Abstract
The tendency of coke formation was investigated using nickel catalysts supported on calcium and barium hexaaluminates, compared with a commercial catalyst of natural gas steam reforming. It was developed a methodology in a microactivity unit using cyclohexane as model compound and hydrogen as gas carrier, at low temperature (300–500 °C). After the coking tests, the catalysts were characterized by elemental analysis (CHN) and thermogravimetric analysis using air and steam. 6NiO-BaAl presented the lowest coke removal rate with air. After that, the methodology was modified for ethanol and acetic acid, important model compounds used in studies of biofuels, steam reforming and bio-oil pyrolysis. All model compounds lead to carbon formation with the same chemical nature, as indicated by the temperature of the oxidation peak. So, the methodology can be used as a tool for selection of catalysts. Additionally, cyclohexane and acetic acid are ideal model compounds, because of the lowest and highest coke removal rates with air.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Coking is a big problem in development of steam reforming catalysts; some authors use in their investigations model compounds of the process of interest, employing conditions of fast deactivation [1, 2]. For this purpose, hydrocarbons that have high rates of coking can be used, as olefins, aromatic compounds and cycloalkanes [3].
Lobo and Trimm [1] used some olefins, paraffins and acetylene in coking experiments with nickel foil using nitrogen and hydrogen as carrier gases, for 1 h at several different temperatures. Deposition from acetylene was found to be rapid, while deposition from olefins is autocatalytic and accelerated by hydrogen. Carbon formation from paraffins is comparatively slow. In another study, a mixture of n-hexane (13.5 mol%), hydrogen (25 mol%) and nitrogen (61.5 mol%) was passed over the catalysts (nickel foil and 18 %Ni/Al2O3) at 600 °C in the presence of steam. The study was conducted in a thermogravimetric balance, with coke formation measured by weight variation and after a certain value, the system was flushed with nitrogen at 600 °C for half an hour, and coke was removed by CO2, H2O and H2 [2].
The main challenge found in studies with renewable sources is to develop a process stable for a long time; thus, catalyst deactivation by coke under real process conditions must be better understood. Some common problems in studies of gas cleaning are the elimination of tar from biomass gasification, generation of hydrogen from ethanol and bio-oils from pyrolysis process [4–6].
Acetic acid and ethanol are used as renewable sources for steam reforming [7, 8]. Acetic acid was chosen because it is a representative compound of pyrolysis bio-oil [6], while ethanol is used directly in studies of hydrogen generation. Cyclohexane was also used to evaluate coking resistance during steam reforming of tar produced in biomass gasification [9].
These streams from gasification and pyrolysis have a lot of known precursors of coke, as aromatic, olefinic and oxygenated compounds. Several reactions can occur in parallel with steam reforming of acetic acid. Some side reactions include reactions of coke formation, as dehydration to ketene and ketonization to acetone that can polymerize by aldol condensation and form coke. Another source of coke is ketene that can form ethene, a well known coke precursor [10]. For steam reforming of toluene, a typical tar model compound, coke can be formed easily by cracking reaction of the aromatic compound [11, 12].
The original contribution of this work is to employ some model compounds, used in these processes, as cyclohexane, acetic acid, and ethanol, in coking studies. Nickel supported on hexaaluminates was chosen as catalysts because our previous studies showed the great potential of these catalysts for tar removal by steam reforming reaction of model compounds [9, 11, 12]. The methodology developed here is suitable as a selection tool for evaluating the resistance to coke formation of steam reforming catalysts, especially for renewable applications.
2 Experimental
2.1 Catalyst Preparation
Calcium and barium hexaaluminates were prepared by the coprecipitation route. Solutions of metal nitrates (Al, Ca and Ba from Vetec, purity of 99.9 %) with appropriate concentrations were dropped slowly into a vessel with a 14.5 wt% NH4OH solution. The precipitation was conducted with slow agitation at room temperature for 1 h after nitrate addition, with pH control at 9–11 and aging during 16 h. Then, the samples were filtered and washed with water until pH 7, followed by drying overnight at 120 °C. The calcination was performed in two steps: at 800 °C for 4 h, and at 1200 °C for 4 h. The prepared supports will be referred to as CaAl for CaAl12O19 and BaAl for BaAl12O19.
The nickel catalysts were prepared by incipient impregnation of the hexaaluminate supports with a solution of nickel nitrate (Vetec) in an appropriate concentration to obtain contents of 6 and 12 wt% of NiO. After impregnation, the samples were dried at 95 °C overnight and calcined at 450 °C for 4 h. The prepared catalysts will be labeled as XNiO-CaAl and XNiO-BaAl, where X = 6 and 12.
For comparison, it was tested a natural gas steam reforming commercial catalyst, named as SCR, with 15 wt% of NiO.
2.2 Characterization of Fresh Catalysts
X-ray powder diffraction (XRD) patterns were recorded in a Rigaku Miniflex II, with a monochromator using Cu Kα radiation (30 kV and 15 mA) over a 2θ range from 2° to 90°, with step of 0.05° and 2 s by step.
The textural characteristics, such as BET specific area, pore volume and average pore diameter (BJH method), were determined by N2 adsorption–desorption at −196 °C in a Micromeritics ASAP 2400. Prior to the analysis the samples were pretreated at 400 °C in vacuum.
Temperature programmed reduction (TPR) was performed using a Micromeritics Autochem II. The gas used was 10 %H2 in Ar, with a flow of 40 mL min−1, and the temperature increased to 1000 °C using a rate of 10°C min−1. Firstly the samples were pretreated with 40 mL min−1 of Ar at 400 °C. The hydrogen consumption was monitored by thermal conductivity detector (TCD).
The basicity of the samples was determined by temperature programmed desorption of CO2 (TPD-CO2) using thermogravimetric (TG) balance of Mettler Toledo (TGA/SDTA 851E). The method consisted of the following steps: (1) Pretreatment with 40 mL min−1 of Ar from 25 to 400 °C at 20 °C min−1, kept at 400 °C for 30 min; (2) Cooling until 25 °C with 40 mL min−1 of Ar at 15 °C min−1; (3) Adsorption of CO2 with 40 mL min−1 at 25 °C for 30 min; (4) Purge with 40 mL min−1 of Ar, at 25 °C for 60 min; (5) Desorption of CO2 with 40 mL min−1 of Ar from 25 to 900 °C at 5 °C min−1; (6) Cooling to room temperature. CO2 signal was monitored by mass spectrometer, Pfeiffer Vacuum GSD 320 T1 Thermostar, using the fragment m/e− = 44. The total basicity was calculated using the weight loss of desorption period (100–900 °C).
The hydrogen chemisorption capacity was used to obtain a measure of the specific Ni surface area, using Micromeritics ASAP 2010C, in a hydrogen pressure range from 0.002 to 260 mmHg and 35 °C. The catalysts were previously reduced at 500 °C with pure hydrogen.
2.3 Coking Experiments
2.3.1 Coking Studies Using Cyclohexane
The coking deposition step was performed using equipment from Micromeritics, AUTOCHEM 2920, and U-tube quartz reactor. It was used 100 mg of catalyst placed on quartz wool. Firstly, it was studied the experimental conditions (temperature and hydrogen to model compound molar ratio—R) using cyclohexane and SCR catalyst. With these results, the conditions of coking tests were defined: 400 °C for 1 h, using 10 %H2/Ar passed through model compound (cyclohexane) saturator kept at 50 °C, under atmospheric pressure. All catalysts were reduced in situ at 650 °C for 2 h. After the experiment, the catalysts were analyzed by thermogravimetry coupled with mass spectrometer (MS), using air or steam (the method will be described in item 2.4), and also by elemental analysis (CHN).
2.3.2 Coking Studies Using Cyclohexane, Acetic Acid and Ethanol
Coking tests were performed in AUTOCHEM 2920 at atmospheric pressure, 400 °C for 1 h, using 10 %H2/Ar passed through model compound (cyclohexane, acetic acid and ethanol). Gas flow was adjusted to obtain hydrogen to model compound molar ratio (R) near to 2.0 for cyclohexane and ethanol, using 48 and 25 mL min−1, respectively. For acetic acid, R was equal to 7.0, using 25 mL min−1; in this case it was impossible to use R = 2.0, because the gas flow should be 8 mL min−1, bellow the equipment limit. Before reaction, the catalysts were reduced in situ at 650 °C for 2 h. The equipment was not connected to a gas chromatograph or a mass spectrometer, so the gases generated were not analyzed during the experiment. After the coking tests, the catalysts were analyzed by TG-MS using air, monitoring the fragment m/e− = 44 (CO2), and CHN, to evaluate the coke removal rate and carbon amount.
2.4 Characterization of Catalysts After Coking Experiments
The amount of coke deposited on the catalysts from the coking tests was determined by elemental analysis (CHN), using a ThermoFinnigan FLASH EA1112 equipment.
Thermogravimetric balance of Mettler Toledo (TGA/SDTA 851E) was used in tests of coke removal. The method consisted of the following steps: (1) Drying with 80 mL min−1 of Ar from 25 to 150 °C at 10°C min−1, kept at 150 °C for 30 min; (2) Coke removal with 40 mL min−1 of Ar and 40 mL min−1 of synthetic air or steam from 150 to 800 °C, at 3 °C min−1. CO2, CO and H2 signals were monitored by mass spectrometer, Pfeiffer Vacuum GSD 320 T1 Thermostar, using fragment m/e− of 44, 28 and 2. Steam was generated by passing 40 mL min−1 of inert gas through a saturator with water at 15 °C. The mass loss was normalized by the metallic area, expressing the coke removal rate by mgcoke gNi m−2 h−1, calculated from TG curve using the linear period of weight loss.
The morphology of the carbon species present in the used catalysts was examined by scanning electron microscopy (SEM) using a JEOL JSM6490LV equipment with secondary electrons, operating with high vacuum at 20 kV and work distance of 10 nm.
3 Results and Discussion
3.1 Catalyst Characterization
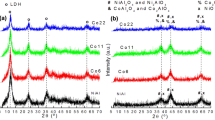
The main crystalline phases found for CaAl support were α-Al2O3, Ca3Al2O6 and CaAl12O19 [11], and θ-Al2O3, BaAl2O4 and BaAl12O19 for BaAl support [12]. Nickel oxide peaks (2θ = 43° and 63°) were identified for all catalysts; it was noted that the calcination at 450 °C after nickel impregnation did not cause any alteration on crystallographic profiles of the supports. It was not possible to identify NiAl2O4 by XRD because of the peak overlap; it can be done by diffuse reflectance spectroscopy in UV–visible [13] or XPS [14]. The formation of these crystalline phases was well discussed previously [11, 12].
TPR profiles of the catalysts were different depending on the support. 6NiO-CaAl catalyst presented only one peak at 482 °C, and 12NiO-CaAl two peaks, at 369 and 478 °C. On the other hand, 6NiO-BaAl showed peaks at 384 and 517 °C and 12NiO-BaAl at 400 and 530 °C. This range of temperature (370–530 °C) is associated with the reduction of nickel species with low interaction with the support [15, 16], that could be explained because of the low temperature used for calcination after Ni impregnation. The two catalysts supported on BaAl also presented a small peak at 760 °C related to reduction of nickel aluminate, formed by strong interaction between nickel and support at high temperatures. TPR profiles of these catalysts were presented previously [11, 12].
Literature teaches that a strong interaction of the metallic phase with support inhibits the sintering phenomenon, increasing the stability of the catalysts. As coke formation is a structure sensitive reaction, it is greatly influenced by the Ni particle size, which increases with sintering [17, 18]. So, it can be said that the catalysts supported on CaAl would be more susceptible to deactivation by coke deposition due to the reduction facility.
Table 1 shows textural analysis and results of hydrogen chemisorption on Ni/hexaaluminate and SCR catalysts. SCR has similar properties of 12NiO-CaAl, with the same Ni dispersion. As it was shown in previous studies [9], the nickel particle size is a significant factor to explain the resistance for filamentous coking, with the increase in the nickel particle size promoting the increase in rate of coke deposition. Catalysts supported on BaAl showed higher Ni dispersion, thus, they are supposed to be less susceptible to filamentous coke deposition. Adsorption curves for 12NiO-BaAl and 12NiO-CaAl are presented in Fig. 1. It should be noticed that the adsorbed amount of hydrogen for the bare supports, although negligible for BaAl, presented positive values for CaAl, probably due to the formation of calcium hydride.
Despite having similar chemical nature, hexaaluminate supports can influence the coke formation in function of their basicity. The order of basicity determined by TPD-CO2 is: 12NiO-CaAl (0.47 mmolCO2/gcat) < 12NiO-BaAl (0.75 mmolCO2/gcat) < 6NiO-CaAl (0.87 mmolCO2/gcat) < 6NiO-BaAl (0.94 mmolCO2/gcat). This order can be correlated to the facility of coke removal; the more basic, the greater tendency to form more reactive coke [3].
3.2 Coking Studies Using Cyclohexane
3.2.1 Preliminary Coking Tests with Subsequent Analysis on TG-MS Using Air
Some preliminary tests were performed in order to select the best conditions to study coke formation from cyclohexane. Table 2 shows results of experiments at 300, 400 and 500 °C, using gas flow of 25, 36 and 48 mL min−1, correspondent to R = 1.2, 1.7 and 2.3. The amount of carbon detected by CHN was not affected by the gas flow or R, at the same temperature: 75–80 % (500 °C), 10–20 % (400 °C) and less than 3 % (300 °C). The profile of CO2 formation with time at 400 °C (Fig. 2) shows an intense peak at around 480 °C and a shoulder at 650 °C, indicating two types of coke, filamentous [21] and “non-structured” [22].
According to Table 2, the amount of coke is very small at 300 °C (<3.0 %) and at 500 °C, the amount is very large (>75 %), causing difficulties to remove the waste catalyst from the reactor (clogging the reactor); so the temperature chosen for subsequent tests was 400 °C, because the amount of coke is reasonable for analysis after the reaction by CHN and thermogravimetric analysis. Moreover, the use of this range of temperature, 400–500 °C, avoids coking due to thermal cracking of model compounds by support effects, and represents a condition with high coking rates, making the comparison of different catalysts easier, in terms of catalytic stability and activity towards a certain model compound, as seen in the literature [1–3, 9, 12, 26].
3.2.2 Comparison of Different Catalysts
Table 3 shows the results of coking methodology using cyclohexane and subsequent removal with air, expressed by coke removal rate (mgcoke h−1 and mgcoke gNi m−2 h−1) and the temperature range of the process. Also, it is shown the carbon content from elemental analysis. Elemental analysis gives the correct value of the amount of organic material present in the catalysts. 6NiO-BaAl presents the lowest rate of coke removal, thus it is the most suitable catalyst to be used in tar steam reforming, as seen in our previous studies [11, 12].
The results shown in Table 3 indicate that the initial amount of coke affects the coke removal rate with air, explaining the rates of coking removal for 6NiO-BaAl and 12NiO-CaAl catalysts, the lowest and highest, respectively.
The coke removal rate was calculated using the linear period of thermogravimetric analysis (Fig. 3); there was some mass increase before this period due to the oxidation of metallic nickel to NiO. As hypothesis, it is suggested that the low amount of adsorbed cyclohexane is removed before 300 °C, not interfering with coking removal at higher temperatures. The SCR commercial catalyst contains potassium in its formulation, which is supposed to assist the removal of deposited coke, explaining the different thermogravimetric analysis profile, while for the nickel hexaaaluminate catalysts the profiles are very similar.
The order of facility to remove coke, considering the metallic area, is: 6NiO-BaAl < 6NiO-CaAl < 12NiO-BaAl < 12NiO-CaAl, evidencing a tendency that coke removal is correlated with the increase of nickel particle size among hexaaluminate supported catalysts (Fig. 4), which is confirmed in previous studies [9, 11, 12] and also by the literature [17, 23]. The SCR commercial catalyst failures this correlation probably because potassium in its formulation helps to prevent coke deposition.
The nature of coke is probably very similar, with CO2 formation peaks at temperatures of 517 °C (12NiO-BaAl), 534 °C (6NiO-CaAl), and 511 °C (12NiO-CaAl), typical temperatures of removal of filamentous coke [13–24], as can be seen in Fig. 5a. Figure 5b shows the profile for SCR, with the peak temperature at about 480 °C and also a shoulder at 610 °C, indicating two species of carbon.
These catalysts were used in steam reforming of toluene, benzene and naphthalene, model compounds for tar gasification, and catalysts supported on barium hexaaluminate have the best stability at 800 °C, S/C = 1.5 and 20,000 mL g −1cat h−1 [11, 12]. Thus, the coking results with cyclohexane at low temperatures can be directly correlated with the coking resistance during steam reforming. Besides of that, the tendency to coke formation found with the methodology presented here is in accordance with the results of other methodologies employed by the authors [9].
3.2.3 Coking Studies with Subsequent Analysis on TG-MS Using Steam
For the study of coke removal with steam, two catalysts were chosen, 12NiO-CaAl and 6NiO-BaAl catalysts, which had higher and lower amount of carbon by elemental analysis, respectively (Table 3).
When the removal of coke is performed with steam, CO2, CO and H2 are generated from the reaction of coke with steam (Eq. 1) and shift reaction (Eq. 2). The removal with hydrogen produces CH4 (Eq. 3), but it wasn’t monitored by mass spectrometer. CH4 and CO2 can be consumed (Eqs. 4 and 5), producing more CO and H2O, favoring hydrogen formation. These are the possible reactions of coke removal [25]. Profiles of CO2, CO and H2 formation during coke removal with steam are displayed in Fig. 6. Cyclohexane dehydrogenation/hydrogenolysis can happen in lower extension (Eqs. 6 and 7), due to the presence of low amount of adsorbed cyclohexane, but it is favored at low temperatures (300–500 °C), producing methane, hydrogen or benzene [9]. Benzene and methane can react with steam by steam reforming producing more carbon monoxide and hydrogen.
The coke nature of 12NiO-CaAl is probably more refractory, explaining the profile shift to higher temperatures, as can be seen in Fig. 6. Although the trend has been maintained using air or steam, it is easier to regenerate 6NiO-BaAl in both conditions (Table 4).
Coke associated with polymer chains is more difficult to remove with steam, CO2 or H2 [2, 26], so this explains the lower value for coke removal rate and the higher temperature, equal to 540 °C, for 12NiO-CaAl in the case of removal with steam instead of air.
3.3 Coking Studies with Other Model Compounds with Subsequent Analysis on TG-MS Using Air
Table 5 shows the results obtained from SCR and 6NiO-BaAl catalysts with three model compounds, ethanol, acetic acid and cyclohexane, using 10 %H2/argon as carrier gas. From these results, it is clear that the coke amount and rate of coke removal are different for each model compound. It should be stressed up that the tests can only be used individually to make a screening of resistance to coking for a set of catalysts, because of GHSV and R were not the same for all three model compounds. The formulation of SCR may help the catalyst to remove coke, because of the presence of potassium, so it is easier to remove coke with air, which explains the higher coke removal rates for SCR than for 6NiO-BaAl, valid for any model compound.
According to Takanabe et al. [27], deactivation by coke is a limiting factor for development of catalysts used on bio-oil steam reforming, due to blockage of active sites by coke/oligomer formed by aldol condensation reaction. The conditions proposed by them were used here because at low temperatures, 200–400 °C, and in the absence of steam, aldol condensation mechanism is favored when using acetone. Acetone may be dehydrated, leading to formation of ketene and mesitylene, producing coke by polymerization and oligomerization reactions, and acetic acid leads to coke with the same mechanism, because of its dehydration to acetone [28].
In the case of ethanol, coke may be formed because of byproducts, as acetic acid, acetone, ethene and methane, produced in accordance with the experimental conditions employed in the tests. Acetone and acetic acid can produce coke by the same reactions described previously, and the dehydration of ethanol to ethylene leads to coke formation due to polymerization reaction [10]. SEM images (Fig. 7) of the SCR catalyst used on experiments with cyclohexane, ethanol and acetic acid showed filamentous and “non-structured” coke [9, 12, 29].
For SCR catalyst, the experiments of coke removal with air (Fig. 8) confirmed that all model compounds lead to carbon formation with the same chemical nature, indicated by the temperature of oxidation peak, in the range of 470–497 °C. Profiles of CO2 formation from ethanol and cyclohexane had shoulders at 617 and 611 °C, pointing to a more aromatic coke [25, 29–31]. The same behavior was found for 6NiO-BaAl, which presented profiles with temperature of oxidation peak at 517, 497 and 532 °C for acetic acid, cyclohexane and ethanol, respectively, and a shoulder at 676 °C.
Coke deposited on experiment with acetic acid is less aromatic, with peak of temperature at 470 °C, which is consistent with the thermogravimetric analysis, exemplified for SCR catalyst (Fig. 9). For cyclohexane and ethanol there were several steps of weight loss, indicating different carbon species, more evident in the case of ethanol because of the olefinic intermediates. For acetic acid, it is assumed that mesitylene, formed in the aldol condensation of acetone [28], leads to a polymeric compound more stable, explaining the less stages of weight loss on profile of thermogravimetric analysis, as seen in the literature [31].
Based on these results, it can be concluded that catalysts for these applications must have suitable formulations. Therefore, there is a lot of effort in simple formulations suitable for steam reforming of aliphatic hydrocarbons; it is expected a rapid deactivation of these catalysts. Exemplifying this, catalysts based on Co, Ni, Fe, Cu, and noble metals, as Pt, Pd, Rh and Ru, largely employed for steam reforming of acetic acid or acetone, are commonly deactivated by coke formation [10].
It is reported in the literature that nickel has activity similar to noble metals (Rh, Pd and Pt), but noble metals are more resistant to carbon formation. One alternative is to employ bimetallic systems, as proposed by Lakhapatri and Abraham [32], who improved the coking resistance by the presence of 2.5 % m/m of Rh in the Rh-Ni catalyst, resulting in inhibition of the nickel particle growth and a decrease in coke deposition on the catalyst [33]. In more recent studies, it can be seen the use of formulations containing noble metals in order to increase reaction stability [34–37].
4 Conclusions
The tendency towards coke formation of nickel catalysts was evaluated using different methodologies and model compounds. When using cyclohexane, the coke removal rate was directly correlated with the nickel particle size for hexaaluminate-supported catalysts. The temperature of CO2 formation during coke removal showed that coke is predominantly filamentous. The coke removal with steam is more difficult than with air, requiring higher temperatures. Among the hexaaluminate-supported catalyst, 6NiO-BaAl presented the highest resistance to coke formation. This catalyst and a commercial catalyst for natural gas steam reforming were also evaluated using other model compounds, acetic acid and ethanol, leading to carbon formation with the same chemical nature. One alternative to minimize coke formation on nickel catalysts is to employ noble metals as promoters, in small quantities, and further studies are being developed in this context.
References
Lobo LS, Trimm DL (1973) J Catal 29:15
Bernardo CA, Trimm DL (1979) Carbon 17:115
Rostrup-Nielsen JR (1974) J Catal 33:184
Han J, Kim H (2008) Renew Sust Energy Rev 12:397
Garcia L, French R, Czernik S, Chornet E (2000) Appl Catal A: Gen 201:225
Vispute TP, Huber GW (2009) Green Chem 11:1433
Wang D, Montané D, Chornet E (1996) Appl Catal A: Gen 143:245
Batista MS, Santos RKS, Assaf EM, Asaaf JM, Ticianelli EA (2003) J Power Sources 124:99
Quitete CPB, Bittencourt RCP, Souza MMVM (2015) Catal Commun 71:79
Trane R, Dahl S, Skjøth-Rasmussen MS, Jensen AD (2012) Int J Hydrogen Energy 37:6447
Quitete CPB, Bittencourt RCP, Souza MMVM (2014) Appl Catal A: Gen 478:234
Quitete CPB, Bittencourt RCP, Souza MMVM (2015) Catal Lett 145:541
Lisboa JS, Santos DCRM, Passos FB (2005) Catal Today 101:15
Wang S, Lu MGQ (1998) Appl Catal B: Environ 19:267
Magrini-Bair KA, Czernik S, French R, Parent YO, Chronet E, Dayton DC, Feik C, Bain R (2007) Appl Catal A: Gen 318:199
Blom R, Dahl IM, Slagtern A, Sortland B, Spjelkavik A, Tangstand E (1994) Catal Today 21:535
Christensen KO, Chen D, Lodeng R, Holmen A (2006) Appl Catal A: Gen 314:9
Bartholomew CH (2001) Appl Catal A: Gen 212:17
Borowiecki T (1984) Appl Catal 10:273
Xu S, Zhao R, Wag X (2004) Fuel Process Technol 86:123
Demicheli MC, Duprez D, Barbier J, Ferreti OA, Ponzi EN (1994) J Catal 145:437
Nandini A, Pant KK, Dhingra SC (2005) Appl Catal A: Gen 290:166
Lercher JA, Bitter JH, Hally W, Seshan K (1996) Stud Surf Sci Catal 101:463
Tracz E, Scholz R, Borowiecki T (1990) Appl Catal 66:133
Rostrup-Nielsen JR, Sehested J, Norksov JK (2002) Adv Catal 47:65
Jackson SD, Thomson SJ, Webb G (1981) J Catal 70:249
Takanabe K, Aika K-I, Seshan K, Lefferts L (2006) Chem Eng J 120:133
Navarro RM, Guil-Lopez R, Ismail AA, Al-Savaris SA, Fierro JLG (2015) Catal Today 242:60
Juan-Juan J, Ramán-Martinez MC, Illán-Gómez MJ (2004) Appl Catal A: Gen 264:169
Trimm DL (1999) Catal Today 49:3
Basagiannis AC, Verykios XE (2007) Catal Today 127:256
Lakhapatri SL, Abraham MA (2009) Appl Catal A: Gen 364:113
Azad AM, Duran MJ, Mccoy AK, Abraham MA (2007) Appl Catal A: Gen 332:225
Steele AM, Polusten S, Magrini-Bair K, Jablonski W (2013) Catal Today 214:74
Oh G, Park SY, Seo MW, Kim YK, Ra HW, Lee J-G, Sang JY (2016) Renewable Energy 86:841
Lida H, Onuki N, Numa T, Igarashi A (2016) Fuel Process Technol 142:397
Dagle VL, Dagle R, Kovarik L, Genc A, Wang Y-G, Bowden M, Wan H, Flake M, Glezakou V-A, King DL, Rousseau R (2016) Appl Catal B: Environ 184:142
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quitete, C.P.B., Tavares, R.P.A., Bittencourt, R.C.P. et al. Coking Study of Nickel Catalysts Using Model Compounds. Catal Lett 146, 1435–1444 (2016). https://doi.org/10.1007/s10562-016-1773-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1773-y