Abstract
Barium hexaaluminate was prepared by coprecipitation method and used as support for nickel catalysts, with 7 and 14 wt% of NiO. After calcination at 1,200 °C the hexaaluminate support consisted of different crystalline phases with specific surface area of 22 m2 g−1. The catalysts exhibited nickel reduction peaks in the range of 400–540 °C. The catalysts were evaluated in steam reforming of different tar model compounds: benzene, toluene and naphthalene (in solution with toluene). The conversions obtained in benzene and toluene reforming at 650 °C were quite similar, while naphthalene is much more difficult to be converted and inhibits toluene conversion at 800 °C. Both catalysts showed good stability with time on stream despite the high amount of carbon deposit. The coke amount and morphology are dependent on the nature of aromatic compound.
Graphical Abstract

.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biomass is one kind of promising new energy sources, due to its extensive distribution, great reserves and renewable capacity [1]. Gasification of biomass is regarded as one of the most promising way to produce syngas, which can be used not only in gas turbines for power generation, but also for catalytic synthesis of methanol, dimethyl ether, liquid hydrocarbons via Fischer–Tropsch reactions and other chemical products [2].
Tar is an inevitable byproduct of biomass gasification that can bring several problems in the downstream process equipment and deposition on catalyst surface [2, 3]. Tar is a complex mixture of aromatic hydrocarbons that depends on biomass composition and gasification conditions; its concentration ranges from 1 to 150 g Nm−3 [4]. The typical composition of tar contains 65 wt% mono-aromatics, 20 wt% di-aromatics, 10 wt% phenolic and heterocyclic compounds with 5 % of high molecular weight. Representative compounds for each of these classes are benzene or toluene, naphthalene, phenol, and pyrene [5, 6]. Most studies in the literature use tar model compounds, such as benzene, toluene and naphthalene, to simulate a real gasification stream; however, few studies compare their reactivity [6–9].
Tar may be removed using the catalytic steam reforming process, because the gas produced in gasification has a high temperature (T > 750 °C) and a high moisture content (20–60 %), favoring the steam reforming [10]. Several catalysts have been proposed in the literature for the catalytic removal of tar, including natural minerals, alkali metal catalysts and Ni-based catalysts [3, 11, 12]. The majority of published work concerns commercial available nickel catalysts designed for steam reforming of natural gas. When reforming aromatic hydrocarbons, the coke formation is particularly severe; moreover, the sintering of the active phase is also critical because of the high temperatures required [13]. Therefore, development of supported Ni catalysts which have high activity, thermal stability and coking tolerance is necessary.
Some researchers have improved the catalytic performance of Ni catalysts in steam reforming of tars adding promoters to the alumina support to inhibit sintering and decrease coke formation. Hexaaluminate compounds offer potential advantages as catalytic supports, including high surface areas, thermal stability and resistance to sintering and deactivation in high-steam environments [14]. The formula of all hexaaluminates is M2O(M´O)–6Al2O3, where M or M´ stand for alkaline or alkaline-earth metal. The structure is lamellar and consists of layers of spinel blocks separated by a monolayer of oxides issued from either bulky alkaline cations, or bulkier alkaline-earth ones [15]. They have been used in combustion, partial oxidation and steam/CO2 reforming reactions, as catalysts [15–19] or catalyst supports [14, 20–23].
The advantage of supporting nickel on hexaaluminates, instead of incorporating it on the hexaaluminate structure, is that the reduction temperature is considerably decreased, while preserving the high coking resistance [14]. Chu et al. [21] reported that Ni/barium hexaaluminate catalyst presents high activity in partial oxidation of methane and capability to suppress carbon deposition. In a previous work we compared the activity of Ni catalysts supported on lanthanum, lanthanum/cerium and calcium hexaaluminates for steam reforming of toluene; La/Ce-containing catalysts presented higher stability during 16 h on stream, with higher resistance to coke formation [24].
The aim of this work is to study the activity of Ni catalysts supported on barium hexaaluminate in steam reforming of tar, using three different model compounds: benzene, toluene and naphthalene, evaluating how the nature of the aromatic compound affects the catalyst performance and the coking resistance.
2 Experimental
2.1 Catalyst Preparation
Barium hexaaluminate was prepared by the coprecipitation route: solutions of metal nitrates (Al and Ba, from Vetec, purity of 99.9 %) with appropriate concentrations were dropped slowly into a vessel with a 14.5 wt% NH4OH solution. The precipitation was conducted with slow agitation at room temperature for 1 h after nitrate addition, with pH control at 9–11 and aging during 16 h. Then, the samples were filtered and washed with water until pH 7, followed by drying overnight at 120 °C. The calcination was performed in two steps: at 800 °C for 4 h and at 1,000 or 1,200 °C for 4 h.
The nickel catalysts were prepared by incipient impregnation of the hexaaluminate support with a solution of nickel nitrate (Vetec) in an appropriate concentration to obtain contents of 7 or 14 wt% of NiO. After impregnation, the samples were dried at 95 °C overnight and calcined at 450 °C for 4 h. The prepared catalysts will be labeled as XNiO–BaAl, where X = 7 or 14, depending on nominal NiO loading.
2.2 Characterization of Fresh Catalysts
X-ray powder diffraction (XRD) patterns were recorded in a Rigaku Miniflex II, using Cu Kα radiation (30 kV and 15 mA) over a 2θ range from 2° to 90°, with step of 0.05° and 2 s by step.
The textural characteristics, such as BET specific area, pore volume and average pore diameter (BJH method), were determined by N2 adsorption–desorption at −196 °C in a Micromeritics ASAP 2400. Prior to the analysis the samples were pretreated at 400 °C in vacuum.
Temperature programmed reduction (TPR) was performed using a Micromeritics Autochem II. The gas used was 10 %H2 in Ar, with a flow of 40 mL min−1, and the temperature increased to 1,000 °C using a rate of 10 °C min−1. Firstly the samples were pretreated with 40 mL min−1 of Ar at 400 °C. The hydrogen consumption was monitored by thermal conductivity detector (TCD).
The hydrogen chemisorption capacity was used to obtain a measure of the specific Ni surface area, using Micromeritics ASAP 2010C, in a hydrogen pressure range from 0.002 to 260 mmHg and 35 °C. Before the experiments, the catalysts were pretreated with hydrogen flow at 500 °C.
Coking rate was determined by thermogravimetric analysis using TGA-SDTA 851 Mettler Toledo. The experiments consisted of the following steps: (1) catalyst drying under 80 mL min−1 of nitrogen, with temperature increase from 25 °C to 400 °C using a heating rate of 20 °C min−1; (2) decrease of temperature to 100 °C that was kept for 30 min; (3) gas exchange to a reducing mixture (10 %H2 in Ar), at 40 mL min−1, saturated with water at 15 °C, and temperature increase to 650 °C with a rate of 10 °C min−1 for 1 h; (4) gas exchange to a synthetic mixture of 5 %ethane, 10 %H2 and 75 %N2 (40 mL min−1), saturated with water at 15 °C, and the coking experiment was performed at 650 °C for 2 h.
2.3 Catalytic Tests
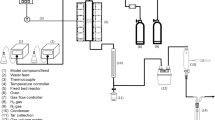
The steam reforming of tar model compounds was performed in a microactivity unit PID Eng&Tech using a fixed bed Inconel reactor (9 mm of internal diameter). It was used 300 mg of catalyst placed between quartz wool and the bed was filled with silicon carbide. The catalysts were reduced in situ at 650 °C for 2 h before reaction.
The aromatic compound and water were pumped separately, vaporized at 180 °C before entering the reactor and mixed with 50 %N2, used as a carrier. Naphthalene was used in solution with toluene (10 wt% of naphthalene). The flow rate was 100 mL min−1, with weight hourly space velocity (WHSV) of 20,000 mL g −1 cat h−1, steam/carbon (S/C) molar ratio of 1.5 and temperature of 650 or 800 °C.
All products were analyzed online by Shimadzu GC-2014 gas chromatograph with two TCDs, one for analysis of the H2 and the other for CO, CO2, and CH4, and a flame ionization detector for aromatic analysis.
Aromatic conversion can be defined by Eq. (1):
where \({\text{C}}_{\text{aromatic}}^{\text{in}}\) and \({\text{C}}_{\text{aromatic}}^{\text{out}}\) are aromatic molar flow rate of the inlet and outlet gases.
Gas product composition was calculated using Eq. (2) and benzene yield was determined by Eq. (3) and (4):
2.4 Characterization of Used Catalysts
The amount of coke deposited on the catalysts was determined by temperature programmed oxidation (TPO) using thermogravimetric balance of Mettler Toledo (TGA/SDTA 851E). The method consisted of the following steps: 1. Drying step with 80 mL min−1 of He from 25 to 150 °C with rate of 10 °C min−1, kept at 150 °C for 30 min; 2. Oxidation step with 40 mL min−1 of He and 40 mL min−1 of synthetic air from 150 to 800 °C, heating at 3 °C min−1. CO2 signal was monitored by mass spectrometer, Pfeiffer Vacuum GSD 320 T1 Thermostar, using fragment m/e− of 44.
The morphology of the carbon species present in the used catalysts was examined by scanning electron microscopy (SEM) using a JEOL JSM6490LV equipment with secondary electrons, operating with high vacuum at 20 kV and work distance of 10 nm.
3 Results and Discussion
3.1 Characterization of Fresh Catalysts
The effect of calcination temperature was investigated on the phase formation and textural characteristics of BaAl support. XRD patterns of BaAl sample after calcination at 800, 1,000 and 1,200 °C can be observed in Fig. 1. The sample calcined at 800 °C presented only peaks of θ-Al2O3 phase (JCPDS 11-517; 2θ ≅ 19°, 31°, 33°, 37°, 45° and 67°); crystalline phases of BaAl2O4 (JCPDS 17-306; 2θ ≅ 19°, 28°, 34°, 40° and 45°) and BaAl12O19 (JCPDS 26-135; 2θ ≅ 19°, 27°, 31°, 33°, 36°, 42° and 45°) appear above 1,000 °C. According to Chu et al. [21] the formation of BaAl12O19 phase is only complete after calcination at 1,200 °C. Xu et al. [18] also showed that the formation of the hexaaluminate-type crystal structure begins at 1,000–1,100 °C and completes at 1,250 °C; an intermediate phase of BaAl2O4 was reported at 1,100 °C. Machida et al. [25] showed that BaAl2O4 was first produced by solid state reaction between γ-Al2O3 and BaCO3 at 1,100 °C; BaAl12O19 phase appeared only after heating above 1,200 °C and its formation was complete at 1,450 °C.
The textural characteristics of the BaAl support at different calcination temperatures are shown in Table 1. There is a great decrease in surface area with increasing calcination temperature, in accordance with other works in the literature [21, 25, 26]. Chu et al. [21] showed a decrease in surface area from 143 to 18 m2 g−1 with increasing calcination temperature from 900 to 1,200 °C, for Ba-hexaaluminates prepared by coprecipitation with ammonium carbonate. There is also a considerable decrease in pore volume and increase in pore size with increasing calcination temperature. The calcination temperature of 1,200 °C was chosen to prepare the support to be impregnated with nickel, because of good phase formation and the surface area is still much higher than that of commercial α-Al2O3 support [20, 21]. It is important to note that after nickel impregnation, characteristic peaks of NiO were identified at 2θ ≅ 43° and 63° (JCPDS 47-1049) for both catalysts, and the textural properties did not change significantly.
TPR profiles (Fig. 2) show that both catalysts exhibited peaks in the range of 400–540 °C, associated with the reduction of NiO to Ni. This range is similar to that of pure NiO, thus the impregnation step did not cause the Ni2+ migration to the hexaaluminate structure, because in this case the profiles would show peaks at much higher temperatures, near to 1,000–1,100 °C [11, 15, 19]. The low calcination temperature used after Ni impregnation (450 °C) is responsible for this behavior, as shown previously [24]. Chu et al. [21] observed Ni incorporation into the structure of BaO·6Al2O3 support after calcination at 800 °C. The low reduction temperature is a great advantage of these catalysts for industrial applications.
The presence of two reduction peaks in the low temperature range indicates that NiOx species have different interaction degrees with the support. According to Scheffer et al. [27], nickel species with reduction at temperatures up to 400 °C are associated with bulk NiO, while those with reduction temperature between 400 and 750 °C are interacting with the support. The small peak at about 760 °C may be related to the presence of nickel aluminate formed by stronger interaction between nickel and support [21].
Typical coking curves are presented in Fig. 3. It is possible to distinguish three different periods: loss of water, NiO reduction, and a mass increase due to coke formation. Chemisorption results and coking rates calculated from TGA experiments are shown in Table 2. The normalized coking rate was calculated from the linear period of increasing mass with time, which is related to deposition of filamentous coke (whiskers) [28–30]. The nickel dispersion decreased with increasing nickel content, as expected. 7NiO–BaAl catalyst, with higher nickel dispersion, presented greater resistance to deposition of filamentous coke [31–33]. Lercher et al. [32] suggested that the rate of whisker carbon formation is proportional to the particle size of Ni and below a critical Ni particle size formation of carbon slowed down dramatically. The critical size of Ni particle to prevent the formation of carbon whisker is about 10 nm [34]. The coke formation is favored by increasing the Ni particle size, which explains the higher coking rate for 14NiO–BaAl catalyst.
3.2 Catalytic Tests
Blank tests with each tar model compound, where the reactor was filled with only silicon carbide, was presented in a previous paper [9]. Figure 4 shows the results of benzene conversion at 650 °C, S/C = 1.5 and WHSV = 20,000 mL g −1 cat h−1. These conditions of temperature and S/C ratio were chosen because they favor the rapid catalyst deactivation according to some studies in the literature, which pointed out that carbon formation is evidenced at temperatures below 650–750 °C and S/C lower than 2.0 [35–37].
From Fig. 4 it can be observed that 7NiO–BaAl presented higher initial benzene conversion than 14NiO–BaAl, but after 12 h on stream both catalysts have approximately the same conversion (~73 %); 14NiO–BaAl catalyst showed a higher stability with time on stream. The catalysts here presented higher benzene conversion than 15 %Ni/Al2O3 reported by Park et al. [38]: the initial conversion of 82 % decreased to 50 % after 5 h at 700 °C, using S/C = 9. The deactivation of 7NiO–BaAl catalyst was similar to that of Ni-catalyst derived from hydrotalcite-like compound, containing 20 wt% of NiO [9]. The benzene conversion in the blank test at this temperature was less than 2 %.
The product composition for 14NiO–BaAl is approximately constant during the whole test: 42 % CO2, 29 % CO, 22 % H2, and 7 % CH4. At this temperature the equilibrium composition is 10 % CO2, 25 % CO, 60 % H2, and 5 % CH4, calculated using PRO-II with a Gibbs reactor and SRK (Soave–Redlich–Kwong) thermodynamic model. Thus, the reactions were conducted outside the thermodynamic regime. It is known that many parallel and consecutive reactions (Eqs. 5–8) can take place during benzene steam reforming and the product distribution is a result of the competition among them [4, 13, 39, 40]. The H2/CO2 ratio of about 0.5 (instead of 2.5 considering the reactions 5 and 6) may be showing a great contribution of H2 consuming by hydrocracking and/or CO2 formation by CO disproportionation.
Steam reforming:
Water–gas shift:
Dry reforming:
Hydrocracking:
Methane steam reforming:
CO disproportionation:
The steam reforming of toluene was also conducted at 650 °C and the results are displayed in Fig. 5. In this case, although the initial conversion for both catalysts was virtually the same (78 %), the 7NiO–BaAl catalyst remained more stable with time on stream, with 73 % of conversion after 16 h, against 64 % of 14NiO–BaAl. The toluene conversions were very close to benzene conversions (Fig. 4), as observed by Mermelstein et al. [6]. Mukai et al. [41] obtained a maximum of 58.2 % of toluene conversion at 600 °C after 3 h of toluene steam reforming, using S/C = 2, over Ni/La0.7Sr0.3AlO3 catalyst. These Ba-hexaaluminate catalysts presented a similar performance to the Ni catalysts supported on La/Ce hexaaluminate, as shown in the previous paper [24], under exactly the same reaction conditions. However, these catalysts were much less active than the Ni catalysts prepared from hydrotalcite precursors [9]. The toluene conversion in the blank test at this temperature was also less than 2 %.
Considering the product distribution, it is similar for both catalysts: approximately 48 % CO2, 23 % CO, 21 % H2, and 8 % CH4. The equilibrium composition is the same as that for benzene reforming. The benzene yield was less than 4 % during the whole test and this can be associated to toluene hydrodealkylation (Eq. 11). The low formation of H2 can be related to its consumption by this reaction, and benzene can also be reformed, as shown in Eq. (5), explaining its low yield.
Hydrodealkylation:
For steam reforming of naphthalene/toluene, the stability test was performed at 800 °C (Fig. 6) because at 650 °C there was almost no conversion. At 800 °C the blank conversions were 15 % for toluene and 30 % for naphthalene [9]. Thus, at this temperature the thermal cracking of naphthalene is favored over toluene. The results shown in Fig. 6 indicate that toluene and naphthalene conversions were very close to each other, and both catalysts presented the same behavior. Adding 10 % of naphthalene to toluene caused a marked reduction in toluene conversion. Naphthalene is strongly adsorbed on the catalyst surface, thereby decreasing the toluene conversion, as already observed by Jess [42]. The mean naphthalene conversion was the same as in the blank test; thus, the conversion is basically due to thermal cracking. It was expected that toluene conversions were higher than naphthalene ones; according to Coll et al. [8] naphthalene is the most difficult compound to steam reform. For Ni catalysts derived from hydrotalcite-like compounds, toluene conversions were slightly higher than naphthalene conversions [9], under the same conditions used here.
In relation to product distribution, CO2 continued to be the main product, but the H2 formation was very low (<3 %) with a great increase in CH4 composition (~23 %). Benzene yield was about 4–5 %. Thus, the presence of naphthalene together with toluene significantly altered the reaction routes, favoring hydrocracking and hydrodealkylation reactions. Yue et al. [43], studying Ni/MgO–Al2O3 catalysts in steam reforming of naphthalene dissolved in toluene, observed that both toluene and naphthalene were completely converted to CO and CH4 at 800 °C and S/C = 0.42, but the feed stream contained 91.7 % of H2, which favors hydrodealkylation reactions.
Initial reaction rates, considering the metallic areas displayed in Table 2, were calculated for all three model compounds (Table 3), showing that 14NiO–BaAl catalyst presented initial reaction rates higher than 7NiO–BaAl catalyst; however, the stabilities on stream were comparable.
3.3 Characterization of Used Catalysts
In general, 7NiO–BaAl and 14NiO–BaAl catalysts presented similar results in steam reforming of tar model compounds; thus, coke evaluation was only performed with 7NiO–BaAl, because it has lower tendency to coke formation, as shown in Table 2.
The amount of coke deposited on used 7NiO–BaAl catalysts was verified by TPO. The CO2 formation profiles from TPO experiments are shown in Fig. 7. All profiles presented a characteristic peak around 600–630 °C, which is usually associated with oxidation of filamentous carbon [36, 44, 45]. The amount of coke obtained from TPO followed the order: benzene (63 wt%) > toluene (57 wt%) ≫ naphthalene (37 wt%). The high amount of carbon formation without significant deactivation with time on stream also suggests the preferential formation of filamentous carbon. It is well known that the accumulation of carbon whiskers can block the catalyst bed and increase pressure drop to unacceptable levels [28, 31]. These effects probably appear only after many hours of reaction, and they were not observed in the present case. The amount of coke formed in steam reforming of toluene on Ba-hexaaluminate catalyst was higher than that formed in other hexaaluminate-supported catalysts [24].
The amount of coke deposition during naphthalene/toluene reforming was much smaller than in the case of benzene and toluene reforming, in contrast with the results of Coll et al. [8], which showed that the tendency towards coke formation increased with the molecular weight of the aromatic molecule. These authors determined the minimum S/C ratio to avoid carbon formation: 2.5 for toluene reforming at 725 °C and 3.7 for naphthalene reforming at 795 °C. The operating conditions in our study are much more severe than those determined by Coll et al. [8].
Figure 8 shows the coke morphology for 7NiO–BaAl catalyst used on toluene and naphthalene/toluene reforming. After reforming of toluene (and also benzene, not shown) there was formation of a mixture of filamentous and structured coke, this last one probably has aromatic characteristic [46]. Using naphthalene/toluene a totally different coke was obtained; probably thermal cracking was favored in the used reaction conditions, leading to polyaromatic compounds such as binaphthalenes and fluoranthenes that can condensate and originate coke [47]. However, the formation of this type of coke did not cause any significant deactivation during 16 h of reaction.
4 Conclusions
Ba-hexaaluminate was prepared by coprecipitation route and used as support for nickel catalysts in steam reforming of tar model compounds. Hexaaluminate support consists in a mixture of crystalline phases with high specific surface area, when considering the high calcination temperature (1,200 °C). The nickel catalysts can be reduced at low temperatures, below 540 °C, which is a great advantage in industrial applications. The Ni loading (7 and 14 wt%) does not have a great influence in the catalyst stability; however, despite the higher metallic dispersion of the catalyst with lower Ni loading, 7NiO–BaAl presented lower initial reaction rates. Naphthalene conversion is much more difficult than benzene or toluene conversions, requiring high temperatures, and inhibiting toluene reforming. The product distribution pointed that H2 formation is lower than that predict by thermodynamic, with CO2 and CO as the main products. It was observed an extensive carbon formation in all reactions, but the amount of coke was higher for benzene and toluene reforming. Despite the high amount of coke deposit, the catalysts presented almost no deactivation with time on stream up to 16 h. The morphology of coke is dependent on the nature of the aromatic compound.
References
McKendry P (2002) Biores Technol 83:37
Han J, Kim H (2008) Renew Sust Energy Rev 12:397
El-Rub ZA, Bramer EA, Brem G (2004) Ind Eng Chem Res 43:6911
Torres W, Pansare SS, Goodwin JG Jr (2007) Catal Rev 49:407
Milne TA, Evans, RJ (1998) Biomass gasifier “Tars”: Their nature, formation, and conversion, National Renewable Energy Laboratory, NREL/TP-570-25357
Mermelstein J, Millan M, Brandon NP (2009) Chem Eng Sci 64:492
Jess A (1996) Fuel 75:1441
Coll R, Salvadó J, Farriol X, Montané D (2001) Fuel Process Technol 74:19
Josuinkas FM, Quitete CPB, Ribeiro NFP, Souza MMVM (2014) Fuel Process Technol 121:76
Rezaiyan J, Cheremisinoff NP (2005) Gasification Technologies—A primer for engineers and scientists. CRC Press, Boca Raton
Sutton D, Kelleher B, Ross JRH (2001) Fuel Process Technol 73:155
Yung MM, Jablonski WS, Magrini-Bair KA (2009) Energy Fuels 23:1874
Xu C, Donald J, Byambajav E, Ohtsuka Y (2010) Fuel 89:1784
Machida M, Teshima T, Eguchi K, Arai H (1991) Chem Lett 134:231
Artizzu-Duart P, Millet JM, Guilhaume N, Garbowskia E, Primeta M (2000) Catal Today 59:163
Machida M, Eguchi K, Arai H (1990) J Catal 123:477
Xu Z, Zhen M, Bi Y, Zhen K (2000) Appl Catal A 198:267
Xu Z, Zhen M, Bi Y, Zhen K (2000) Catal Lett 64:157
Gardner TH, Spivey JJ, Kugler EL, Pakhare D (2013) Appl Catal A 455:129
Majocchi L, Groppi G, Cristiani C, Forzatti P, Basini L, Guarinoni A (2000) Catal Lett 65:49
Chu W, Yang W, Lin L (2001) Catal Lett 74:139
McGuire NE, Sullivan NP, Keea RJ, Zhu H, Nabity JA, Engel JR, Wickham DT, Kaufman MJ (2009) Chem Eng Sci 64:5231
Li D, Ishikawa C, Koike M, Wang L, Nakagawa Y, Tomishige K (2013) Int J Hydrogen Energy 38:3572
Quitete CPB, Bittencourt RCP, Souza MMVM (2014) Appl Catal A 478:234
Machida M, Eguchi K, Arai H (1987) J Catal 103:385
Groppi G, Cristiani C, Forzatti P (2001) Appl Catal B 35:137
Scheffer B, Molhoek P, Moulijn JA (1989) Appl Catal 46:11
Trimm DL (1999) Catal Today 49:3
Rostrup-Nielsen JR, Sehested J, Norskov JK (2002) Adv Catal 47:65
Borowieck T, Denis A, Grzegorczuk W, Gloebiowski A (2002) React Kinet Catal Lett 77:163
Rostrup-Nielsen JR (1984) J Catal 85:31
Lercher JA, Bitter JH, Hally W, Niessen W, Seshan K (1996) Studies Surf Sci Catal 101:463
Christensen KO, Chen D, Lodeng R, Holmen A (2006) Appl Catal A 314:9
Xu S, Zhao R, Wang X (2004) Fuel Process Technol 86:123
Simell P, Kurkela E, Stahlberg P, Hepola J (1996) Catal Today 27:55
Swierczynski D, Courson C, Kiennemann A (2008) Chem Eng Process 47:508
Zhao B, Zhang X, Chen L, Qu R, Meng G, Yi X, Sun L (2010) Biomass Bioenergy 34:140
Park HJ, Park SH, Sohn JM, Park J, Jeon J, Kim S, Park Y (2010) Bioresour Technol 101:S101
Li C, Hirabayashi D, Suzuki K (2009) Fuel Process Technol 90:790
Rönkkönen H, Simell P, Reinikainen M, Krause O, Niemelä MV (2010) Fuel 89:3272
Mukai D, Tochiya S, Murai Y, Imori M, Hashimoto T, Sugiura Y, Sekine Y (2013) Appl Catal A 453:60
Jess A (1996) Chem Eng Process 35:487
Yue B, Wang X, Ai X, Yang J, Li L, Lu X, Ding W (2010) Fuel Process Technol 91:1098
Demicheli MC, Duprez D, Barbier J, Ferretti OA, Ponzi EN (1994) J Catal 145:437
Lisboa JS, Santos DCRM, Passos FB, Noronha FB (2005) Catal Today 101:15
Jackson SD, Thomson SJ, Webb G (1981) J Catal 70:249
Devi L, Ptasinski KJ, Janssen FJJG (2005) Fuel Process Technol 86:707
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quitete, C.P.B., Bittencourt, R.C.P. & Souza, M.M.V.M. Steam Reforming of Tar Model Compounds Over Nickel Catalysts Supported on Barium Hexaaluminate. Catal Lett 145, 541–548 (2015). https://doi.org/10.1007/s10562-014-1405-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1405-3