Abstract
A series of Ni–Cu–Mo/Al2O3 catalysts with different metal contents were prepared by sequential impregnation method. The performance of the catalysts for carbon dioxide reforming of methane was investigated in a quartz tubular reactor at CH4/CO2 ratio of 4/6, feed gas flux of 100 mL/min, catalysts 460 mg, temperature range of 600–900 °C and atmospheric pressure. The catalysts were characterized by XRD, XPS, BET, CO2-TPD, TG, EDS and SEM. The performance and characterization results showed that the addition of Cu and Mo was beneficial for the reaction. The formation of spinel (NiMoO4, NiAl2O4, Cu6Mo4O15) and alloy (Ni3Mo, Cu3.8Ni) structures could significantly improve the catalytic activity and prevent the generation of carbon deposition. XRD patterns of the catalyst with the mass ratio of Ni:Mo = 0.75 after reaction suggested Mo2C formation which could eliminate the coke and extend the stability. The catalyst with the mass ratio of Ni:Mo = 0.75 showed better activity and still remained highly active under the condition of 800 °C for 60 h. Therefore, the highly effective Ni–Cu–Mo/Al2O3 catalyst could be a potential catalyst for carbon dioxide reforming of methane.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Carbon dioxide reforming of methane has great potential and attracts many researchers’ attention during recent years. It is of great significance because of the greenhouse effect, as well as energy and resource challenges. Nickel based catalysts have been extensively used in carbon dioxide reforming of methane due to relatively high activity and low cost, but sintering and carbon deposition over them prevent their application [1]. Improvement of nickel based catalysts is favorable.
To improve the performance of nickel based catalysts, various metallic active components were added into nickel based catalysts. Xinfa Dong et al. [2] investigated the effect of transition metal (Cu, Co and Fe) on the modification of nickel based catalysts for the autothermal reforming of methane. The results showed that the addition of transition metal Cu improved the dispersion of NiO, inhibited the formation of NiAl2O4, and thus significantly increased the activity of catalyst. Jae-Sung Choi et al. [3] studied CO2 reforming of methane over modified Ni/Al2O3 catalysts. Catalysts modified with Cu showed slightly improved activity.
Moreover, investigations before had shown that Mo, as well as the oxides and carbides of Mo were excellent promoters to improve the performance of nickel based catalysts. The addition of Mo into nickel catalysts could effectively improve the metal-support interaction and lead to a decrease in carbon deposition [4–6]. The addition of MoO3 enhanced the electron density of Ni and improved the stability of the catalysts [7]. Chuan Shi et al. [8] studied Ni-modified Mo2C catalysts for methane dry reforming. Ni-Mo2C was a typical bi-functional catalyst for methane dry reforming, and a catalytic redox cycle could be established by regulating the mass ratio of Ni and Mo2C. The formation of the alloy between the active ingredients could basically eliminate carbon deposition on the catalyst and improve the catalytic performance of catalysts [9]. A. Djaidja [10] studied the Ni–M–Mg/Al (M = Fe or Cu) catalysts for CH4–CO2 reforming, the formation of Ni–Fe or Ni–Cu alloys led to a very good dispersion of the metal dispersion of the metal particles which explained the good catalytic stability observed in the CH4−CO2 reforming.
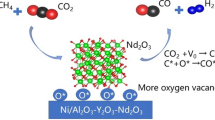
In the present work, Ni–Cu–Mo/Al2O3 catalyst was proposed for carbon dioxide reforming of methane. Comparing the properties and catalytic performance of Ni/Al2O3, Ni–Cu/Al2O3, Ni–Mo/Al2O3, Ni–Cu–Mo/Al2O3 catalysts, Ni–Cu–Mo/Al2O3 catalyst showed higher activity for the carbon dioxide reforming of methane reaction, affording high selectivity of CO and H2.
2 Experimental
2.1 Catalyst Preparation
The 12 wt% Ni/Al2O3, Ni–Cu/Al2O3, Ni–Mo/Al2O3 and Ni–Cu–Mo/Al2O3 (Cu proportional content: 10 wt%) catalysts were prepared by impregnation method as shown in Fig. 1. γ-Al2O3 was first impregnated with aqueous solution of (NH4)6Mo7O24·H2O, and dried at 110 °C for 5 h, followed by calcination in a muffle furnace at 500 °C for 4 h to form MoO3/Al2O3 compound. Trimetallic catalysts Ni–Cu–Mo/Al2O3 were prepared by impregnation of MoO3/Al2O3 with mixtures of aqueous solutions of Ni(NO3)2·6H2O and Cu(NO3)2·3H2O in corresponding amount. Then, the catalysts were dried at 110 °C for 5 h, followed by calcination in a muffle furnace at 500 °C for 4 h and reduction at 700 °C for 2 h.
2.2 Catalytic Performance Tests
The catalytic performance tests were carried out in a quartz fixed-bed reactor at 600–900 °C. The feed mixture of 100 mL·min−1 with CH4:CO2 mol ratio of 4:6 was introduced into the reactor, and 460 mg of catalyst was charged in the reactor. The product composition was analyzed by an on-line gas chromatography (GC-1100) equipped with a TDX-01column (2 m × 3 mm) and with TCD detector (Fig. 2).
The conversions (X) of CH4 and CO2, the selectivity (S) of CO and H2 were shown in form (1)–(4).
2.3 Catalyst Stability Test
The reforming reaction was carried out at 800 °C for 80 h in order to study the long term stability of the catalyst with Ni:Mo mass ratio of 0.75. The feed mixture of 100 mL min−1 with CH4:CO2 mol ratio of 4:6 was introduced into the reactor, and 460 mg of catalyst was charged in the reactor.
2.4 Catalyst Characterization
X-ray diffraction (XRD) of the catalysts was recorded with an X-ray diffractometer (RINT2000, Japan) using monochromatic Cu Kα radition at 40 kV and 150 mA. The diffraction patterns were recorded in the 2θ range of 10–80° with a scanning speed of 10°min−1 and a step size of 0.02°s−1. X-ray photoelectron spectra (XPS) was measured on an Thermo ESCALAB 250Xi (US) spectrometer using monochromatized Al Kα radiation at electron gun power of 150 W, and the Al Kα monochromatized line (1486.6 eV) was adopted during the analysis. The BET surface area measurements were performed with N2 adsorption at −196 °C using an adsorption unit ASAP2020. CO2 temperature-programmed desorption (CO2-TPD) was performed on an AutoChemII 2920 Analyzer. 400 mg of catalyst was heated to 350 °C and kept for 60 min in Ar stream. Then, the catalyst was cooled down to ambient temperature and followed by adsorption with pure CO2. After adsorption, the catalyst was purged with He stream. The desorption of CO2 was carried out with a heating rate of 6 °C·min−1 to 500 °C and measured by a mass spectrometer. Scanning electron microscope (SEM) images were carried out with a JSM-6700F instrument operated at 8 kV. The thermal analyses were carried out on a NETZSCH TG/209/F1 thermoanalyzer. The measurements were conducted in dynamic air atmosphere (50 mL min−1), using the alumina plates crucibles of 150 mL. The heating rate was of 20 K min−1 in the range of temperature 30–900 °C and the mass samples were about 4 mg. Energy Dispersive Spectroscopy (EDS) was also used for qualitative detection of Ni, Cu, Mo elements in the catalyst matrix.
3 Results and Discussion
3.1 Catalytic Performance
The Ni content 12 wt% with the Ni/Al2O3, Ni-Cu/Al2O3, Ni-Mo/Al2O3, Ni–Cu–Mo/Al2O3 catalytic performances for carbon dioxide reforming of methane were shown in Fig. 3, which showed the reactants conversion as a function of temperature. From the results, it was apparent that the catalytic activities of four kinds of catalysts were presented the same trend, namely the reactants conversion increased significantly with increasing temperature. In fact, the increase in catalytic performance with temperature conformed to the thermodynamics of the process since it was an endothermic reaction. The Ni/Al2O3 catalyst conversion rate was the lowest. The conversion rates of the Ni–Cu/Al2O3 and Ni-Mo/Al2O3 were similar and higher and the conversion rate of the Ni–Cu–Mo/Al2O3 was the highest. This showed that on the basis of Ni catalysts added Cu or Mo fertilizer could enhance the catalytic activity of catalysts, adding the two Cu and Mo fertilizers could further increase the catalytic activity of the catalysts, especially at low temperatures (650–750 °C). The reason may be that between Ni and Cu or Mo formed some kinds of new structure and this could be achieved by characterization of catalysts.
Figure 4 illustrated the catalytic performance of the Ni–Cu–Mo/Al2O3 catalyst with the different Ni:Mo mass ratio for carbon dioxide reforming of methane, showing the reactants conversion and products selectivity as a function of temperature. From the results, it was apparent that the reactants conversion increased with increasing temperature, while there was negligible difference in products selectivity. In 600–800 °C temperature range, the conversions increased rapidly with the increase of reaction temperature. But as the temperature increased, the conversion rate increased slower. When temperature was higher than 750 °C, as the temperature increased, the conversion rates basically remained unchanged. Therefore, the proper temperature for the Ni–Cu–Mo/Al2O3 catalysts was 750 °C. The reactants conversion and products selectivity also varied with the Ni:Mo mass ratio. The catalyst with the mass ratio of Ni:Mo = 0.75 showed higher activities. The conversions of CH4 and CO2 reached 97.68 and 99.12 %, and the selectivity of CO and H2 reached 92.96 and 90.01 % at 800 °C, respectively.
3.2 Catalyst Stability
Figure 5 illustrated the stability of the catalyst with the mass ratio of Ni:Mo = 0.75. The stability of the catalyst was tested in the quartz fixed-bed reactor at 800 °C. As to the catalyst with Ni:Mo mass ratio of 0.75, the conversions of methane and carbon dioxide were over 90 %, and the conversions still remained at 90 % till 60 h. The Reaction conversion rate after 60 h began to gradually reduce. This showed that the Ni–Cu–Mo/Al2O3 catalyst was highly active.
3.3 Catalyst Characterization
Figure 6 showed the XRD patterns in the range of 10–80° of the catalysts with different mass ratio of Ni:Mo. It appeared that nickel was mainly in the form of elemental distribution in the catalyst, a few in the form of spinel (NiAl2O4) or alloy (Ni3Mo, Cu3.8Ni) and the Cu or Mo additives formed the Cu6Mo4O15 metal oxides. The structures of NiMoO4 and NiAl2O4 could effectively prevent the sintering of nickel and improve the activity of catalysts. The broadness of Ni3Mo and Cu3.8Ni characteristic peaks increased with the increasing mass ratio of Ni:Mo. The NiMoO4 characteristic peak of the catalyst with Ni:Mo mass ratio of 0.75 was high and sharp, which explained that the crystal shape was perfect. The characteristic peaks of the alloy Ni3Mo and Cu3.8Ni were wide and low, suggesting that the formation of the grain size was small and the dispersion was high. It suggested that the alloy structure formed between Ni and Cu or Mo could significantly improve the catalytic activity of catalysts, which was consistent with the results of the catalytic performance tests. The Ni surface area and average Ni particle size as shown in Table 1 were calculated according to the method described in Ref. [11].
Figure 7 Showed the XRD patterns of Ni–Cu–Mo/Al2O3 catalyst with the mass ratio of Ni/Mo = 0.75 after reaction. The spinel and alloy structures disappeared after reaction, while MoO2, Mo2C, Al0.9Ni4.22 and Cu0.8Ni0.19 species formed. The results suggested that the addition of Cu and Mo induced strong metal-support interaction, which was reported as one of the contributions to good catalytic performance [12]. Mo2C, which was also catalytically active for carbon dioxide reforming of methane, contributed to the prevention of carbon deposition and extended the stability of the catalyst.
X-ray photoelectron measurements were performed over the Ni–Cu–Mo/Al2O3catalyst with Ni:Mo mass ratio of 0.75 to investigate the surface properties and elemental oxidation states. Figure 8 showed the XPS patterns of the catalyst with Ni:Mo mass ratio of 0.75, and the Al Kα monochromatized line (1486.6 eV) was adopted during the analysis. The samples were pressed into a thin pellet, and the binding energys (BEs) were corrected with the reference BE of C1 s (284.6 eV).
The spectra of Ni2p, Cu2p, Mo3d, Al2p, O1 s were taken at the constant analyzer energy mode (30 eV pass energy) and C1 s at the 50 eV pass energy. XPS analysis was performed to examine the elemental composition and to determine the valence state of elements of the catalysts. For the O1 s spectra, the O1 s peak is generally complicated because of the O ions from different oxides. As per the literature, the O2− was existed in CeO2 at the peak of 529.6 eV [13]. And the O1 s binding energy value reported for Rh2O3 was 530.0 eV [14]. On this basis, the peak at 529.87 eV may be attributed to O2− of the NiAl2O4, NiMoO4 and Cu6Mo4O15. The BEs of Al2p on the Ni/Al2O3 catalysts were found to be in the range of 73.7–74.1 eV without significant variations [15], which suggested the Al3+ of the formation of spinel NiAl2O4 species. For the Ni2p spectra, Ni mainly existed as Ni0 (852.48 ± 0.5 eV) in Zr-based catalyst [16] and Ni0 (852.7 eV) in Ni–Co catalyst [17]. Ni mainly existed as Ni2+ (853.5–855.6 eV) [18] or (856 or 874 eV) [19]. As it is shown in Fig. 8, the low-intensive peak of 852.06 eV could be assigned to Ni elemental. The peak of 852.06 and 872.74 eV were attributed to Ni2+, which may be the spinel NiAl2O4 or the NiMoO4. In addition, the higher energy peak (860.85 eV) may be associated with the Ni3Mo or Cu3.8Ni alloy, which combined with the XRD analysis. By the peak intensity, it could also find that the more the alloy component content, the better the CH4 and CO2 conversions. The observed binding energy values for various Mo phases differ from the one reported in the Ref. [19] and the BEs were performed Mo0 (227.9–228.2 eV), Mo4+ (229.4–229.6 eV) and Mo6+ (232.5–232.6 eV) [19]. We could speculate that the peak of 227.52 eV was the metal state Mo0 (which was consistent with the results of XRD). The 234.41 eV may be Mo6+ existed in the NiMoO4 and the 231.2 eV may be the Ni3Mo alloy structure. Due to the different chemical environment of the Cu element, the observed binding energy values for various Cu phases were very complex. The satellite peak at 940–950 eV was the copper oxide [20]. The peak at 932.4 and 934.3 eV could be attributed to presence of Cu1+ and Cu2+, respectively [21]. The binding energies at 952.2 and 932.3 eV can be attributed to Cu0 and Cu1+, respectively [22]. From the XRD analysis, we could know that the Cu element existed in the Cu6Mo4O15 metal oxides and the Cu3.8Ni alloy. Due to the Ni2+, Mo6+, Al3+, O2−, the valence state of the Cu element should be Cu1+. On this basis, the peak at 944.74 and 954.48 eV may be the Cu6Mo4O15 metal oxides. The higher-intensive BEs (934.64 and 936.65 eV) may be attributed to Cu3.8Ni alloy.
On the one hand, the NiAl2O4, Cu6Mo4O15 and NiMoO4 played a fixed role for the active components; on the other hand, they also provided the oxygen in the process of the reaction to prevent the generation of carbon deposition. Since the presence of the metal alloys could increase the dispersion of the active components, the existence of Ni3Mo and Cu3.8Ni alloy could also improve the catalytic activity of the Ni–Cu–Mo/Al2O3 catalyst.
Figure 9 presented the SEM images of Ni–Cu–Mo/Al2O3 catalysts with different mass ratio of Ni:Mo. The images showed that the metals dispersed uniformly on the support.
Table 2 showed the surface and pore properties of Ni–Cu–Mo/Al2O3 catalysts with different mass ratio of Ni:Mo. The BET surface area, average pore diameter and pore volume did not vary much between Ni–Cu–Mo/Al2O3 catalysts with different mass ratio of Ni:Mo. The catalyst with Ni:Mo mass ratio of 0.75 exhibited the biggest BET surface area, which might be favorable for the catalytic reaction.
The CO2-TPD curves of Ni–Cu–Mo/Al2O3 catalysts with different mass ratio of Ni:Mo were presented in Fig. 10. It was apparent from TPD profiles that all catalysts showed three CO2 desorption peaks at about 100, 200 and 450 °C, respectively. The research estimated that the CO2 adsorbed on weaker sites was desorbed at low temperature while that adsorbed on strong sites was desorbed at high temperature [23]. The increase in Ni:Mo mass ratio led to the increase in peak areas toward high temperature, which were assigned to medium and strong sites.
In order to further verify the Ni–Cu–Mo/Al2O3 catalyst with the Ni/Mo ratio of 0.75 could simultaneously lead to both high stability and high activity, the catalyst was tested in the same reaction for 80 h at the 800 °C.
Zhang et al. [24] found that on the surface of Ni/Al2O3 catalyst there were three forms of carbon deposition: the surface of the catalyst was mainly Cα at 150–220 °C, which was a kind of active carbon mainly responsible for generating the syngas; At 500–600 °C, the catalyst surface were mainly Cβ, which may be involved in generating CO at more than 600 °C; When temperature greater than 650 °C, the catalyst surface mainly existed in the form of Cγ, which was the main factor that led to the catalyst deactivation. Figure 11 showed the TG analysis of the Ni–Cu–Mo/Al2O3 catalyst which reacted in CH4–CO2 reforming reaction at 800 °C for 80 h. Figure 11 suggested that the amount of carbon deposition Cα and Cβ were 1.17 and 51.57 %, respectively. And the Cγ was not found in the catalyst after reaction. Since the Cγ was the main factor that led to the catalyst deactivation, the absent of Cγ may be the reason for high catalytic stability of the Ni–Cu–Mo/Al2O3 catalyst for CH4–CO2 reforming reaction at 800 °C after 80 h. TG analysis further verified the existence of the alloy structure may inhibit carbon deposition.
The EDS quantitative analysis was carried out on the catalyst surface element and the relative contents of surface Ni, Cu and Mo were obtained for Ni–Cu–Mo/Al2O3 catalyst with the Ni/Mo ratio of 0.75. Data results as shown in Table 3 indicated that Ni–Cu–Mo/Al2O3 catalyst had good dispersion which could promote the reaction.
4 Conclusions
With the addition of Cu and Mo, a series of Ni–Cu–Mo/Al2O3 catalysts with different mass ratio of Ni:Mo were prepared and applied for CH4–CO2 reforming. The spinel (NiMoO4, NiAl2O4, Cu6Mo4O15) and alloy (Ni3Mo, Cu3.8Ni) structures were formed. The chemical composition of the Ni–Cu and Ni–Mo alloys were found to have a higher dispersed state in the Ni–Cu–Mo/Al2O3 catalyst. The Ni–Cu–Mo/Al2O3 catalysts had high BET surface area and uniform distribution, and showed potential for carbon dioxide reforming of methane. The formation of Mo2C after reaction contributed to the prevention of carbon deposition and high stability of the catalyst.
References
Nader R, Mohammad H, Ali AB, Somaiyeh A, Mahdi FJ (2014) Energy Convers Manag 84:50–59
Dong XF, Cai XL, Song YB, Lin WM (2007) J Nat Gas Chem 16(1):31–36
Choi JS, Moon KI, Kim YG, Lee JS, Kim CH, David L (1998) Catal Lett 52(1–2):43–47
Huang T, Huang W, Huang J, Ji P (2011) Fuel Process Technol 92(10):1868–1875
Quincoces CE, Vargas SP, Grange P, Gonzάlez MG (2002) Mater Lett 56(5):698–704
Borowiecki T, Gac W, Denis A (2004) Appl Catal A 270(1–2):27–36
Liu HT, Tian H, Wang XL (2007) J Mol Catal (China) 21(4):304–307
Shi C, Zhang AJ, Li XS, Zhang SH, Zhu AM, Ma YF, Au CT (2012) Appl Catal A 431–432:164–170
Chen L, Hao ZD, Yang TZ (2014) Int J Hydrogen Energy 39:15474–15481
Djaidja A, Messaoudi H, Kaddeche D (2015) Int J Hydrogen Energy 40(14):4989–4995
Donald DG, Bartholomew CH (1981) J Catal 67:186–206
Wang SB, Lu GQM (1988) Appl Catal B 16(3):269–277
Reddy BM, Rao KN, Reddy GK, Khan A, Park SE (2007) J Phys Chem C 11(50):18751–18758
Sheerin E, Reddy GK, Smirniotis P (2016) Catal Today 263:75–83
Lee HY, Kim AR, Park MJ, Jo JM, Lee DH, Bae JW (2015) Chem Eng J 280:771–781
Survilienè S, Cešūuniené A, Jasulaitiené V, Jureviciūte I (2012) Appl Surf Sci 258:9902–9906
Jian Li.(2014) Studies on Stability and Coke-Resistant properties of CoNi Alloy Catalysts for Dry Reforming [D].East China University of Science and Technology.
Ivanova TM, Kochur AG, Maslakov KI, Kiskin MA, Savilov SV, Lunin VV, Novotortsev VM, Eremenko IL (2015) J Electron Spectrosc Relat Phenom 205:1–5
Kukushkin RG, Bulavchenko OA, Kaichev VV, Yakovlev VA (2015) Appl Catal B 163:531–538
Zhao J, Li YX, Zhu YQ, Wang Y, Wang CY (2015) Appl Catal A 510:34–41
Rahemi N, Haghighi M, Babaluo AA (2014) Energy Convers Manag 84:50–59
Sato AG, Volanti DP, Meira DM, Damyanova S, Longo E (2013) J Catal 307:1–17
Naeem MA, Al-Fatesh AS, Abasaeed AE, Fakeeha AH (2014) Fuel Process Technol 122:141–152
Tsipouriari VA, Efstathiou AM, Zhang ZL, Verykios XE (1994) Catal Today 21(2–3):589–595
Acknowledgments
Financial supports from Shandong Excellent Young Scientists Fund (BS2011NJ006) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tao, X., Wang, G., Huang, L. et al. Effect of Cu–Mo Activities on the Ni–Cu–Mo/Al2O3 Catalyst for CO2 Reforming of Methane. Catal Lett 146, 2129–2138 (2016). https://doi.org/10.1007/s10562-016-1814-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1814-6