH and PEG, and different temperatures. Reaction in the PEG was chosen as an effective medium for this reaction. As shown in Table 9, 0.05 g catalyst at 120 °C in PEG were found to be the ideal reaction conditions for the synthesis of 5-substituted 1H-tetrazoles (Table 9, entry 4).
After optimization the reaction, as shown in Table 10 the wide range of nitriles were employed to afford the corresponding 5-substituted 1H-tetrazole derivatives (Scheme 9). The derivatives of 5-substituted 1H-tetrazole (5a–5h) were synthesized in good to excellent yields (70–94%).
A proposed mechanism for the preparation of 5-substituted1H-tetrazol is shown in Scheme 10. Initially, activated nitrile reacted with sodium azide to afford the intermediate G. Finally the intermediate G converted to compound H that the 5-substituted 1H-tetrazoles was obtained with rearrangement [45].
6 Recyclability of the Catalysts
Recyclability and reusability of the catalysts was studied for oxidation of tetrahydrothiophene under the optimization conditions. After completion of the reaction, the catalyst was separated from the reaction mixture by an external magnet, then the product was extracted with CH2Cl2 (4 × 5 mL) and water (20 mL). Anhydrous Na2SO4 was used for dried of the organic phase, filtered and solvent was evaporated. Finally, the separated catalysts were dried and reused for the same reaction again. As shown in Fig. 7, the two catalysts were recovered and reused five times without significant loss of catalytic efficiency.
Also reusability of the Fe3O4@SiO2@DOP-BenPyr-Ni(II) was tested for synthesis of polyhydroquinoline. Reusability of the Fe3O4@SiO2@DOP-BenPyr-Ni(II) was confirmed by the reaction of 4-chlorobenzaldehyde with dimedone, ethylacetoacetate and ammonium acetate as a model reaction in solvent-free condition at 100 °C. After completion of the reaction, catalyst was separated magnetically and washed several times with ethanol to removal of any organic residuals. The separated catalyst was reused for many times in the next cycles without any significant loss of its activity. The results shown in Fig. 7.
7 Comparison Results of Fe3O4@SiO2@DOP-BenPyr-M(II) (M=Ni, Cu) with Other Catalysts
To demonstrate the merit of the synthesized catalysts in this work, the result of oxidation of methyl phenyl sulfide (Table 11, entry 1–4), synthesis of 2,3-dihydroquinazolin-4(1H)-one (Table 11, entry 5–7), synthesis of polyhydroquinoline (Table 11, entry 8–10) and synthesis of 5-substituted 1H-tetrazole (Table 11, entry 11–13) compared with the previous reported in the literature. These results indicated the efficiency of the proposed methodology in terms of the reaction yield and rate in comparison with other literature reports.
8 Conclusions
In summary, we have designed two nano catalysts of Fe3O4@SiO2@DOP-BenPyr-Ni(II) and Fe3O4@SiO2@DOP-BenPyr-Cu(II). These compounds were efficient, recyclable and heterogeneous catalysts that employed for the high yielding and room temperature one-pot procedure for the oxidation of sulfides and oxidative coupling of thiols. In addition to, the catalyst activity of Fe3O4@SiO2@DOP-BenPyr-Ni(II) was investigated for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones, polyhydroquinolines and 5-substituted 1H-tetrazoles. These most applicable prepared catalysts offers several advantages in various organic reactions, such as short reaction times, simple workup, simple separation of heterogeneous catalysts by external magnet, easy recyclability of catalysts, and high yields of products. Also the prepared catalysts were characterized by FT-IR spectroscopy, TGA, XRD, VSM, EDX, ICP and SEM techniques.
References
Lu AH, Salabas EL, Schuth F (2007) Angew. Chem. Int. Ed. 46:1222
Ghorbani-Choghamarani A, Darvishnejad Z, Norouzi M (2015) Appl. Organometal. Chem. 29:707
Shokouhimehr MR, Piao Y, Kim J, Jang Y, Hyeon T (2007) Angew. Chem. Int. Ed. 46:7039
Zhu Y, Stubbs LP, Ho F, Liu R, Ship CP, Maguire JA, Hosmane NS (2010) Chem. Cat. Chem. 2:365
Qiu J, Peng H, Liang R (2007) Electrochem. Commun. 9:2734
Lv G, Mai M, Jin R, Gao L (2008) Synlett 9:1418
Mojtahedi MM, Abaee MS, Eghtedari M (2008) Appl. Organometal. Chem. 22:529
Baruwati B, Polshettiwar V, Varma RS (2009) Tetrahedron Lett. 50:1215
Zeng T, Yang L, Hudson R, Song G, Moores AR, Li CJ (2011) Org. Lett. 13:442
Sreedhar B, Suresh Kumar A, Surendra Reddy P (2010) Tetrahedron Lett. 51:1891
Dalaigh CO, Corr SA, Gunko Y, Connon SJ (2007) Angew. Chem. Int. Ed. 46:4329
Shylesh S, Schweizer J, Demeshko S, Schunemann V, Ernst S, Thiel WR (2009) Adv. Synth. Catal. 351:1789
Polshettiwar V, Luque R, Fihri A, Zhu H, Bouhrara M, Basset JM (2011) Chem. Rev. 111:3036
Zhang ZH, Lu HY, Yang SH, Gao JW (2010) J. Comb. Chem. 12:643
Ghorbani-Choghamarani A, Zamani P (2012) J. Iran. Chem. Soc. 9:607
Safari J, Gandomi-Ravandi S (2013) C. R. Chim. 16:1158
Chen J, Wu D, He F, Liu M, Wu H, Ding J, Su W (2008) Tetrahedron Lett. 49:3814
Narasimhulu M, Lee YR (2011) Tetrahedron 67:9627
Hajjami M, Gholamian F (2016) RSC Adv. 6:87950
Safari J, Gandomi-Ravandi S (2013) J. Mol. Catal. A 371:135
Niknam K, Jafarpour N, Niknam E (2011) Chin. Chem. Lett. 22:69
Sivaguru P, Parameswaran K, Kiruthiga M, Vadivel P, Lalitha A (2015) J. Iran. Chem. Soc. 12:95
Ghorbani-Choghamarani A, Tahmasbi B (2016) New J. Chem. 40:1205
Ghorbani-Choghamarani A, Azadi G (2015) RSC Adv. 5:9752
Mohammadi Ziarani G (2010) Iran. J. Chem. Chem. Eng. 29:1
Pokhodylo NT, Matiichuk VS, Obushak ND (2010) Russian J. Org. Chem. 46:556
Patil DR, Deshmukh MB, Dalal DS (2012) J. Iran. Chem. Soc. 9:799
Fazeli A, Oskooie HA, Beheshtiha YS, Heravi MM, Valizadeh H, Bamoharram FF (2013) Monatsh. Chem. 144:1407
Abdollahi-Alibeik M, Moaddeli A (2016) J. Chem. Sci. 128:93
Dehghani F, Sardarian AR, Esmaeilpour M (2013) J. Organomet. Chem. 743:87
Zolfigol MA, Khazaei A, Safaiee M, Mokhlesi M, Rostamian R, Bagheri M, Shiri M, Kruger HG (2013) J. Mol. Catal. A 370:80
Gogoi P, Kalita M, Bhattacharjee T, Barman P (2014) Tetrahedron Lett. 55:1028
Kon Y, Yokoi T, Yoshioka M, Tanaka S, Uesaka Y, Mochizuki T, Sato K, Tatsumi T (2014) Tetrahedron 70:7584
Jalilian F, Yadollahi B, Riahi Farsani M, Tangestaninejad S, Amiri Rudbari H, Habibi R (2015) Catal. Commun. 66:107
Rajabi F, Kakeshpour T, Saidi MR (2013) Catal. Commun. 40:13
Zhang Z, Li W, Liu J, Chen X, Bu YJ (2012) J. Organomet. Chem. 706:89
Tamhankar BV (2014) Int. J. Res. Org. Chem. 4:4
Dharmarathna S, Kingondu CK, Pahalagedara L, Kuo CH, Zhang Y, Suib SL (2014) Appl. Catal. B 147:124
Bahrami K, Khodaei MM, Yousefi BH, Arabi MS (2010) Tetrahedron Lett. 51:6939
Hajjami M, Sharifirad F, Gholamian F (2017) App. Org. Chem. 31:e3844
Bayraq SS, Nikseresht A, Khosravi I (2013) Phosphorus Sulfur 188:1236
Silveira CC, Mendes SR (2007) Tetrahedron Lett. 48:7469
Ghorbani-Choghamarani A, Moradi P, Tahmasbi B (2016) RSC Adv. 6:56458
Hajjami M, Nejat R, Sharifirad F, Gholamian F (2018) Org. Chem. Res. 4:23
Ghorbani-Choghamarani A, Moradi P, Tahmasbi B (2016) RSC Adv. 6:56638
Rostamnia S, Doustkhah E, Bahrami K, Amini S (2015) J. Mol. Liq. 207:334
Rostami A, Navasi Y, Moradi D, Ghorbani-Choghamarani A (2014) Catal. Commun. 43:16
Acknowledgements
Financial support to this work by the Ilam University and Bu-Ali Sina University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Conflict of interest authors declare that there is no conflict of interest involved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hajjami, M., Sheikhaei, S., Gholamian, F. et al. Synthesis and Characterization of Magnetic Functionalized Ni and Cu Nano Catalysts and Their Application in Oxidation, Oxidative Coupling and Various Multi-Component Reactions. Catal Lett 151, 2420–2435 (2021). https://doi.org/10.1007/s10562-020-03495-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03495-3