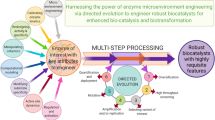
Abstract
The current industrial revolution signifies the high-value of protein engineering. The development of multipurpose biocatalysts is significantly expanding as a result of increased access and enzyme tailoring ability to satisfy the ever-increasing industrial demands. Enzyme-catalyzed processes offers multi-benefits at a time, e.g., low catalyst loading, high specificity, selectivity, mild processing for a complex and chemically unstable compounds, capability to reduce or eliminate reaction by-products, overall reusability and cost-effective ratio via immobilization, and potential to carry out conventional multi-stage processes via one-pot reaction. In this review, we critically elaborated recent achievements in applying new and/or state-of-the-art sophisticated protein engineering approaches to tailor the catalytic properties of enzymes or design enzymes with new and improved activities to catalyze desired biochemical transformations by orders of magnitude. We focused on different protein engineering approaches such as substrate engineering, medium engineering, and post-translational enzyme modification, structure-assisted protein tailoring, advanced computational modeling, and the exploration of inimitable synthetic scaffolds to develop multipurpose biocatalyst and improve the performance of multi-enzyme systems. In short, this study demonstrates an array of molecular biology insights and computational designs speeding up the tailored design of new and industrial biocatalysts. Continuous key developments in this direction together with protein engineering in unique ways might offer the ever-increasing opportunities for impending biocatalysis research for industrial bioprocesses.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Biocatalysis refers to the use of enzymes in chemical biotransformations either as isolated enzyme preparations or as whole cell format. Over the last few decades, biocatalysis emerges as an appealing technology for several industrial sectors, in particular, pharmaceutical industry because enzyme-driven catalysis addresses the ever-growing demands for safe, renewable, and highly selective industrial bioprocesses. In contrast to chemo-catalysts, biocatalysts possess a bulky three-dimensional structural configuration that creates numerous contact points with a target substrate and thus allowing for impeccable selectivity [1,2,3,4]. Biocatalytic reactions are carried out under mild environment obviating the requisite for functionalization of an active group, and tiresome blocking and deblocking steps commonly required in region- and enantio-selective chemical production [5]. Additionally, biocatalysis offers both economic and environmental incentives over chemocatalytic techniques. Enzymes are versatile, and nature’s green biocatalysts produced from sustainable resources and are essentially non-toxic, biocompatible, and amenable to biodegradability. These desired attributes fulfill the central tenants of sustainable development and immobilization of enzymes using magnetic nanoparticles (MNPs) [6]. The utilization of MNPs represents a noteworthy green chemistry approach, since it lengthens the biocatalyst lifetime through multiple recovery cycles [6]. Furthermore, these enzymatic features translate to the production of energy and resource-efficient purer products in processes in a highly selective way, and generate no undesirable by-products, thus creating enzymes an ecologically responsive substitute to chemocatalysts. More importantly, enzyme-driven catalytic reactions are executed under the identical pressure and temperature conditions that render easy to consolidate several reactions into the eco-efficient one-pot biocatalytic cascade. The wide-ranging application of biocatalytic processes might be ascribed to its numerous economic and environmental benefits [2,3,4].
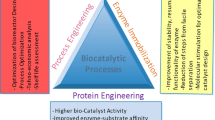
Over millions of years, wild-type native enzymes have evolved to in vivo transform their native substrates at greater rates. Therefore, it is not unanticipated that retaining an enhanced catalytic productivity and performance in vitro with unnatural substrates is problematic under unfavorable environments, such as non-aqueous media and high substrate concentrations. However, in some cases, promising outcomes have been achieved with the use of native enzymes. Recently, Martinez et al. [7] designed a highly efficient chemoenzymatic process to synthesize pregabalin using the lipase from Thermomyces lanuginosus. The main enzymatic step was steered at a striking substrate concentration (765 g L−1) in an aqueous phase with the markedly less usage of organic solvent. The implication of this novel process afforded a higher product yield in contrast to the common production bioprocess. Despite impressive industrial potentialities of pristine enzyme catalysts with unnatural substrates, optimizing biocatalytic process is indispensable for scale-up feasibility, either by manipulating the reaction system or enzyme. An entire enzyme-driven catalytic process encompasses many parameters, and biocatalyst is the only one part amongst these. This review article elaborates different state-of-the-art sophisticated protein engineering approaches to tailor the catalytic properties of enzymes or design enzymes with new and improved activities to catalyze desired biochemical transformations by orders of magnitude. We focused on different protein engineering approaches such as substrate engineering, medium engineering, and post-translational enzyme modification, structure-assisted protein tailoring, advanced computational modeling, and the exploration of inimitable synthetic scaffolds to develop multipurpose biocatalyst and improve the performance of multi-enzyme systems (Fig. 1). The protein engineering approach might offer economically viable and environmentally acceptable biotransformation following the optimization of all the variables mentioned above.
2 Protein Engineering: A Driving Force for Applied Biocatalysis
Despite the increasing number of obvious advantages of biocatalyst-mediated processes, there have been a modest historical number of industrial applications, while the use of biocatalysis is dramatically increased within the last 20 years [5, 8]. The rationale for this is lack of hasty ability to recognize, acquire, test, and optimize biocatalyst attributes for the manufacturing of pharmaceutical compounds [9]. Protein engineering is considered one of the most significant factors leading to the implementation of catalytic bioprocesses for industrial-level biotransformation [10]. The last century has witnessed three distinct phases of biocatalysis, and now entering into a fourth wave. All these biocatalysis phases were distinguished by the tendency to manipulate or tailor an enzyme catalytic attributes according to the target purposes [1, 11]. The first phase of biocatalysis involved the use of natural biocatalysts to catalyze the desired chemical transformation. The chemical mechanism implicates wild-type enzyme’s inherent ability to transform a substrate into the product. In the second biocatalysis wave between the 1980s and 1990s, structural information directed preliminary protein engineering technologies were exploited to broaden the substrate preference of biocatalysts toward non-natural and unusual products. The third phase fast-tracked the optimization of biocatalyst using directed evolution techniques [12, 13]. The fast generation of different enzyme variants by means of molecular approaches accompanied by effective screening methods allowed significant improvement in enzymes desired catalytic properties at an accelerating rate. This universal strategy was applied to innumerable enzymes and regarded as the principal approach to boosting biocatalyst features nowadays. Indeed, it is worthy to mention that directed evolution-assisted protein engineering allows envisaging a perfect bioprocess and building biocatalysts to suit that bioprocess. Notwithstanding decades of research developments in the front, the too long timeframe to optimally design a biocatalyst is the paramount impediment to applying biocatalysis in pharmaceutical industrial processes [1].
3 Protein Engineering Approaches
In spite of an unprecedented number of industrial and biotechnological applications; usually, biocatalysts do not display satisfactory catalytic performance, i.e. stability, selectivity, and activity. Some of the limitations mentioned above can be solved by manipulating substrates, reaction medium or enzyme tailoring via immobilization [2,3,4, 14,15,16,17,18,19,20,21,22,23,24,25,26,27].
3.1 Substrate Engineering
Substrate engineering implicates manipulation of the substrate specificity of the enzyme that results in both the optimization and improvement of existing biotransformations and the discovery of innovative reactions. Substrate engineering can be categorized as (1) switching, (2) expanding, and (3) narrowing the substrate specificity of the enzyme as shown in Fig. 2. Often, it is intimated as the use of unnatural substrates, which derives the term enzyme promiscuity [11]. Use of substrate engineering has been widely investigated to boost up the efficiency of enzymatic reactions and might lead to the invention of entirely differential reaction types. For instance, a class of serine hydrolases, i.e. lipases, catalyzes in vivo triglycerides conversion to a mixture of glycerol and fatty acids. A shared characteristic feature of these serine hydrolases is the presence of a serine-histidine-aspartate triad, where serine acts as a nucleophile, histidine as a proton donor and aspartate forms a charge relay network. The acyl-enzyme intermediate is generated as a result of substrate reaction with the hydroxyl group of a serine residue in the catalytic site, which subsequently reacts with water to release free fatty acid (FFA). A deep assessment and scrutiny of this reaction mechanism lead to the notion that other unnatural nucleophilic acyl acceptors could also be utilized as a substitute for water [3].
Another remarkable example of non-natural nucleophiles involves the use of halohydrin dehalogenases (HHDHs) in an enzymatic reaction. Notably, HHDHs catalyze the reversible in vivo ring closure of chlorohydrins to epoxides. The mechanism implicates a halide ion-binding site that can be substitutable with other nucleophiles, which could subsequently react with the epoxide. Due to great promiscuous nature, HHDHs can recognize and accept nine other anions as nucleophiles in the epoxides ring opening that form the basis for pure enantioselective manufacturing of an array of β-substituted alcohols [28]. The cyanide ion is particularly interesting to use as the nucleophile since it generates a new C–C linkage and has been used to produce an important intermediate in the synthesis of atorvastatin [29], an industrial process commercialized by Codexis [3]. Generating alterations in the substrate structure has also investigated to accomplish improvements in biocatalytic processes. The enzyme traits such as enantioselectivity, conversion efficiencies, and catalytic steadiness were noticeably improved using vinyl esters of chiral carboxylic acids than routinely used ethyl esters [30, 31]. Moreover, the application of numerous substrate-engineering approaches has also been demonstrated in biohydroxylation [32].
3.2 Medium Engineering
Generally, enzymes optimally function in aqueous solution but are also active in organic media. Undeniably, biocatalysis exhibits several benefits in organic solvents. Several organic substrates are miscible with water, however, some catalytic reactions such as amidations and (Trans)-esterifications cannot be carried out in aqueous phase due to product hydrolysis computation or equilibrium limitations. Easier product retrieval from organic solvents accompanied by lack of microbial contamination is the additional advantages of non-aqueous catalytic reactions. Consequently, medium engineering can be employed to optimize and improve the biosynthetic perspective of enzyme-mediated biotransformations [3].
Though enzymes can perform in organic solvents as suspension forms, their catalytic efficacies are considerably diminished than those detected in water. Furthermore, the environmental concern connected with the consumption of volatile organic solvents (VOCs) represents another disadvantage of catalytic reactions in the organic phase. In this milieu, numerous biocatalysis reactions are conducted on laboratory as well as industrial level, in an aqueous biphasic system, comprising of water and environmentally compatible organic solvent. For this, the key reaction occurred in water, and the substrate and resulting product are primarily dissolved in the organic media [2, 4]. Three enzyme processes for atorvastatin intermediate carried out in aqueous ethyl acetate is a notable example of this type of biocatalytic reaction. The catalytic performance of biocatalysts in organic media can be amplified by lyophilization using high quantities of salts (i.e., KCl). This directed to the insight that enzyme suspension in a room temperature ionic liquid (IL) along with its salt- and water-like character leads to considerable rate improvements in comparison to organic solvents [3]. Notably, ILs composed of ions is liquid at room temperature, and have been extensively contemplated as potential alternatives to VOCs.
The suspension of CaLB-catalyzed amidations and transesterifications is the foremost illustrations of biocatalytic reactions in a water-free IL [3, 33]. Though, the reaction proportions were only marginally greater compared to the best organic solvents; the results revealed the biocompatibility of enzyme catalysts with ILs and highlighted the biocatalysis advent in ILs. Employment of ILs as reaction media can lead to an amplified stability or enantioselectivity consequence from conformational modifications of enzymes in IL media. A significant driving force for ILs utilization is the VOCs substitution prospect with non-volatile ILs, thus eliminating the menace of air contamination. Nonetheless, the substantial ILs solubility in water and in particular, first-generation ILs, such as dialkyl imidazolium and tetraalkylammonium salts possesses poor biodegradability and aquatic eco-toxicity and involve expensive preparation procedures. Therefore, the current inclination concerning the rationally designed target-oriented biocompatible and biodegradable ILs is considered as cost-effective with minimized ecological apprehension [2].
3.3 Enzyme Engineering and Immobilization
After the identification of an appropriate enzyme for the pre-designed biotransformation, and engineering substrate and reaction medium, the enzyme should be expressed in a GRAS (Generally Regarded as Safe) status microbial chassis enabling its hyper-production in large amounts at low-cost and environmentally friendlier environment [34]. Being soluble in water, enzymes are costly and even challenging to recover from the aqueous medium and employed on a single use, throwing-away basis. The shortcoming above can be overcome by immobilization of the enzyme to develop a heterogeneous biocatalyst with easy recovery and reusability (Fig. 3) [35]. It results in procedure simplification together with a high bioproduct quality and minimum environmental issues [21,22,23,24,25, 36,37,38,39,40,41,42,43,44]. Additionally, enzyme immobilization, i.e. post-translational biocatalyst engineering generally led to elevated stability and protects enzyme inactivation, which consequently allows its use in wide-ranging solvents [1, 44, 45].
Physical and chemical based enzyme immobilization methods along with considerable limitations and potentialities. Reprinted from Bilal et al. [35], with permission from Taylor & Francis. Copyright (2018) Informa UK Limited, trading as Taylor & Francis Group
For example, Truppo et al. [1] carried out the immobilization of transaminase on different polymer-based resins and comparatively assessed the immobilizate characteristics with the lyophilized native counterpart. Use of a highly hydrophobic octadecyl-activated polymethacrylate resin displayed the optimum results for adsorbed transaminase with 4.0% and 45% of loading efficiency and active recovery, respectively. Interestingly, the polymethacrylate resin-attached enzyme was found to be vigorous in a broad range of organic solvents. However, isopropyl acetate as environmentally attractive solvent showed a favorable solubility of the ketone substrate along with remarkable biocatalyst steadiness than other solvents. Further, the immobilized transaminase exhibited robust activities in dry isopropyl acetate at a high temperature of 50 °C, with a very slow inactivation rate up to 6 days. Notably, no deactivation was detected in water-saturated isopropyl acetate over the same time duration, and immobilized derivative was reusable for ten repeated batches with no noticeable activity decrease for the synthesis of enantiomeric pure chiral amines. On the other hand, the pristine soluble enzyme was entirely deactivated retaining no activity in the organic solvent. It is important to mention that utilizing organic solvents showed evident advantages over the aqueous procedure, which necessitates the use of a buffer to continuously control pH throughout the bioprocess. The resulting product is then easily extracted using the organic solvent and the denatured enzyme is eliminated from the mixture by filtration. The deployment of an immobilized enzyme in an environmentally compatible organic solvent circumvents the requisite for constant pH control, use of a buffer, and difficult exclusion of the denatured enzyme. Overall, this protocol significantly shortens the work-up, trim-down the processing duration and waste generation, and the enzyme can be reused for many times [35]. Despite the existence and advancements in immobilization strategies, a rapid and economical biocatalysts immobilization protocol still evades us. Overcoming this obstacle requires a multidisciplinary integrated research effort among molecular biology, chemical engineering, and organic chemistry and material science [4].
3.4 Carrier-Free Cross-Linked Enzyme Aggregates
Over the past few years, the synthesis and use of cross-linked enzyme aggregates (CLEAs) have appeared as a hopeful and promising carrier-free immobilization technology. Owing to its several advantages (Fig. 4) [46], and procedural simplicity, robustness, and use of unpurified enzymes, this method has attracted increasing researcher’s attention as a very attractive approach. Generally, CLEAs exhibit some interesting features such as greater catalytic activities enhanced operational and storage stabilities against denaturing agents, and excellent reusability/recoverability [46,47,48]. The preparation of CLEAs implicates simple precipitation of the enzyme extract from an aqueous buffer using precipitating agents (a salt, nonionic polymer, or an organic solvent) followed by cross-linking of the precipitated enzymes with a suitable cross-linker, i.e. glutaraldehyde (Fig. 5) [49, 50]. Physical aggregation or precipitation of enzymes may be induced by the addition of a precipitant without denaturing the three-dimensional enzyme conformation [51].
Potential advantages of CLEAs. Reprinted from Bilal et al. [46], with permission from Taylor & Francis. Copyright (2019) Informa UK Limited, trading as Taylor & Francis Group
3.5 Carrier-Supported CLEAs Strategies
The particle sizes of the most of the literature-reported CLEAs are below 10 µm [49], which results in difficult recovery from the reaction system, and thereby hinders their continuous application. Also, they might be considered too soft for several industrial applications [52]. To overcome these inadequacies, recently carrier-supported CLEAs strategies have been intended as alternative solutions and endeavored to design biocatalysts with outstanding mechanical properties [53, 54].
3.5.1 Magnetic CLEAs
The use of magnetic support materials is a simple and promising approach to separating and recovery of the enzyme, improving stability for repeated usage, and greater control over the biocatalytic process [55]. Cruz-Izquierdo group developed a technique for the synthesis of magnetic cross-linked enzyme aggregates (mCLEAs) by cross-linking insolubilized Candida antarctica lipase B (CALB) to aminated MNPs by glutaraldehyde. Figure 6 illustrates a simplified schematic development of MNPs and mCLEAs. The resulting mCLEAs show greater storage and thermal stabilities and can be continuously recycled following their separation from the reaction mixture by a magnet [56]. Magnetic α-amylase-CLEAs developed through integrating NH2-functionalized MNPs to an enzyme solution results in 100% of the α-amylase activity recovery than nonmagnetic CLEAs (only 45% recovery). The magnetic CLEAs exhibited a broader limit of operating temperature and elevated thermal and shelf life properties and can be easily recovered and eliminated from the reaction medium without filtration or centrifugation or because of the magnetic character of the MNPs [57]. Magnetic cutinase-CLEAs fabricated by Sekhon et al. [58] preserved 55% of its original activity after carrying out 50 rounds for the degradation of polycaprolactone. Cui et al. [55] prepared a novel hybrid magnetic CLEAs of phenylalanine ammonia lyase (HM-PAL-CLEAs) by co-aggregating enzyme molecules with MNPs followed by glutaraldehyde crosslinking. The resulting HM-PAL-CLEAs were readily recovered from the reaction system by means of external magnetic field and revealed a wide-working optimal pH than PAL-CLEAs and native enzyme. Furthermore, the engineered CLEAs also showed the increased storage and denaturant resistance as well as elevated thermal stability. High activity retention after eleven reusability cycles revealed that the developed magnetic CLEAs-based technology might serve as an efficient and practical solution for boosting up the catalytic performance of immobilized.
3.5.2 Mesoporous Silica Supported CLEAs
In recent years, enzymes entrapped in the pores of mesoporous materials led to highly loaded, active, and robust biocatalysts, which are considerably stabilized compared to biocatalysts formed by adsorption [52, 59]. Prime immobilization techniques including adsorption and others applied for attachment of enzymes in mesoporous support materials are listed in Table 1 [35]. Enormous research has been focused towards a ship-in-a-bottle approach for enzyme immobilization. Using this approach, CLEAs shipped in hierarchically ordered meso-cellular mesoporous silica showed a significant improvement of enzymes’ functional stability [60, 61]. Recently, Jiang et al. [62] synthesized Candida sp. 99-125 lipase CLEAs using three-dimensionally ordered macroporous silica as support material (CLEAs-LP@ 3DOM-SiO2). For this, lipase was first precipitated in the pores of 3DOM SiO2 by saturated ammonium sulfate followed by cross-linking by glutaraldehyde to develop CLEAsLP@3DOM-SiO2. In contrast to the free form of lipase, the resulting CLEAs-LP@3DOM-SiO2 presented outstanding mechanical and thermal steadiness retaining above 80% of original activity after 16 days of shaking in aqueous and organic solutions. Moreover, engineered CLEAs also exhibited improved catalytic performance and recyclability for esterification and transesterification reaction. These improved features might be attributed to the structural firmness of the lipase in the dehydrated form and its stability towards covalent reactions that otherwise cause irreversible thermal denaturation in the aqueous phase [63]. Significantly increased enzyme stability and high enzyme immobilization yield have been reported for mesoporous silica-assisted α-chymotrypsin-CLEAs [64]. Nevertheless, the smaller pore size of mesoporous silica limited the highest immobilization of CLEAs. In order to increase the immobilization efficiency, Wang et al. [52] developed a simple strategy to synthesize CLEAs based on a single step cross-linking into the pores of macroporous silica material. More recently, a new enzyme immobilization strategy was utilized to develop mesoporous enzymes-silica composite microparticles. The method involves co-entrapment of gelatinized starch and phenylalanine ammonia lyase (PAL) cross-linked aggregates comprising gelatinized starch into biomimetic silica (Fig. 7) [65]. The engineered mesoporous CLEAs-silica composite micro-particles possessed higher catalytic potential and stability characteristics than conventional CLEAs, free PAL, and biomimetic silica entrapped PAL. Furthermore, superior catalytic performances, storage stability and excellent reusability of the mesoporous CLEAs-silica composite microparticles were attributed suitable size, unique structure, and improved mechanical properties. The results demonstrated this approach as a highly advantageous for the development of a great variety of mesoporous biocomposites with noteworthy catalytic potentialities [65].
Schematic sketch for the synthesis of mesoporous CLEAs-silica composite microparticles. a CLEAs, b PAL-Si, and c P-CLEAs-Si. Reprinted from Cui et al. [65], an open-access article licensed under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)
3.6 New Concepts in CLEAs Immobilization
3.6.1 Combined CLEAs (Combi-CLEAs)
Combined cross-linked enzyme aggregates (combi-CLEAs) approach is regarded as a fascinating prospect for the insolubilization of a combination of different enzymes (Fig. 8) [66]. Fine chemical productions entail numerous steps such as the separation of reaction intermediates and metabolites, whereas combi-CLEAs were developed to combine multi-steps into one-pot catalytic cascade system [67]. Talekar et al. [68] prepared combi-CLEAs of three enzymes including α-amylase, glucoamylase, and pullulanase for the starch breakdown in the one-pot cascade process. The combi-CLEAs results in 100% starch conversion efficiency in one-pot starch hydrolysis as compared to the free enzyme and separate CLEAs mixture that gave 40% and 60% hydrolysis conversions, respectively. Co-immobilized enzymes like combi-CLEAs were stable against elevated temperature and maintained 100% original activity after five repeated cycles. Combi-CLEAs of xylanase, cellulase, and β-1,3-glucanase were attempted for one-pot saccharification of sugarcane bagasse (SCB). Along with enhanced temperature and storing properties, the combi-CLEAs were able to retain 90% activity after six consecutive cycles. In contrast to free enzymes (73%), Combi-CLEAs catalyzed almost 84% hydrolysis of ammonia-treated SCB in a reaction time of 48 h [69]. Ketoreductase and d-glucose dehydrogenase derived combi-CLEAs exhibited greater activity, long-term functioning stability, and recyclability and potential ability to catalyze the highly selective reduction of the prochiral keto ester [70]. More recently, Su et al. [71] synthesized magnetic combi-CLEAs d-glucose dehydrogenase and containing ketoreductase. Though magnetic combi-CLEAs displayed identical pH and temperature optima, and thermal stability, catalytic performance and working stability of magnetic combi-CLEAs were significantly improved in both aqueous and biphasic media than that of original CLEAs. Moreover, the magnetic CLEAs were used for multiple times due to easy recovery with a magnetic field. Recently, combi-CLEAs have shown potential applications in pharmaceutical industry. For example, Jung et al. [72] reported the successful cascade biosynthesis of trehalose from sucrose using combi-CLEAs consisting of trehalose hydrolase, sucrase, and trehalose synthase. Five-enzyme integrated CLEAs of adenylate kinase, phosphoribosyl pyrophosphate synthetase, pyruvate kinase, ribokinase, and hypoxanthine phosphoribosyltransferase has also been used in the one-pot manufacturing of nucleotide analogs [73].
A schematic illustration of cross-linked enzyme aggregates (CLEAs) and COMBI-CLEAs development in the presence of cross-linker and Fe2O3 particle, respectively. Reprinted from Bilal et al. [66], with permission from Elsevier. Copyright (2017) Elsevier B.V
3.6.2 Multipurpose CLEAs (Multi-CLEAs)
After the development of combi-CLEA technology, it was found promising that combined CLEAs can also drive non-cascade biochemical reactions. At this juncture, Dalal et al. [74] referred multi-CLEA as “biocatalyst able to catalyze many distinct biological activities”. The same group synthesized a multi-CLEA of pectinase, xylanase, and cellulase to accomplish three different independent catalytic reactions. The developed multipurpose biocatalyst was highly thermostable, recyclable and exhibited no activity loss after three repeated cycles. Neutrase and papain based multi-CLEAs were found to be pH and thermally more resilient than native enzymes. They preserved greater catalytic activities in hydrophilic and nonpolar solvents with no activity reduction at 4 °C storage for about 6 months [75]. The multi-CLEAs showed superior bean protein and zein hydrolysis activities in contrast with free enzymes indicating a broad range of biotechnological potential as a multifunctional biocatalyst. More recently, Mahmod et al. [76] reported the formation of a novel multi-CLEA biocatalyst exhibiting protease and lipase properties and able to catalyze unrelated reactions in the one-pot application. Notably, more than 34% of the initial activity of the multi-CLEAs was found after five successive batches, and the multi-CLEA produced biodiesel from vegetable oil with a 51.7% transformation efficiency. An efficient and promising biocatalyst containing Palatase and two different lipases CAL B has also been engineered to synthesize biodiesel from oil [77], whereas the triglycerides level in serum was determined by co-immobilizing glycerol-3-phosphate oxidase, glycerol kinase, peroxidase, and lipase [78]. In conclusion, all the results mentioned above demonstrated the potential feasibility of the multi-CLEA as a versatile and promising biocatalyst for myriads of industrial and biotechnological perspectives.
3.7 Structure-Guided Biocatalyst Engineering
Most of the protein structures are predicted or solved through modeling, and rational design using protein libraries. Moreover, the directed evolution screening can be substituted with more informed, structure-directed combinatorial designs envisioned to be functionally enriched and minimizing the total number of variants to be screened and created [11]. With the aid of structure-guided engineering, oligonucleotides are designed to introduce modifications at sites intended to possess significant impact on substrate recognition or catalytic activity. The oligonucleotides are often designed such that the target mutation regions encode all or a subset of possible amino acids [79]. In an earlier study, cytochrome P450-cam was rationally re-design directed by the crystal structure of the enzyme to fine-tune site-selectivity in the oxidative hydroxylation of (+)-a-pinene [80]. WT P450-cam results in a combination of oxidation products including (+)-cis-verbenol, (+)-myrtenol, (+)-a-pinene epoxide, (+)-verbenone and unidentified compounds. Different point mutations including F87 W, F87A, V247L, and Y96F were made, and their combinations were deliberated. Results showed that F87W/Y96F/L247A variant demonstrated superior biocatalytic performance to form (+)-cis-verbenol with high regioselectivity of 86%.
3.7.1 Engineering Cofactor Preference and Substrate Specificity
Modifying an enzyme’s cofactor and substrate recognition and specificity is a sophisticated approach to expand the enzyme applicability in industrial scale bioprocess for producing chiral compounds of pharmaceutical interest or biofuels. It may also be important to accomplishing oxidation–reduction equilibrium in recombinant metabolic pathways [81]. Currently, Ehsani group compared the sequence of NAD(H)-dependent 2,3-butanediol dehydrogenase (Bdh1) with NADP(H)-dependent yeast alcohol dehydrogenase (Adh6) and found that three contiguous substitutions of amino acid (I222R/A223S/E221S) produced a Bdh1 mutant with a comparable activity to wild-type but complete switching in cofactors specificity for NADPH [82]. On the contrary, Hasegawa et al. [83] obtained an NADH-utilizing specific variant of ketol-acid reductoisomerase from Corynebacterium glutamicum to increase redox balance and improve l-valine biosynthesis under oxygen-deficient environment. The resulting triple substituted novel variant exhibited no accelerated activity using NADH, and catalytic efficiency was rather more than 700-fold diminished using NADPH. Nevertheless, the resulting variant led to redox balancing accompanied by significantly improved l-valine titer by engineered strain.
l-Homoalanine is an important chiral precursor to manufacture a variety of commodity pharmaceutical compounds. Zhang et al. [84] obtained a glutamate dehydrogenase (GDH) variant able to biosynthesize a non-natural amino acid (l-homoalanine) following the reductive amination of 2-ketobutyrate. Due to the similar structure of 2-ketobutyrate and 2-ketoisovalerate, authors speculated that a GDH variant capable of 2-ketoisovalerate amination to l-valine might also generate l-homoalanine from 2-ketobutyrate. Guided by the GDH crystal structure from Clostridium symbiosum and sequence comparison with E. coli enzyme, simultaneous saturation mutagenesis was targeted at four amino acid positions in the E. coli GDH binding pocket. The resulting GDH variant showed pronounced activity on 2-ketobutyrate yielding 5.4 g L−1-homoalanine when cultivated on glucose. Iterative saturation mutagenesis (ISM) has emerged as a noteworthy approach for boosting the catalytic activity of the enzyme at low or medium throughput screening systems [85]. In this technique, amino acid regions surrounding the substrate-binding pocket are distributed into numerous groups consisting of one to three residues each and subsequently subjected to a combinatorial active-site saturation mutagenesis test (CAST). In CAST, all plausible mutants within a group are screened, and better variants were sued as prototypes for succeeding saturation mutagenesis rounds. Unlike other less efficient methods, ISM is intended to diminish the screening effort to identify unique variations in a designated territory of the protein sequence.
3.8 Improving Multi-enzyme Catalysis by Protein Engineering
Natural biosynthetic pathways are often designed to couple and equilibrate sequential reactions by increasing substrate or local enzyme concentrations, or by preventive diffusion of toxic or unstable reaction metabolites [86]. For examples, in eukaryotes, the subunits of fatty acid synthase enzyme are systematically organized and arranged in a head-to-tail fashion that renders it convenient to immediately deliver each intermediate formed from one subunit to the next subunit [87]. Similarly, a large hydrophobic tunnel helps tryptophan synthase complex to sequester the intermediate indole and deliver it between active sites [88]. Motivated by these natural systems, researchers have developed the state-of-the-art synthetic strategies to ameliorate the efficacy of the multi-enzyme catalytic system. Figure 9 illustrates a mechanistic approach to improve multi-enzyme catalysis by protein engineering via gene manipulation.
3.8.1 Physical Fusion and Synthetic Scaffolds
Physical fusion is the simplest technique to get enzymes in the constant close vicinity. In order to enhance patchoulol biosynthesis in Saccharomyces cerevisiae. Albertsen et al. [89] designed fusion protein variants of patchoulol synthase (PTS) and farnesyl diphosphate synthase (FPPS). Both PTS-FPPS and FPPS-PTS fusions were found to be functional in vivo, and among different linkers of varying compositions and lengths, the short flexible linker Gly-Ser-Gly was utmost promising for the biosynthesis of patchoulol. As compared to a strain expressing free FPPS and PTS, a 9.5 mg L−1 patchoulol titer (1.6-fold higher) was achieved by yeast strain expressing the FPPS-GSG-PTS. In another study, Asadollahi et al. [90] recorded 2.8-fold improvement in patchoulol titer by repressing the squalene synthase through switching native promoter with regulatable MET3, indicating the potential applicability of the enzyme fusion approach together with metabolic engineering techniques.
Linear physical fusions through linkers represent the limitation to only a small number of proteins, as multi-enzyme fusions result in diminished catalytic activity or misfolding. The use of synthetic protein scaffolds overcomes this apprehension by adding flexibility to varying enzyme ratios for stoichiometric control. Dueber et al. [91] developed a novel protein scaffold comprising varying combinations of protein–protein interaction domains such as GBD, PDZ, and SH3. Metabolic pathway enzymes labeled with peptide ligands are explicitly recognized by one of the domains in the synthetic scaffold that led to increased local concentration and posttranslational assemblage of each component in the pathway. The enzymatic stoichiometry was optimized to enhance pathway flux through this modular scaffold. Identically, resveratrol production was fivefold improved in S. cerevisiae than non-scaffold-free system [92]. In another study, Lee et al. [93] exploited a DNA scaffolding system to integrate three enzymes converting aspartate semialdehyde to l-threonine in 50% shortened culture duration to synthesize the same product concentration than non-scaffold-control. Moreover, the concentration of inhibitory intermediate, i.e. homoserine was also considerably diminished by using the DNA-scaffolding system.
3.9 Advanced Computational Design for Protein Engineering
Computational-based design and modeling increasingly contribute to improving catalytic features of enzymes, such as enzyme activity, substrate preference, and stability for expanding their applications. Before experimental construction and characterization of a subset of variants, molecular dynamics (MD) simulations and powerful energy calculations assist in identifying potential regions for mutations and in silico estimation of resulting mutant properties. Gordon and coworker [94] redesigned the substrate specificity of an acidic endopeptidase kumamolisin-As using Rosetta Software Suite. The computational modeling results in 261 variants with one to seven amino acid replacements. Experimentally characterization revealed that 50% of the variants exhibited improved proteolytic activity toward the new substrate PQLP. Among the variants, the best one containing seven substitutions displayed 116-folds elevated activity and 877-folds shift in substrate preference towards the new substrate in contrast with native enzyme. By computational-guided protein engineering approach together with cofactor binding energy calculations, Khoury et al. [95] obtained utilize NADH utilizing 10 variants of xylose reductase as a substitute of the native cofactor NADPH. Experimental testing evidenced more specific behavior of some variants for NADH, while rest of the variants exhibited dual substrate preference. Along with activity enhancement, the enzyme stability can also be improved by harnessing the power of the computational design and methods.
Joo et al. [96] selected flexible residues on the protein surface, and residue flexibility was characterized using FRODA (Framework Rigidity Optimized Dynamics Algorithm) in order to ameliorate the thermal resistance profile of the xylanase Bcx from Bacillus circulans. Among the eight substitutions predicted by RosettaDesign algorithm [97], three substitutions were experimentally corroborated to evaluate thermal stability. D52Y and the quadruple substitution variant identified using the Standard Dynamics Cascade of Discovery Studio package presented fivefold and 30-fold enhancements in half-life at an elevated temperature of 50 °C over the pristine enzyme, respectively, along with moderately improved catalytic performance.
3.10 Cell-Free Biocatalytic Cascade Processes
The multi-step synthesis of one-pot cell-free catalytic cascades eliminates the requisite for laborious isolation and purification of product intermediates. Such Cascading-based syntheses represent the several advantages of minimum solvent and reaction volume, fewer unit operations, diminished cycle times, higher volumetric yields and less waste generation, which deciphers to considerable environmental and economic benefits. Nevertheless, catalysts incompatibility with each other, differential optimum conditions and complicated catalyst recovery and recycling are the associated problems that need to be overcome. Since nature deciphers the incompatibility issues by cataloging enzymes in different portions of the cell, therefore compartmentalization through immobilization might be the solution in enzymatic cascades. The catalytic processes largely proceeded under the identical conditions, i.e. at ambient temperature and pressure that additionally simplifying their incorporation in cascade processes. In recent years, biocatalytic cascade processes have become a focus of considerable research attention largely owing to their economic and environmental benefits and to build up molecular complexity from cheap starting feedstocks in one-pot, while driving reaction balance toward the target products [98, 99]. The rates of consecutive catalytic cascades can be markedly amplified by simulating the proximity of the enzymes in microbial cells by combi-CLEA-based co-immobilization of the enzymes. The tri-enzyme CLEA consisting of amidase, nitrilase, and (S)-hydroxynitrile lyase is a good illustration of a combi-CLEA with significantly higher rates than observed mixtures of the individual CLEAs [100].
4 Concluding Remarks and Future Prospects
The impact of biocatalysis continues to expand rapidly with the enormous advances in biotechnology and molecular engineering approaches. In recent years, biocatalysis has developed as a distinguished technology for the sustainable manufacturing of pharmaceutical compounds, commodity chemicals, food and beverage processing applications and many more. Dramatically accelerating the speed of protein engineering could fully take advantages of low cost and more sustainable biocatalytic processes. In the near future, biocatalysis application is anticipated to further grow in myriads of biotechnological sectors due to the paradigm shift from a non-renewable fossil fuels-based economy to a sustainable bio-economy. Biocatalytic cascade processes and the capability to catalyze in non-polar solvents with carrier-immobilized enzymes get us another step closer to the eventual goal. Nevertheless, there is a pressing need to standardize industrial transformation bioprocesses from both an economic and ecological perspective, and state-of-the-art protein engineering strategies will play a futuristic role in this development.
References
Bilal M, Cui J, Iqbal HMN (2019) Int J Biol Macromol 130:186–196
Sheldon RA (2016) Chem Eur J 22(37):12984–12999
Bilal M, Asgher M, Iqbal HMN et al (2016) Catal Lett 146(11):2221–2228
Bilal M, Rasheed T, Zhao Y et al (2018) Int J Biol Macromol 119:278–290
Schmid A, Dordick JS, Hauer B et al (2001) Nature 409(6817):258
Bilal M, Zhao Y, Rasheed T, Iqbal HMN (2018) Int J Biol Macromol 120:2530–2544
Martinez CA, Hu S, Dumond Y et al (2008) Org Process Res Dev 12(3):392–398
Choi J-M, Han S-S, Kim H-S (2015) Biotechnol Adv 33:1443–1454
Pollard DJ, Woodley JM (2006) Trends Biotechnol 25:66–73
Woodley JM (2013) Curr Opin Chem Biol 17:310–316
Bornscheuer UT, Huisman GW, Kazlauskas RJ et al (2012) Nature 485:185–194
Arnold FH (2015) Q Rev Biophys 48:404–410
Denard CA, Ren H, Zhao H (2015) Curr Opin Chem Biol 25:55–64
Adamczak M, Krishna SH (2004) Food Technol Biotechnol 42(4):251–264
Asgher M, Noreen S, Bilal M (2017) Int J Biol Macromol 95:54–62
Bilal M, Asgher M, Iqbal M et al (2016) Int J Biol Macromol 89:181–189
Bilal M, Asgher M, Shahid M et al (2016) Int J Biol Macromol 86:728–740
Bilal M, Iqbal HMN, Shuqi G et al (2017) Int J Biol Macromol 108:893–901
Bilal M, Asgher M, Iqbal HMN et al (2017) Int J Biol Macromol 102:582–590
Bilal M, Iqbal HMN, Hu H et al (2017) Sci Total Environ 575:1352–1360
Bilal M, Asgher M, Parra-Saldivar R et al (2017) Sci Total Environ 576:646–659
Bilal M, Rasheed T, Iqbal HMN et al (2017) Int J Biol Macromol 105:328–335
Bilal M, Iqbal HMN, Hu H et al (2017) J Environ Manag 188:137–143
Ali M, Husain Q (2018) Int J Biol Macromol 116:463–471
Ali M, Husain Q, Sultana S et al (2018) Chemosphere 202:198–207
Bilal M, Rasheed T, Iqbal HMN et al (2018) Int J Biol Macromol 113:983–990
Husain Q (2018) J Nanosci Nanotechnol 18(1):486–499
Hasnaoui-Dijoux G, Majerić Elenkov M, Lutje Spelberg JH et al (2008) ChemBioChem 9(7):1048–1051
Ma SK, Gruber J, Davis C et al (2010) Green Chem 12(1):81–86
Henke E, Schuster S, Yang H et al (2000) Biocatalysis. Springer, Vienna, pp 107–112
Ottosson J, Hult K (2001) J Mol Catal B 11(4–6):1025–1028
De Raadt A, Griengl H (2002) Curr Opin Botechnol 13(6):537–542
Madeira Lau R, Van Rantwijk F, Seddon KR et al (2000) Org Lett 2(26):4189–4191
Sheldon RA, van Pelt S (2013) Chem Soc Rev 42(15):6223–6235
Bilal M, Asgher M, Cheng H et al (2019) Crit Rev Biotechnol 39(2):202–219
Ansari SA, Husain Q (2012) Biotechnol Adv 30(3):512–523
Arica MY, Salih B, Celikbicak O et al (2017) Chem Eng Res Design 128:107–119
Antecka K, Zdarta J, Siwińska-Stefańska K et al (2018) Catalysts 8(9):402
Asmat S, Husain Q, Khan MS (2018) New J Chem 42(1):91–102
Bayramoglu G, Karagoz B, Arica MY (2018) J Ind Eng Chem 60:407–417
Khan N, Husain Q (2019) Environ Sci Pollut Res 26(4):3517–3529
Zdarta J, Meyer AS, Jesionowski T et al (2018) Adv Colloid Interface Sci 258:1–20
Zdarta J, Pinelo M, Jesionowski T et al (2018) ChemCatChem 10(22):5164–5173
Bayramoglu G, Salih B, Akbulut A et al (2019) Ecotoxicol Environ Saf 170:453–460
Li H, Moncecchi J, Truppo MD (2015) Org Process Res Dev 19:695–700
Bilal M, Zhao Y, Noreen S et al (2019) Biocatal Biotransform 37(3):159–182
Bayramoglu G, Altintas B, Arica MY (2012) Bioprocess Biosyst Eng 35(8):1355–1365
Yang X, Zheng P, Ni Y et al (2012) J Biotechnol 161:27–33
Cao L (2005) Curr Opin Chem Biol 9(2):217–226
Cao L, van Langen L, Sheldon RA (2013) Chem Soc Rev 42:6223–6235
Bilal M, Iqbal HMN (2019) Coord Chem Rev 388:1–23
Wang MF, Qi W, Yu QX et al (2010) Biochem Eng J 52:168–174
Jung D, Paradiso M, Hartmann M (2009) J Mater Sci 44:6747–6753
Park JM, Kim M, Park HS et al (2013) Int J Biol Macromol 54:37–43
Cui J, Cui L, Ping Zhang S et al (2014) PLoS ONE 9(5):e97221
Cruz-Izquierdo Á, Picó EA, Anton-Helas Z et al (2012) New Biotechnol 29S:S100–S101
Talekar S, Ghodake V, Ghotage T et al (2012) Bioresour Technol 123:542–547
Sekhon SS, Ahn J-Y, Shin W-R et al (2017) J Nanosci Nanotechnol 17:9306–9311
Hwang ET, Lee B, Zhang ML et al (2012) Green Chem 14:1884–1887
Lee J, Kim J, Kim J et al (2005) Small 1:744–753
Kim YH, Lee L, Choi SH et al (2013) J Mol Catal B 89:48–51
Jiang Y, Shi L, Huang Y et al (2014) ACS Appl Mater Interface 6:2622–2628
Pizarro C, Brannes MC, Markovits A et al (2012) J Mol Catal B 78:111–118
Kim MI, Kim J, Lee J et al (2007) Biotechnol Bioeng 96:210–218
Cui J, Jia S, Liang L et al (2015) Sci Rep 5:14203
Bilal M, Iqbal HMN, Guo S et al (2018) Int J Biol Macromol 108:893–901
Sheldon RA, Woodley JM (2018) Chem Rev 118:801–838
Talekar S, Pandharbale A, Ladole M et al (2013) Bioresour Technol 147:269–275
Periyasamy K, Santhalembi L, Mortha G et al (2016) RSC Adv 6:32849–32857
Ning C, Su E, Tian Y et al (2014) J Biotechnol 184:7–10
Su E, Meng Y, Ning C et al (2018) J Biotechnol 271:1–7
Jung D-H, Jung J-H, Seo D-H et al (2013) Bioresour Technol 130:801–804
Scism RA, Bachmann BO (2010) ChemBioChem 11:67–70
Dalal S, Sharma A, Gupta MN (2007) Chem Cent J 1(1):16
Chen Z, Wang Y, Liu W et al (2017) Int J Biol Macromol 95:650–657
Mahmod SS, Yusof F, Jami MS et al (2015) Process Biochem 50(12):2144–2157
Banerjee A, Singh V, Solanki K et al (2013) Sust Chem Process 1(1):14
Pundir CS (2008) Indian J Biochem Biophys 45:111–115
Li Y, Cirino PC (2014) Biotechnol Bioeng 111(7):1273–1287
Bell SG, Yang W, Dale A et al (2013) Appl Microbiol Biotechnol 97(9):3979–3990
Wang Y, San K-Y, Bennett GN (2013) Curr Opin Biotechnol 24(6):994–999
Ehsani M, Fernández MR, Biosca JA et al (2009) Biotechnol Bioeng 104(2):381–389
Hasegawa S, Uematsu K, Natsuma Y et al (2012) Appl Environ Microbiol 78(3):865–875
Zhang K, Li H, Cho KM et al (2010) Proc Natl Acad Sci USA 107(14):6234–6239
Reetz MT (2011) Angew Chem Int Ed 50(1):138–174
Agapakis CM, Boyle PM, Silver PA (2012) Nat Chem Biol 8:527–535
Garrett RH, Grisham CM (1999) Biochemistry. Philadelphia Saunders College Publishing, Philadelphia, p 1127
Hyde C, Ahmed S, Padlan E et al (1988) J Biol Chem 263(33):17857–17871
Albertsen L, Chen Y, Bach LS et al (2011) Appl Environ Microbiol 77(3):1033–1040
Asadollahi MA, Maury J, Møller K et al (2008) Biotechnol Bioeng 99(3):666–677
Dueber JE, Wu GC, Malmirchegini GR et al (2009) Nat Biotechnol 27(8):753–759
Wang Y, Yu O (2012) J Biotechnol 157(1):258–260
Lee JH, Jung S-C, Bui LM et al (2012) Appl Environ Microbiol 79(3):774–782
Gordon SR, Stanley EJ, Wolf S et al (2012) J Am Chem Soc 134(50):20513–20520
Khoury GA, Fazelinia H, Chin JW et al (2009) Protein Sci 18(10):2125–2138
Joo JC, Pohkrel S, Pack SP et al (2010) J Biotechnol 146(1–2):31–39
Liu Y, Kuhlman B (2006) Nucleic Acids Res 34:W235–W238
Xue R, Woodley JM (2012) Bioresour Technol 115:183–195
Sigrist R, da Costa BZ, Marsaioli AJ et al (2015) Biotechnol Adv 33:394–411
Chmura A, Rustler S, Paravidino M et al (2013) Tetrahedr Asymmet 24(19):1225–1232
Acknowledgements
The literature facilities provided by the representative institutes/universities are thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest in any capacity.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bilal, M., Iqbal, H.M.N. Tailoring Multipurpose Biocatalysts via Protein Engineering Approaches: A Review. Catal Lett 149, 2204–2217 (2019). https://doi.org/10.1007/s10562-019-02821-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02821-8