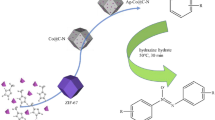
Abstract
A Heterogeneous catalyst developed for selective reduction of nitroarenes to the analogous anilines using formic acid as hydrogen source. This catalytic procedure offers a simplistic path to prepare aromatic amines in good to excellent yields. Especially, even anilines functionalized with other potentially reducible moieties are obtained with high selectivity. Herein, we report convenient and stable bimetallic AgPd nanocatalyst supported on metal organic framework coated with polyaniline. Hydrogenation of nitroarenes gave analogues anilines with excellent yields at 90 °C in 6 h with no use of additives. Catalyst maintained stable performance in five repeated cycles.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Aromatic primary amines (Ar-NH2) are important feed stocks and essential ingredients for the synthesis of several pharmaceutical, agrochemicals, dye, polymer, pigments, fine chemicals and natural products [1,2,3]. The method generally used for the production of Ar-NH2 is hydrogenation of functionalised aromatic nitro compounds using a stoichiometric amount of reducing agents in presence of metal catalyst based on gold, palladium, rhodium, ruthenium, and iridium [4,5,6]. However, a disadvantage of commercially offered Pd catalysts is their lack of chemo-selectivity. These catalytic systems are unfortunately related with environmental issues or formation of enormous undesired by-products. Supplementary alteration of noble metals with suitable additives was essential to improve the selectivity, but not at cost of activity. While, the existence of other reducible functional groups in the nitroarenes creates the dual necessities of activity and selective reduction of nitro group pretty challenging. Leaching of metal from catalyst is also a major issue. The degree of leaching is strongly sensitive to the nanoparticle size, support material and most importantly reaction media and conditions. Although amazing improvements have been achieved but still the development of novel catalysts with broad functional group tolerance and high activity signifies an important challenge.
Recently, metal organic frameworks (MOFs) have established as a promising class of porous materials with very large precise surface area, high porosity, and chemical tunability [7]. Because of these advantages and facile synthesis of MOFs, have been accepted for common applications in many fields, including gas storage, gas separation, luminescence, drug delivery, and catalysis [8,9,10,11,12]. MOFs allow access to guest molecules similar to their pore size. A variety of approaches have been taken to create comparatively large pores in MOFs, including use of longer linkers, modulators, defective crystallization, and templates [13,14,15,16,17,18] but the silver-catalysed decarboxylation (silver etching) [7] is quite interesting. It creates heterogeneous pores, even in highly stable MOFs, without changing the unique structure. This alteration in MOFs generates meso porosity which can allow comparatively large guest molecules.
To defeat the leaching obstruction and to improve the performance of catalyst, alternative support materials must be developed to achieve high dispersion, utilization, activity, and stability. To fulfil these requirements intrinsically conducting polymer (ICP) class has attracted significant attention. After intensive research it is found that among all ICPs polyaniline (PANI) is a finest choice because of controllable conductivity, good chemical stability, high conductive property via doping with acids and, easy synthesis using tremendously simple chemical oxidation of the low price monomer (aniline) in aqueous solutions [19]. For PANI, It is expected that the N atoms in carbon matrix may not only act as an electron donor but also serve as anchoring sites for the precursor.
AgPd nanoparticles (NPs) are found to be promising in catalytic dehydrogenation of formic acid in aqueous medium [20,21,22]. The AgPd NPs was supported on metal organic framework for preparation of a heterogeneous nanocatalyst. To enhance the activity of catalyst MOF is firstly coated with PANI. The catalytic enhancement is due to the synergic effect between Ag, Pd and PANI. The improved activity is because of electron delocalization between the d orbitals of Pd and the PANI π-conjugated ligand. The PANI coating also protects the MOF support from direct exposure to the corrosion and leaching. Several advantages of PANI coating is also there. First AgPd NPs supported on PANI is quite stable, furthermore the method of growing NPs is awfully simple. In this work a series of MOF composites were synthesized.
Herein, we reported heterogeneously catalysed selective reduction of nitroarenes using green reductant formic acid as the source of hydrogen. We synthesised MOF-PANI-Metal alloy composite (UiO-66-D-PANI-AgPd). In the first step, MOF (Zr based metal organic framework UiO-66) particles were prepared by hydrothermal route. MOF was decarboxylated by silver etching path. Then PANI was coated on the surface of decarboxylated MOF (UiO-66-D) by chemical (oxidative) polymerization method. In conclusion, AgPd nanoalloy grown on the PANI coated MOF surface.
1.1 Silver Etching Method
5 mL Acetonitrile solution of UiO-66 (100 mg), Potassium persulfate (135 mg, 0.5 mmol) and Silver nitrate (50 mg, 0.3 mmol) were allowed to react in Teflon-lined autoclave, and placed in a preheated silicon oil bath at 120 ℃ for 1 h. Decarboxylated MOF was centrifuged and washed three times with water and acetone. It was activated by keeping under vacuum for 1 h at 120 °C. This method was first introduced by Joeng et al. [7], they have described selective breaking of carboxylic bond during modification of MOFs, this will create pores in MOFs.
1.2 Preparation of Catalyst
Preparation of UiO-66-D-PANI-AgPd: Decarboxylated MOF (UiO-66-D) solution (250 mg, in 50 mL water) and Aniline solution (93 µL, 1 mmol in 50 mL water with 5 mg Sodium dodecyl sulphate SDS) were sonicated separately for 1 h. Then both the solutions were mixed together on an ice bath and acidic solution of Ammonium persulfate (APS) (229 mg, 1 mmol in 25 mL 1 M HCl) was added drop wise with continuous stirring. Solution remained on stirring until green colour appeared. In this green suspension 10 mL aqueous solution of AgNO3 (10% by weight of MOF) was added and stirred for 1 h. Further 10 mL aqueous solution of Pd(NO3)2 (10% by weight of MOF) was added and stirred for another 1 h, followed by addition of Hydrazine hydrate (3 mL, 65%). Suspension was maintained at 90 °C for 4 h with stirring. Catalyst was collected by centrifugation, washed three times with water and acetone and finally activated by keeping under vacuum for 1 h at 120 °C.
2 Results and Discussion
To evaluate the efficiency and selectivity of catalyst, nitrobenzene was chosen as model substrate and solvothermal one-pot reactions (90 °C 6 h) between nitrobenzene and formic acid in aqueous medium was done. It is noted that Pd could be the main active component of the catalyst responsible for the selective hydrogenation. Recycled catalyst UiO-66-D-AgPd losing its efficiency because of leaching. We also observed that after loading PANI the results were enhanced. Furthermore, high selectivity of the catalyst was retained with no leaching through up to five cycles. Recycling via simple centrifugation with loaded NPs (Tables 1, 2).
2.1 Catalyst Characterization
AgPd NPs were synthesized from AgNO3 and Pd(NO3)2 in water reducing by Hydrazine hydrate at 90 °C in 4 h. Figure 1 shows the XRD patterns of UiO-66-D-PANI-AgPd, UiO-66-D-AgPd and UiO-66-D. Similar to palladium, silver also has fcc structure with a cell constant of 0.40853 nm [23].
Decaroxylation of MOF confirmed by comparative IR data. Figure 2a, b provides the FTIR spectra of the decarboxylated MOF (UiO-66-D). It can be clearly seen that, after treatment with AgNO3 and K2S2O8 in Acetonitrile, intensity of the peak at 1676 cm−1 belongs to the stretching vibration of carbonyl decreases [24].
3 Conclusion
We have introduced a novel heterogenous catalyst UiO-66-D-PANI-AgPd for selective reduction of aryl nitro group. Our catalyst is effective in dehydrogenating formic acid to form H2 and subsequently utilizing the hydrogen generated in situ for reduction of nitro group into amine. The catalyst is easy to synthesize, stable, easily separable, and readily recyclable without loss of activity, and the reactions can be carried out in water, making the process environmentally friendly.
References
Ergen S, Nişancı B, Metin Ö (2018) One-pot reductive amination of aldehydes with nitroarenes using formic acid as the hydrogen donor and mesoporous graphitic carbon nitride supported AgPd alloy nanoparticles as the heterogeneous catalyst. New J Chem 42(12):10000–10006
Gomez S, Peters JA, Maschmeyer T (2002) The reductive amination of aldehydes and ketones and the hydrogenation of nitriles: mechanistic aspects and selectivity control. Adv Synth Catal 344(10):1037–1057
Tripathi RP, Verma SS, Pandey J, Tiwari VK (2008) Recent development on catalytic reductive amination and applications. Curr Org Chem 12(13):1093–1115
Liang S, Monsen P, Hammond GB, Xu B (2016) Au/TiO2 catalyzed reductive amination of aldehydes and ketones using formic acid as reductant. Org Chem Front 3(4):505–509
Liu J-T, Yang S, Tang W, Yang Z, Xu J (2018) Iridium-catalyzed efficient reduction of ketones in water with formic acid as a hydride donor at low catalyst loading. Green Chem 20(9):2118–2124
Jiang L, Zhou P, Zhang Z, Chi Q, Jin S (2017) Environmentally friendly synthesis of secondary amines via one-pot reductive amination over a heterogeneous Co–Nx catalyst. New J Chem 41(20):11991–11997
Jeong G-Y, Singh AK, Kim M-G, Gyak K-W, Ryu U, Choi KM et al (2018) Metal-organic framework patterns and membranes with heterogeneous pores for flow-assisted switchable separations. Nat Commun 9(1):3968
Han X, Yang S, Schröder M (2019) Porous metal–organic frameworks as emerging sorbents for clean air. Nat Rev Chem 3(2):108–118
Zhao P, Fang H, Mukhopadhyay S, Li A, Rudić S, McPherson IJ et al (2019) Structural dynamics of a metal–organic framework induced by CO2 migration in its non-uniform porous structure. Nat Commun 10(1):999
Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM (2013) The chemistry and applications of metal-organic frameworks. Science 341(6149):1230444
Shen K, Zhang L, Chen X, Liu L, Zhang D, Han Y et al (2018) Ordered macro-microporous metal-organic framework single crystals. Science 359(6372):206–210
Long JR, Yaghi OM (2009) The pervasive chemistry of metal–organic frameworks. Chem Soc Rev 38(5):1213–1214
Carrington EJ, McAnally CA, Fletcher AJ, Thompson SP, Warren M, Brammer L (2017) Solvent-switchable continuous-breathing behaviour in a diamondoid metal–organic framework and its influence on CO2 versus CH4 selectivity. Nat Chem 9:882
Eddaoudi M, Kim J, Rosi N, Vodak D, Wachter J, O'Keeffe M et al (2002) Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295(5554):469–472
Cai G, Jiang H-L (2017) A modulator-induced defect-formation strategy to hierarchically porous metal-organic frameworks with high stability. Angew Chem Int Ed 56(2):563–567
Fang Z, Bueken B, De Vos DE, Fischer RA (2015) Defect-engineered metal-organic frameworks. Angew Chem Int Ed 54(25):7234–7254
Peng L, Zhang J, Xue Z, Han B, Sang X, Liu C et al (2014) Highly mesoporous metal–organic framework assembled in a switchable solvent. Nat Commun 5:4465
Zhang W, Liu Y, Lu G, Wang Y, Li S, Cui C et al (2015) Mesoporous Metal-organic frameworks with size-, shape-, and space-distribution-controlled pore structure. Adv Mater 27(18):2923–2929
Ramohlola KE, Monana GR, Hato MJ, Modibane KD, Molapo KM, Masikini M et al (2018) Polyaniline-metal organic framework nanocomposite as an efficient electrocatalyst for hydrogen evolution reaction. Composites B Eng 137:129–139
Sanchez F, Motta D, Roldan A, Hammond C, Villa A, Dimitratos N (2018) Hydrogen generation from additive-free formic acid decomposition under mild conditions by Pd/C: experimental and DFT studies. Top Catal 61(3):254–266
Nabid MR, Bide Y, Etemadi B (2017) Ag@Pd nanoparticles immobilized on a nitrogen-doped graphene carbon nanotube aerogel as a superb catalyst for the dehydrogenation of formic acid. New J Chem 41(19):10773–10779
Ma X, Feng Y, Li Y, Han Y, Lu G, Yang H et al (2015) Promoting effect of polyaniline on Pd catalysts for the formic acid electrooxidation reaction. Chin J Catal 36(7):943–951
Tabrizi NS, Xu Q, van der Pers NM, Lafont U, Schmidt-Ott A (2008) Synthesis of mixed metallic nanoparticles by spark discharge. J Nanopart Res 11(5):1209
Zhang X, Yang Y, Huang W, Yang Y, Wang Y, He C et al (2018) g-C3N4/UiO-66 nanohybrids with enhanced photocatalytic activities for the oxidation of dye under visible light irradiation. Mater Res Bull 99:349–358
Acknowledgements
The authors are thankful to CSIR-HRDG for their financial assistance [File no: 09/172(0084)/2017-EMR-1].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Babel, V., Hiran, B.L. Heterogeneous AgPd Alloy Nanocatalyst for Selective Reduction of Aromatic Nitro Compounds Using Formic Acid as Hydrogen Source. Catal Lett 150, 1865–1869 (2020). https://doi.org/10.1007/s10562-020-03098-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03098-y