Abstract
A new nitrogen ligand, i.e. 1,3-di-(o-aminophenoxy)-2-propyl propargyl ether (DPPE), has been synthesized and characterized. Magnetic mesoporous silica composite (MNP@SiO2-SBA) was obtained via embedding magnetite nano-particles (MNPs) between SBA-15 channels. DPPE palladium dichloride (MNP@SiO2-SBA-DPPE-Pd(II)) was then prepared via click chemistry and fully characterized. The activity and recyclability of supported magnetic Pd(II) catalyst were evaluated in Heck coupling reaction after optimizing the optimal reaction conditions including solvent, amount of catalyst, base and temperature. Aryl iodides and aryl bromides showed enhanced activity compared to those of aryl chlorides in the Heck reaction. The catalyst was easily separated magnetically, reused in five runs sequentially, and no significant loss of activity was observed.
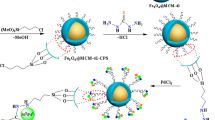
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Heck, Hiyama, Suzuki, Sonogashira, and Stille cross-coupling reactions are those reactions that they are widely used for C–C bond formation in the modern organic synthesis [1]. The palladium-catalyzed arylation reaction of olefinic compounds with aryl iodides in the presence of potassium acetate was independently reported by Mizoroki [2] and Heck [3] and then developed by Heck, Scheme 1.
Aryl, benzyl, and styryl halides were found to undergo the reaction however, among the aryl compounds only the iodides reacted rapidly [3]. Among various metals for catalyzing these reactions, palladium is more preferred and widely used due to enhanced activity and selectivity [4]. The Heck reaction is one of the most noted transformations within the Pd-involved organic preparations. Nowadays, Heck-type chemistry is a known method to assemble molecules via the formation of C–C bonds in both conventional and modern organic syntheses [5]. The reaction has a great potential for industrial applications, however, due to the properties and activities of the existing catalysts, limited industrial applications have been observed for the Heck reaction [6]. During the last decades, the preparation of a broad spectrum of supported Pd(II) complexes with different ligands as catalysts for coupling reactions have been reported. Improving the reaction yields and decreasing reactions time, processes costs, and byproducts are some of the results of these investigations [7].
In recent years, anchoring catalysts on MNPs has been extensively studied [8,9,10,11]. Magnetite nanoparticles are nontoxic, highly dispersible, biocompatible, and they have a high surface-to-volume ratio. Furthermore, MNP-supported catalysts enjoy the benefit of easy and rapid magnetic separation from different reaction mixtures [12,13,14].
It is highly recommended to coat MNPs prior to any treatment because they agglomerate intrinsically due to strong magnetic dipole–dipole interactions and high specific surface area [15, 16]. The most popular method to overcome this problem is using MNPs in the form of a composite with other metal oxides. Compositions of MNPs with different forms of mesoporous silica such as MCMs and SBAs have been widely investigated in recent years [17, 18]. This combination will also give the advantages of both mesoporous materials and magnetic nanoparticles to the MNP/silica nanocomposite materials. High selectivity and activity by providing high density of single reaction sites via heterogenization of homogenous catalysts, and easy catalyst separation and reusability are some of these advantages [19, 20].
There are plenty of reports on the incorporation of magnetic mesoporous particles as support for catalyst and preparing magnetically retrievable catalyst supports in the literature [14, 21]. For example, Fu et al. reported that magnetic mesoporous silica functionalized with poly(m-aminothiophenol) can rapidly adsorb Hg(II) ions. They showed that, the Hg(II)-complexed adsorbent can be used as an efficient catalyst for transformation of phenylacetylene to acetophenone [22]. In another work, Jiang et al. supported the polyoxometalate-based ionic liquid [(C4H9)3NCH3]3PMo12O40 with short carbon chain onto magnetic mesoporous silica microspheres with rough surface. The prepared catalyst showed enhanced activity toward oxidative desulfurization of diesel with H2O2 as an oxidant in compared with the smooth surface catalyst [23]. Sorokina et al. reported that, pyridylphenylene dendrons immobilized on to magnetic mesoporous silica act as efficient stabilizing molecules of Pd species. Their amphiphilic nanocomposite demonstrates excellent performance in Suzuki–Miyaura cross-coupling reaction in the ethanol/water mixture due to the combination of hydrophilic support and hydrophobic dendrons [24].
Among the Pd-involved organic preparations, the Heck reaction is one of the most noted transformations. Nowadays, Heck-type chemistry is a well-known method to assemble molecules via the formation of C–C bonds in both common and modern organic syntheses [5, 25]. In recent years, the preparation of a wide spectrum of supported Pd(II) complexes with different ligands as catalysts for coupling reactions has been reported. Improving the reaction yields and decreasing reactions time, processes costs, and byproducts are some of the results of these investigations [26, 27].
In the present study, a new ligand, i.e. 1,3-di-(o-aminophenoxy)-2-propyl propargyl ether (DPPE), has been synthesized and characterized. The prepared ligand can be supported on the surface of metal oxides via a simple click reaction. Separatedly, magnetic mesoporous silica was obtained via embedding the MNPs between mesoporous silica channels and then functionalized with azide. The combination of SBA-15 porous structure with the magnetic property of MNPs provides high density of catalytic sites for enhanced activity along with the advantage of simple magnetic decantation of the catalyst at the end of reaction. DPPE was grafted onto the surface via click reaction and then treated with a Pd(II) solution in EtOH to give a new Pd(II)-supported magnetic catalyst (MNP@SiO2-SBA-DPPE-Pd(II)). The obtained magnetic catalyst is a quite effective catalyst in the Heck reaction. Furthermore, it is compatible with the green chemistry principles and can be easily separated from the reaction medium, recycled, and re-used many times without significant loss of activity.
2 Experimental
2.1 Materials
MNP@SiO2-SBA magnetic composite [28] and 3-azidopropyltriethoxysilane [29] were prepared according to reported methods in the literature. 1,3-Di-(o-nitrophenoxy)-2-propanol (1) was synthesized via the method described by Zhang et al. [30] via the reaction of o-nitrophenol and 1,3-dichloro-2-propanol in boiling DMF and in the presence of K2CO3. Laboratory grade solvents used in this work were dried according to reported procedures in the literature [31]. The remaining chemicals were obtained from Merck and used as received.
2.2 Synthesis of 1,3-Di-(o-nitrophenoxy)-2-Propyl Propargyl Ether (3)
To a stirred solution of 1,3-di-(o-nitrophenoxy)-2-propanol 1 (0.830 g, 2.5 mmol) in dry DMF (5 ml) at 0 °C was added NaH (0.132 g, 3 mmol; 60% dispersion in mineral oil) in small portions under argon. The mixture was stirred in an ice bath for 1 h and then propargyl bromide (0.31 ml, 6 mmol) was added dropwise. The reaction mixture was warmed to room temperature, stirred for additional 10 h, and quenched by MeOH (7 ml). The white precipitate was filtered in vacuum, washed cold EtOH and dried to give 0.630 g product [32]. (Yield: 66%, mp: 100–103 °C). FT-IR (KBr, cm–1): 3269 (m), 3061 (w), 2932 (w), 2869 (w), 1607 (m), 1521 (s), 1350 (s), 1280 (m), 1250 (m), 743 (m).
2.3 Synthesis of 1,3-Di-(o-aminophenoxy)-2-Propyl Propargyl Ether (DPPE)
To a rapidly stirred suspension of powdered zinc (1.740 g, 26.6 mmol) in 4.25 ml of 37% aqueous ammonia was added a solution of 3 (1.000 g, 2.65 mmol) in 4.25 ml of THF. The mixture was refluxed for 6 h and then filtered. The filtrate was extracted with three 15 ml portions of ethyl acetate/water (50:50) mixture, and the combined extracts evaporated to dryness. The obtained residual orange oil chromatographed on 20 cm × 20 cm silica gel covered glass plates using 75:25 petroleum ether/acetone eluent to afford to give 0.510 g of orange oily product [33]. (Yield: 38%). FT-IR (KBr, cm–1): 3294 (s), 3048 (w), 2935 (w), 2869 (w), 1648 (s), 1526 (m), 1448 (w), 1310 (w), 1032 (m), 735 (m). 1H-NMR (250 MHz, CDCl3 δ): 6.85–7.00 (m, 2H); 6.70–6.80 (m, 2H); 4.47–4.50 (m, 2H); 4.40–4.46 (m, 1H); 4.23–4.32 (m, 4H); 3.90 (br, s, 4H); 2.54–2.56 (m, 1H). 13CNMR (62.5 MHz, CDCl3,δ): 141.1 (C–O, Ar.), 136.6 (C–N, Ar.), 122.0, 118.5, 115.4, 112.2, 79.8 (CH–O), 75.5 (≡C–C H2), 75.1 (≡C–H), 68.3 (O–CH2–CH), 57.9 (≡C–C H2). Mass: (m/z)+: 312.15 (M+, 84%).
2.4 Synthesis of Azide Functionalized Magnetic Nanocomposite MNP@SiO2-SBA-N3
A suspension of MNP@SiO2-SBA (1.000 g) in 50 ml of toluene was irradiated in an ultrasonic bath for 10 min. To this suspension, 3-azidopropyltriethoxysilane (2.0 ml, 9.7 mmol) was added, and the mixture was stirred for 16 h at 80 °C under argon. The reaction mixture was then cooled, filtered and washed with toluene several times and then dried at 45 °C in a vacuum oven [29]. FT-IR (KBr, cm–1): 2935 (w), 2869 (w), 2104 (m), 1628 (m), 1526 (w), 1448 (w), 1088 (s), 735 (m).
2.5 Synthesis of MNP@SiO2-SBA-DPPE via Click Reaction
In a round-bottomed flask MNP@SiO2-SBA-N3 (1.000 g) was dispersed in 20 ml of t-BuOH/water (1:1) containing CuSO4 (0.330 g, 1.32 mmol), sodium ascorbate (1.070 g, 5.4 mmol) and DPPE (1.200 g, 4.8 mmol). The mixture was irradiated in an ultrasonic bath for 15 min and stirred at 80 °C for 24 h under argon. The magnetic particles were then magnetically decanted, washed with EtOH and then dried in a vacuum oven at 80 °C [29]. FT-IR (KBr, cm−1): 3397 (br, s), 3067 (w), 2935 (w), 2869 (w), 2104 (m), 1628 (m), 1526 (w), 1448 (w), 1088 (s), 735 (m).
2.6 Synthesis of MNP@SiO2-SBA-DPPE-Pd(II)
DPPE functionalized magnetic mesoporous silica particles (1.000 g) were dispersed in a PdCl2 (0.03 g, 0.17 mmol) solution in EtOH (8 ml) in an ultrasonic bath for 15 min, and then stirred at room temperature for 12 h. The catalyst was decanted magnetically, washed several times with EtOH and then dried in vacuum at 45 °C [34].
2.7 General Procedure for the Heck Reaction
Typically, the supported catalyst (1.10 mol%) was dispersed in 1 ml N-methyl-2-pyrrolidone (NMP) through 8 min ultrasonication. Olefin (1.00 mmol), aryl halide (1.00 mmol), and K2CO3 (1.5 mmol) were then added and the reaction mixture was stirred at 120 °C. The catalyst was magnetically decanted as the reaction was completed (followed by TLC) and the mixture was analyzed by GC. The samples for NMR analysis were chromatographed on glass plates covered by silica gel.
3 Results and Discussion
3.1 Preparation and Characterization of the Catalyst
The new ligand (DPPE) has been prepared in two steps as shown in Scheme 2. In the first step, 1,3-di-(o-aminophenoxy)-2-propanol (1) was etherified with propargyl bromide 2 [32]. Selective reduction of nitro groups in the presence of ethynyl group by conventionl methods such as Pd/C/hydrazine; Pd/C/NaBH4; Fe/HCl; Sn/HCl, and Zn/HCl were unsuccessful. This was done by the specific method described by Kovar and Arnold for preparation of the ethynyl substuted o-diamines [33].
Common spectroscopy methods were applied to confirm the structure of DPPE. In the mass spectrum (Fig. 1), molecular ion peak appears at m/e = 312.15 (84%).
Figure 2a and b present the 1H NMR and 13C NMR spectra of DPPE, respectively. As Fig. 1a shows, the acetylenic proton is observed as a multiplet at 2.54–2.56 ppm. The protons related to amino groups and methylenes adjacent to oxygens of the aromatic rings appear as a broad singlet centered at 3.90 ppm, and a multiplet at 4.23–4.32 ppm, respectively. The protons of chiral carbon and methylene group adjacent to C≡C bond are seen as two multiplets at 4.40–4.47 ppm and 4.47–4.50 ppm, respectively. The aromatic rings protons appear at 6.70–6.80 ppm. In Fig. 2b, the observed 11 signals are compatible with the structure of DPPE. The prepared ligand can be supported on the surface of metal oxides via a simple click reaction.
Magnetite nanoparticles were prepared via co-precipitation method and then covered by a silica layer and then embedded into mesoporous silica (SBA-15) [28]. The combination of SBA-15 porous structure with the magnetic property of MNPs provides high density of catalytic sites for enhanced activity along with the advantage of simple magnetic decantation of the catalyst at the end of reaction. The as-prepared MNP@SiO2-SBA particles were then silylated with 3-azidopropyltrimethoxysilane via post-synthesis grafting technique described by Malvi et al. [29]. The azide functionalized magnetic mesoporous silica was further functionalized with DPPE via the click methodology, and then treated with PdCl2 solution in EtOH to afford supported Pd(II)-complex, Scheme 3.
The FT-IR spectra of DPPE and MNP@SiO2-SBA-DPPE are shown in Fig. 3a and b. Figure 3b shows the characteristic peak of magnetite at 560 cm−1. The strong absorption band observed at 1095 cm−1 (νas Si–O–Si) reveals the formation of MNP@SiO2 nano-composite [8]. Figure 3b clearly shows the characteristic peaks related to the ligand. The adsorption bands observed at 1451 cm−1, and 1520 cm−1 are related to C=C stretching vibrations of aromatic ring. The N–H bending vibration appeared at 1625 cm−1. The stretching vibrations related to aliphatic C–H, aromatic C–H, and NH2 groups appear at 2935 cm−1, 3050 cm−1, and 3375 cm−1, respectively. The FT-IR data are summarized in Table 1.
Surface qualitative elemental analysis at random points by EDX confirmed the presence of palladium atoms, Fig. 4. In addition, the EDX spectrum shows the signals of carbon, nitrogen, oxygen, silicon, and iron, and the presence of these elements in the supported catalyst. Atomic absorption spectroscopy revealed that the palladium loading is 0.10 mmol g−1 (1.10 wt%).
Figure 5a and b shows the XRD patterns of MNP and MNP@SiO2-SBA-DPPE-Pd(II). Both samples exhibit well-resolved diffraction peaks that can be indexed as (220), (311), (400), (422), (511) and (440) planes associated with cubic magnetite nanoparticles (JCPDS-ICDD Copyright 1938, file No. 01–1111) with the Fd − 3 m space group [35]. However, due to reposing of MNPs between the channels of mesoporous silica, intensities of these characteristic diffraction peaks were attenuated in the pattern of supported catalyst, Fig. 5b.
The image obtained by TEM also confirmed ordered structure of magnetic SBA (Fig. 6). Highly ordered arrays of 1D mesoporous channels in which MNPs with 20 to 50 nm diameter are located between the channels of MNP@SiO2@SBA are seen in this image.
Figure 7 shows the hysteresis loops of MNP, MNP@SiO2 and MNP@SiO2-SBA-DPPE-Pd(II) at room temperature and the results are tabulated in Table 2. Superparamagnetic behavior of the samples is shown by small remanence, hysteresis, coercivity values, and narrow width of the loop as seen in the inset of Fig. 7 [36, 37]. The saturation magnetizations of MNP, MNP@SiO2 and MNP@SiO2-SBA-DPPE-Pd(II) are 78.14, 34.52, and 1.56 emu g−1, respectively. Dropping of the maximal saturation magnetization of supported catalyst may be due to the entangling of MNPs between SBA channels.
3.2 Heck Coupling Reaction
Styrene and 1-bromonaphthalene were chosen as the model compounds for optimizing the Heck coupling reaction conditions. Reaction parameters such as the solvent, base, amount of base, amount of catalyst, and reaction temperature were studied. The results are shown in Table 3 and Fig. 8a–c. According to solvent screening (Entries 1 to 9), the NMP solvent is the best choice due to the highest yield (Entry 9, 90% yield). The effect of the base and its quantity were also evaluated (Entries 9 to 13). The highest conversion is achieved with the K2CO3 base (Entry 9, 90% yield). Higher or lower quantities of the base than that of 1.5 molar ratio diminish the reaction conversion (Entries 14 and 15). The optimization of catalyst dosage shows that using the catalyst containing 0.1 mol% Pd(II) is the optimal value (Entry 9, 90% yield). It should be noted that the reaction does not proceed properly at the temperatures lower or higher than 120 °C (Entries 19 to 21) and in the absence of the catalyst (Entry 22).
Various aryl halides were subjected to the optimized reaction conditions with different olefins, Table 4. As shown, aryl iodides and aryl bromides showed excellent conversions and TOFs (Entries 1 to 13), excluding 2-bromopyridine (Entry 13, conversion 34%). Aryl chlorides showed moderate reactivity under the applied reaction conditions excluding chlorobenzene (Entries 14 to 16).
A possible mechanism for the Heck cross-coupling reaction catalyzed by a supported Pd(II) complex can be proposed as Scheme 4. The mechanism is suggested based on the mechanism described by Shaw et al. [38, 39], in which a Pd(II)/Pd(IV) cycle is involved. In the proposed mechanism, olefin coordinates of to the supported Pd(II), and then an alkyl σ-complex is formed by attacking the nucleophile (KCO3−) on the coordinated complex. The aryl halide oxidizes the Pd(II) center, the attached nucleophile is released and the alkene coordinates to the Pd(IV) complex, again. The catalyst is regenerated via β-hydride elimination and base-induced removal of hydrogen halide that gives the desired product.
3.3 Characterization of the Recycled Catalyst
In order to indicate the stability of MNP@SiO2-SBA-DPPE-Pd(II), after using in the coupling reaction of bromonaphthalene and styrene, the catalyst was collected and characterized by FT-IR, Fig. 9a and b. As seen, the spectra of the recovered catalyst does not show any significant changes in comparison with the fresh catalyst.
3.4 Comparison of the Catalyst
The comparison of the prepared magnetic catalyst with a few literature reported catalysts in the coupling reaction of iodobenzene with styrene reveals that our prepared magnetic catalyst has enhanced reactivity, Table 5. In addition, the mesoporous structure of the prepared catlyst provides a very high srface area in compared with the most catalysts listed in Table 5. The prepared catlyst has also enjoys the benefit of magnetic separation and easy recovering process as the major advantage. High thermal stability is an other advantage of the our prepared catlyst over the catalysts listed catalyst in Table 5.
3.5 Recyclability of the Catalyst
Supported catalysts are usually prepared via complicated and expensive routes. Therefore, it is necessary to pay sufficient attention to retrieving of a used catalyst. The stability and re-usability of the magnetic catalyst were tested in the Heck coupling reactions of 1-bromonaphthalene and ethyl acrylate, Fig. 10. After each reaction, the catalyst was collected magnetically, washed with ethyl acetate, and then dried. As shown, the conversion reaches to 97.3% in the first run and then slightly dropped to 90.1% during five consecutive runs of the Heck reaction. Therefore, it can be concluded that the prepared supported catalyst is active and recyclable.
3.6 Leaching Study
In order to confirm the stability and heterogeneous nature of MNP@SiO2-SBA-DPPE-Pd(II), after using in the coupling reaction of bromonaphthalene and styrene, the catalyst was collected. The palladium concentration was measured in the remaining aliquat by atomic absorption which was found to be 0.0000116 mmol ml−1. The very low Pd concentration proves that no considerable metal leaching occurred in the reaction medium. Therefore, the heterogeneous palladium is responsible for completion of the reaction.
4 Conclusion
In summary, the synthesis, characterization, and application of an efficient and retrievable magnetic catalyst are reported. The catalyst was obtained by supporting a Pd(II) complex on magnetic mesoporous silica. For this purpose, DPPE was synthesized, characterized, and then supported on magnetic SBA via the click reaction. The supported ligand was treated with a Pd(II) solution to afford a supported Pd(II) catalyst. The obtained magnetic catalyst was fully characterized by the FT-IR, TGA, SEM, TEM, XRD, EDX, BET, and VSM analyses. It was shown that, the prepared catalyst could efficiently catalyze the Heck cross-coupling reaction of aryl halides and olefins. Excellent conversions were obtained and the recovering ability of the catalyst was shown by magnetic separation and re-using of the recovered catalyst. The recovered catalyst was reused several times with significant deactivation.
References
Chen Y, Wang M, Zhang L, Liu Y, Han J (2017) RSC Adv 7:47104
Tsutomu M, Kunio M, Atsumu O (1971) Bull Chem Soc Jpn 44:581
Heck RF, Nolley JP (1972) J Org Chem 37:2320
Nasrollahzadeh M, Issaabadi Z, Tohidi MM, Mohammad Sajadi S (2018) Chem Rec 18:165
Beletskaya IP, Cheprakov AV (2000) Chem Rev 100:3009
C.P. Mehnert, J. Y. Ying, Chem Commun, 2215 (1997)
Polshettiwar V, Molnár Á (2007) Tetrahedron 63:6949
Kang T, Li F, Baik S, Shao W, Ling D, Hyeon T (2017) Biomaterials 136:98
Moon SH, Noh S-H, Lee J-H, Shin T-H, Lim Y, Cheon J (2017) Nano Lett 17:800
Tayeb Oskoie P, Mansoori Y (2018) J Part Sci Technol 4:1
Rahimi L, Mansoori Y, Nuri A, Esquivel D (2020) ChemistrySelect 5:11690
Arnaudov M (1999) Spectrosc Lett 32:165
Baig RN, Varma RS (2013) Chem Commun 49:752
Nuri A, Mansoori Y, Bezaatpour A, Shchukarev A, Mikkola J-P (2019) ChemistrySelect 4:1820
Rutnakornpituk M, Puangsin N, Theamdee P, Rutnakornpituk B, Wichai U (2011) Polymer 52:987
Donescu D, Raditoiu V, Spataru CI, Somoghi R, Ghiurea M, Radovici C, Fierascu RC, Schinteie G, Leca A, Kuncser V (2012) Eur Polym J 48:1709
Yasuhara A, Kasano A, Sakamoto T (1999) J Org Chem 64:2301
Yang J-S, Wang C-M, Hwang C-Y, Liau K-L, Chiou S-Y (2003) Photochem Photobiol Sci 2:1225
Ghorbani-Choghamarani A, Tahmasbi B, Hudson RHE, Heidari A (2019) Microporous Mesoporous Mater 284:366
Tahmasbi B, Ghorbani-Choghamarani A (2019) New J Chem 43:14485
Martínez-Edo G, Balmori A, Pontón I, Martí del Rio A, Sánchez-García D (2018) Catalysts 8:617
Fu Y, Sun Y, Chen Z, Ying S, Wang J, Hu J (2019) Sci Total Environ 691:664
Jiang W, Jia H, Fan X, Dong L, Guo T, Zhu L, Zhu W, Li H (2019) Appl Surf Sci 484:1027
Sorokina SA, Kuchkina NV, Lawson BP, Krasnova IY, Nemygina NA, Nikoshvili LZ, Talanova VN, Stein BD, Pink M, Morgan DG, Sulman EM, Bronstein LM, Shifrina ZB (2019) Appl Surf Sci 488:865
Nuri A, Mansoori Y, Bezaatpour A (2019) Appl Organomet Chem 33:e4904
Kanagaraj K, Pitchumani K (2013) Chem Eur J 19:14425
Nuri A, Vucetic N, Smått J-H, Mansoori Y, Mikkola J-P, Murzin DY (2019) Catal. Lett. 149(7):1941–1951
Wang S, Wang K, Dai C, Shi H, Li J (2015) Chem Eng J 262:897
Malvi B, Sarkar BR, Pati D, Mathew R, Ajithkumar TG, Sen Gupta S (2009) J Mater Chem 19:1409
Zhang W, Liu S, Ma C, Jiang D (1998) Polyhedron 17:3835
Armarego WLF, Chai CLL (2003) Purification of laboratory chemicals, 5th edn. Butterworth-Heinemann, Burlington
Lv G, Mai W, Jin R, Gao L (2008) Synlett 2008:1418
R.F. Kovar, F.E. Arnold, US Patent 3,975,444 (1976)
Peng X, Zhao Y, Yang T, Yang Y, Jiang Y, Ma Z, Li X, Hou J, Xi B, Liu H (2018) Microporous Mesoporous Mater 258:26
Hanawalt JD, Rinn HW, Frevel LK (1938) Ind Eng Chem Anal Ed 10:457
Choubey J, Bajpai AK (2010) J Mater Sci Mater Med 21:1573
Basavaraja C, Jo EA, Huh DS (2010) Mater Lett 64:762
B.L. Shaw, Chem Commun, 1361 (1998)
Shaw B (1998) New J Chem 22:77
Nieva Lobos ML, Sieben JM, Comignani V, Duarte M, Volpe MA, Moyano EL (2016) Int J Hydrogen Energy 41:10695
Moradi P, Hajjami M, Tahmasbi B (2020) Polyhedron 175:114169
Danon A, Stair PC, Weitz E (2011) J Phys Chem C 115:11540
Mansoori Y, Khodayari A, Banaei A, Mirzaeinejad M, Azizian-Kalandaragh Y, Pooresmaeil M (2016) RSC Adv 6:48676
Rahmaninia A, Mansoori Y, Nasiri F, Bezaatpour A, Babaei B (2019) J Part Sci Technol 5:47
Sun P, Zhu Y, Yang H, Yan H, Lu L, Zhang X, Mao J (2012) Org Biomol Chem 10:4512
Górski B, Talko A, Basak T, Barbasiewicz M (2017) Org Lett 19:1756
Inomata S, Hiroki H, Terashima T, Ogata K, Fukuzawa S (2011) Tetrahedron 67:7263
Huang S-H, Chen J-R, Tsai F-Y (2010) Molecules 15:315
Yu L, Huang Y, Wei Z, Ding Y, Su C, Xu Q (2015) J Org Chem 80:8677
Prevost MS, Delarue-Cochin S, Marteaux J, Colas C, Van Renterghem C, Blondel A, Malliavin T, Corringer P-J, Joseph D (2013) J Med Chem 56:4619
Firouzabadi H, Iranpoor N, Kazemi F, Gholinejad M (2012) J Mol Catal A Chem 357:154
Khalafi-Nezhad A, Panahi F (2014) ACS Sustain Chem Eng 2:1177
Gruber AS, Zim D, Ebeling G, Monteiro AL, Dupont J (2000) Org Lett 2:1287
Cassez A, Kania N, Hapiot F, Fourmentin S, Monflier E, Ponchel A (2008) Catal Commun 9:1346
Mondal J, Modak A, Bhaumik A (2011) J Mol Catal A Chem 350:40
Han W, Liu N, Liu C, Jin ZL (2010) Chin Chem Lett 21:1411
Rosol M, Moyano A (2005) J Organomet Chem 690:2291
Acknowledgements
The financial support provided by Graduate Council of the University of Mohaghegh Ardabili is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mousavi, S., Mansoori, Y., Nuri, A. et al. A New Nitrogen Pd(II) Complex Immobilized on Magnetic Mesoporous Silica: A Retrievable Catalyst for C–C Bond Formation. Catal Lett 151, 1923–1936 (2021). https://doi.org/10.1007/s10562-020-03458-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03458-8