Abstract
Esters of cinnamyl alcohol find many applications in food, cosmetic and pharmaceutical industries as flavor and fragrance compounds. The current work focuses on the synthesis of cinnamyl acetate from cinnamyl alcohol and vinyl acetate, including screening optimization of reaction conditions such as organic solvents, temperature, catalyst loading and mole ratio. Conversion (93%) was achieved after 4 h when transesterification was carried out at vinyl acetate/cinnamyl alcohol 2:1, 4.0 g L−1 of lipase loading, and at 40 °C in hexane as solvent. Also the catalytic behaviors of lipase LipBA for synthesis different carbon chain lengths of cinnamyl esters were determined. Among the different acyl donors employed, vinyl propionate was found to be the best acyl donor which presents a 96% conversion. Enzymatic synthesis of cinnamyl esters is an efficient process vis-à-vis chemical catalysis.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Flavor esters have various applications in food, perfume, flavors, and pharmaceuticals because of their aromatic qualities. Traditionally, esters can be obtained through extraction from natural sources or by chemical synthesis [1]. In recent years, application of enzymes to the synthesis of esters has aroused widespread interest because of its high efficiency, moderate reaction condition, and environmental friendliness [2]. The selection of enzymes, acyl donors, and solvents is the critical factor to an enzyme catalyzed reaction. Lipase (triacylglycerol ester hydrolase, EC 3.1.1.3) is specific toward the ester bond and hence has been widely used in ester synthesis. Many reports of synthesis of esters with various lipases have been published [3].
The ester can be synthesized by lipase through direct esterification and transesterification with different acyl donors. Compared with the esterification reaction, transesterification is preferred because little water is used in the reaction and the lipase is usually stable under this condition [1]. Vinyl esters are the most popular acyl donors in transesterification reactions because the produced vinyl alcohol can tautomerize to acetaldehyde, which can promote the synthesis process. Mahapatra et al. successfully synthesized n-butyl acetate and n-propyl acetate by transesterification using vinyl acetate as both solvent and acyl donor [4]. The maximum conversion of 50% for n-butyl acetate and 56% for n-propyl acetate was achieved after 24 h of reaction at 30 °C. However, the deactivation of acetaldehyde on the lipase has also been proved. Most of these lipase-catalyzed reactions are carried out in organic solvents such as n-hexane, heptane, or toluene [5–7].
Synthesis of cinnamyl acetate through a chemical method has already been reported, but it was often performed under an undesirable high temperature and low substrate concentration. So far, there are few reports about cinnamyl acetate synthesis via the enzyme catalysis method. Previously, the lipase LipBA from Bacillus amyloliquefaciens was firstly reported by our research team [8]. Lipases from Bacillus sp. were considered to be the lidless and small (actually the smallest lipases known). These lipases seem to be well-suited for biotechnological applications, the synthesis of chiral drugs in particular. In this work, cinnamyl acetate was synthesized by lipase (LipBA) under the optimal reaction conditions (vinyl acetate/cinnamyl alcohol 2:1, 4.0 g L−1 of lipase loading, and at 40 °C in hexane as solvent). Among the different acyl donors employed, vinyl propionate was chosen as the best acyl donor with the highest conversion of 96%.
2 Materials and Methods
2.1 Materials
The lipase LipBA was obtained from B. amyloliquefaciens Nsic8 previously reported in our research team, and the enzyme activity was 1750 U mg−1 [8]. Cinnamyl alcohol and various vinyl esters were purchased from Sigma. Toluene, n-hexane and other reagents were of analytical grade and obtained from common commercial sources. All other chemicals referred in this paper were of analytical grade.
2.2 Enzymatic Synthesis of Cinnamyl Acetate
The cells containing lipase LipBA were centrifuged, washed once with 100 mM Tris–HCl (pH 8.0), and then lyophilized by vacuum freezing. The transesterification reaction was carried out in a 2 mL eppendorf tube and initiated by adding the lipase LipBA. The effect of reaction temperature was studied at 20, 25 30, 35, 40 and 45 °C, respectively. The reactor was shaken at 200 rpm and immersed in a thermostatic water bath to keep the system within ±2 °C of the desired temperature. To investigate the effects of reaction conditions on the conversion of cinnamyl alcohol, the enzyme loading was studied in the range from 0.5 to 4.0 g L−1, and then different mole ratios between vinyl acetate and cinnamyl alcohol (1:1, 2:1, and 3:1) was prepared as the reactant. The optimal reaction conditions were obtained through investigating each factor when other factors were kept constant. A parallel reaction under the same conditions without the addition of the enzyme was used as a control. All experiments were performed three times. The conversion rate (%) for ester synthesis was calculated from the conversion of alcohol to ester after a given time.
2.3 Cinnamyl Esters Synthesis With Different Acyl Donors
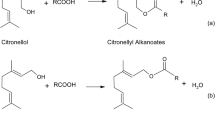
To study the catalytic behavior of lipase LipBA with different acyl donor, cinnamyl esters were synthesized through the transesterification of cinnamyl alcohol with various acyl donors (vinyl acetate, vinyl propionate, vinyl butyrate, vinyl pivalate, vinyl neononanoate, vinyl decanoate and vinyl laurate). Recent experimental studies in cinnamyl esters synthesis by enzymatic catalysis was shown in Table 1. The preparation of cinnamyl esters through transesterification was shown in Fig. 1a.
2.4 Analysis Method
At appropriate intervals during the reaction, samples were withdrawn from the reaction mixtures. The reactants were analyzed by thin-layer chromatography (TLC) and high performance liquid chromatography (HPLC) respectively. The prepared samples were spotted onto the silica gel TLC plate, and then the plate was placed in a chamber containing a solvent system of n-hexane and Ethyl acetate (18:2). After the plate was dried, the products bands were dyed by solid iodine (Fig. 1b). For quantitative analysis, the reactants were analyzed by HPLC with an OB-H column and a mobile phase/ n-hexane: isopropyl alcohol about 90:10 at 254 nm (Fig. 1c). The flow rate was kept at 1.0 mL min−1. The concentrations of reactants and products were obtained according to standard curves.
3 Results and Discussion
3.1 Effect of Different Solvents
Choice of solvents is very important for a successful industrial process. Solvent affects the catalytic power of enzyme by changing the three dimensional conformation of protein, and therefore significantly alters conversion [12]. Therefore, the effect of different organic solvents with varying log P values, such as acetonitrile (log P = −0.34), acetone (log P = −0.24), chloroform (log P = 1.97), toluene (log P = 2.69), hexane (log P = 3.2), heptane (log P = 4.0), isooctane (log P = 4.5) were studied under similar conditions. As shown in Fig. 2, LipBA works better in non-polar solvents than in polar solvents during cinnamyl acetate synthesis in this study. This is consistent with the stated characteristics of this commercial lipase, i.e. Novozym 435 prefers inert solvents such as petroleum ether or hexane [13]. The conversion rates of more than 80% were obtained in the solvents with the log P value ranging from 3.2 to 4.5. Maximum conversion of 93% was obtained by employing hexane as solvent which has high log P value among those studied. Heptane and isooctane led to a conversion of 91 and 89%, respectively. It has been reported that biocatalysts are more stable in non-polar solvents than polar solvents or in other words, solvents which have high log P value showed good compatibility with enzymes molecules that leads to improved activity [14]. The conversion rates of cinnamyl alcohol were all <30% in acetonitrile, acetone and chloroform. It clearly indicated that enzymes in hydrophobic solvent with high log P value show good activity compared to organic solvents with low log P value. Therefore, further experiments were carried out by using hexane as the solvent.
3.2 Effect of Reaction Temperature
In lipase-catalyzed reactions, temperature significantly influences both the conversion rate of the reaction and stability of the enzyme. In most cases, reaction conversion rate increases with temperature, while the stability of enzymes declines. The results in Fig. 3 showed that there is a sharp increase in the initial rate from 20 to 30 °C and a smooth increase from 30 to 45 °C. Increasing temperature may reduce mixture viscosity, enhance mutual solubility, improve diffusion process of substrates, and enhance the interactions between catalytic particles and substrates. In the case of biocatalytic process, high temperature may disrupt the active conformation of enzyme which leads to loss of activity and selectivity [15]. So it is important to find the desired temperature for optimum enzyme activity. The effect of temperature was studied in the range of 20–45 °C by maintaining other parameters constant (Fig. 3). It was observed that the cinnamyl alcohol conversion increased with temperature raise up to 40 °C. Above this temperature, there was relatively smaller decrease in conversion of reaction; it also showed that the enzyme remained active at 45 °C. Small changes of the conversion rates of cinnamyl alcohol ranging from 89 to 93% were observed at a temperature range from 30 to 55 °C, where the enzyme had a higher activity and stability. At lower temperature, the conversion rate increased from 53% (20 °C) to 82% (30 °C). The result of the present study is in confirmation with the earlier reports where the optimum temperatures for lipase action lie between 30 and 62 °C [16]. Yong and Al-Duri suggested that almost all enzymes suffer from thermal denaturation at temperatures above 45 °C [17]. Therefore, 40 °C was chosen as the reaction temperature to carry out further reactions.
3.3 Effect of Enzyme Concentration
The amount of enzyme is a crucial economic factor for any bioconversion processes. The increase of enzyme loading will accelerate the reaction rate and enhance the conversion [18]. To select the appropriate amount of enzyme, a number of experiments were performed in the range of 0.5–4.0 g L−1 enzyme loading under similar conditions [1]. Initial rates of transesterification as a function of enzyme concentration have been represented in Fig. 4. There was a general tendency for the initial rates under different enzyme concentrations, to rise as the enzyme concentration increased up to 4.0 g L−1. Not the initial rates, the conversion rates increased with enzyme loading increasing up to 4.0 g L−1; beyond which there was a marginal increase in conversion which may be due to the lack of substrate to access the active site of enzyme, and/or difficulty in maintaining uniform suspension of the biocatalysts at higher enzyme concentration [7, 19]. From Fig. 4, it was observed that 93% molar conversion for cinnamyl acetate synthesis could be achieved after 4 h of reaction at an enzyme concentration of 4.0 g L−1.
Effect of the enzyme loading on the conversion of cinnamyl alcohol. Reaction conditions: enzyme loading filled square 0.5 g L−1, filled circle 1.0 g L−1, filled triangle 2.0 g L−1, filled inverted triangle 4.0 g L−1; cinnamyl alcohol 0.1 M L−1 in hexane; vinyl acetate/cinnamyl alcohol = 2:1; T = 40 °C; 4 h and 200 rpm
3.4 Effect of Mole Ratio
Enzymatic reactions, especially transesterification, were often challenged by high substrate concentration due to the inactivation or inhibitory effects of acyl donor or alcohol. The influence of the substrate concentration and mole ratio was not part of the initial study in the batch system, but might have a strong impact on the reaction course [20]. The effect of vinyl acetate/cinnamyl alcohol molar ratio (R) on cinnamyl acetate synthesis was studied by increasing the R value from 1.0 to 3.0 at a fixed cinnamyl alcohol concentration of 0.1 M L−1 [18]. Vinyl acetate is relatively cheap, but increasing the vinyl acetate/cinnamyl alcohol ratio cannot help much to enhance the conversion. The result in Fig. 5 showed that the conversion rate increased with the R value increasing, and reaching a maximum value of 93% (R = 2.0). The cinnamyl alcohol conversion rate was the lowest of 82% (R = 1.0). This may be due to the inhibition of the enzyme caused by the excess cinnamyl alcohol [18]. To maximize the utilization of the substrate, the further enzymatic catalyzed transesterification experiments were carried out with an R value of 2.0.
3.5 Transesterification of Cinnamyl Alcohol With Different Acyl Donors
The transesterification of cinnamyl alcohol with different acyl donors (vinyl acetate, vinyl propionate, vinyl butyrate, vinyl pivalate, vinyl neononanoate, vinyl decanoate and vinyl laurate) were investigated. As indicated in Table 2, cinnamyl esters of different acyl donors could be efficiently prepared in this system. The specific activities towards different acyl donors were significantly different. The highest conversion 96% was obtained when using vinyl propionate as acyl donor. It is reported in the literature that the immobilized lipase Pseudomonas cepacia (PCL) provided 67% conversion when conventional technique (absence of sonication) was used for reaction [11]. Under the optimized conditions, the cinnamyl acetate yield would reach 93%. Using the lipase from procine pancreas, Wu et al. achieved 62.56% of conversion under the optimal reaction conditions for the synthesis of cinnamyl acetate [10]. Comparison of the specific activities to short chain and medium acyl donors, LipBA revealed higher conversion rates of short chain than medium. The LipBA displayed similar catalytic activities towards the vinyl acetate, vinyl propionate and vinyl butyrate, ranging from a conversion of 93–96%.
4 Conclusion
In this study, the effects of various parameters on the conversion and rate of lipase catalyzed transesterification of cinnamyl alcohol with vinyl esters in non-aqueous medium were studied. Among the different acyl donors, Vinyl propionate was found to be the most suitable in n-hexane as solvent. Overall conversion of 96% was achieved in 4 h with a mole ratio of vinyl acetate to cinnamyl alcohol of 2:1 using 4.0 g L−1 of catalyst at 40 °C. Compared to traditional methods, this enzymatic process is more greener.
References
Geng B, Wang M, Qi W, Su R, He Z (2012) Biotechnol Appl Biochem 59:270–275
Devulapelli VG, Weng H-S (2009) Catal Commun 10:1638–1642
Gao W, Wu K, Chen L, Fan H, Zhao Z, Gao B, Wang H, Wei D (2016) Microb Cell Fact 15:41–53
Mahapatra P, Kumari A, Kumar Garlapati V, Banerjee R, Nag A (2009) J Mol Catal B 60:57–63
Hou M, Wang R, Wu X, Zhang Y, Ge J, Liu Z (2015) Catal Lett 145:1825–1829
Rosset IG, Cavalheiro MCHT, Assaf EM, Porto ALM (2013) Catal Lett 143:863–872
Yadav GD, Dhoot SB (2009) J Mol Catal B 57:34–39
Cai X, Ma J, Wei DZ, Lin JP, Wei W (2014) Antonie Van Leeuwenhoek 106:1049–1060
Yadav GD, Devendran S (2012) Process Biochem 47:496–502
Wu Z, Qi W, Wang M, Su R, He Z (2014) J Mol Catal B 110:32–38
Badgujar KC, Pai PA, Bhanage BM (2016) Bioprocess Biosyst Eng 39:211–221
Iyer PV, Ananthanarayan L (2008) Process Biochem 43:1019–1032
Wang R, Hou M, Zhang Y, Ge J, Liu Z (2015) Catal Lett 145:995–999
Guillaume B, Shareck Fo, Hurtubise Y, Le´pine Fo, Doucet N (2014) PLoS ONE 9:1–10
Huang J, Liu Y, Jin Q, Wu X, Wang X, Song Z (2012) J Am Oil Chem Soc 89:1627–1632
Malcata FX, Reyes HR, Garcia HS, Hill CG, Amundson CH (1992) Enzyme Microb Technol 14:426–446
Yong YP, Al-Duri B (1996) J Chem Technol Biot 65:239–248
Yan H-D, Zhang Q, Wang Z (2014) Catal Commun 45:59–62
Wolfson A, Atyya A, Dlugy C, Tavor D (2009) Bioprocess Biosyst Eng 33:363–366
Damnjanović JJ, Žuža MG, Savanović JK, Bezbradica DI, Mijin DŽ, Bošković-Vragolović N, Knežević-Jugović ZD (2012) J Mol Catal B 75:50–59
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. C31570795), the National High Technology Research and Development Program of China (No. 2013AA102109), the Shanghai International Science and Technology Cooperation Project (No. 14520720500), the Minhang District Leading Talent Project (No. 201541), the Shanghai Talent Development Project (No. 201531).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cai, X., Wang, W., Lin, L. et al. Cinnamyl Esters Synthesis By Lipase-Catalyzed Transesterification in a Non-Aqueous System. Catal Lett 147, 946–952 (2017). https://doi.org/10.1007/s10562-017-1994-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-1994-8