Abstract
Several acidic ionic liquids (ILs) with different cations and anions were used as catalysts in the transesterification of methyl acetate with n-butanol. The IL [omim][PF6] can enhance the catalytic activity of the partially soluble IL catalyst. The kinetics for the transesterification using the catalysts [HSO3bmim][p-TS] and [HSO3bmim][OTF] were measured. The ideal homogeneous model and the nonideal homogeneous (NIH) model were used to correlate the kinetic data. The NIH model was more reliable to describe the reaction rate.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Transesterification, as a method to produce useful esters via the catalytic reaction of aliphatic esters and alcohols, has been widely used in chemical industry [1–3]. An important application of transesterification is to transform methyl acetate with n-butanol to n-butyl acetate and methanol [4, 5]. Methyl acetate is a by-product in poly(vinyl alcohol) (PVA) process and its application in chemical industry is limited, but butyl acetate is a widely used chemical for manufacturing paint, varnish and coating materials [6], and methanol is a feed stock for PVA, so the transesterification process is a good choice to deal with methyl acetate. Transesterification is a catalytic reaction, and the commonly used catalysts are solid heterogeneous catalysts such as acidic ion-exchange resins [7, 8], heteropolyacids [9] and molecular sieves [10]. Nevertheless, these catalysts have some drawbacks, such as easy deactivation, high mass transfer resistance and especially difficult installation in a reactive distillation column, which restrain their practical applications [11].
To overcome the above problems, many researchers began to use ionic liquids (ILs) as catalysts because of their advantages over the traditional catalysts, such as negligible vapor pressure, wide liquid range, high catalytic activity and thermal stability [12, 13]. For example, comparing with acidic ion-exchange resin, the IL possesses high thermal stability and catalytic activity. Moreover, the IL is also easy to be used and has no mass transfer problems. So far, ILs have been successfully utilized in laboratory for some transesterification reactions [14–16]. For example, Qureshi et al. have used a halogen free Bronsted acidic IL N-methyl-2-pyrrolidone hydrogen sulfate as catalyst for the transesterification of β-ketoesters with alcohols and found the IL shows high catalytic activity and reusability with high yields of the desired products [17]. Kuschnerow et al. have carried out a continuous IL-catalyzed transesterification of butanol with ethyl acetate to produce ethanol and butyl acetate and they found the ILs did not show a relevant loss of activity after 1000 h of reaction [18]. Peng et al. have catalyzed the transesterification of methyl acetate and ethanol by 4-(3-methyl-1-imidazolio)-1-butanesulfonic acid triflate and 4-(3-methyl-1-imidazolio)-1-butanesulfonic acid hydrogen sulfate respectively and measured the reaction kinetics [19, 20]. Wang et al. have synthesized five acidic imidazolium ILs as catalysts for the preparation of sec-butanol via the transesterification of sec-butyl acetate (SBAC) with methanol and a high conversion of the SBAC of 97.72 % was obtained by the reactive distillation in the presence of 1-(3-sulfopropyl)-3-methylimidazolium hydrogen sulfate [21]. However, published information about the effects of cations or anions on the transesterification is still limited and to the best of our knowledge, there is no research work on transesterification catalyzed by mixed ILs.
To describe the reaction rate of the transesterification of methyl acetate and n-butanol, different kinetic models were applied by researchers. Bozek-Winkler et al. have used two kinetic models, namely, the pseudohomogeneous and the Langmuir–Hinshelwood model to describe the kinetic for the transesterification of methyl acetate and n-butanol catalyzed by ion-exchange resin Amberlyst 15 [22]. Jimenez et al. have also applied pseudohomogeneous model to describe the kinetic of the transesterification of methyl acetate and n-butanol using acid ion-exchange resin as catalyst [23]. But the transesterification catalyzed by ILs is different from that catalyzed by acidic ion-exchange resin, because the former is homogenous and the latter is heterogeneous. Cui et al. have proposed two kinetic models, the ideal homogeneous (IH) model and the nonideal homogeneous (NIH) model, for the transesterification of methyl acetate and n-butanol catalyzed by an acidic IL [24].
In this paper, the effects of cations ([HSO3bmim]+, [HSO3bpy]+) and anions (Cl−, [p-TS]− and [OTF]−) on the catalytic performance of ILs in the transesterification of methyl acetate with n-butanol were investigated. The effects of mixed IL catalysts on the transesterification reaction were also studied. Furthermore, the kinetics of the transesterification catalyzed by [HSO3bmim][p-TS] and [HSO3bmim][OTF] were measured and correlated by IH model and NIH model, respectively.
2 Experimental Section
2.1 Materials
2.1.1 Chemicals
n-Butanol and methanol was purchased by Kewei Chemical Reagents Co., Tianjin, China. Methyl acetate and n-butyl acetate was obtained from Guangfu Chemical Reagents Co., Tianjin, China. The purities of these chemicals were greater than 0.997 (mass fraction) as confirmed by gas chromatography and used without additional purification.
2.1.2 Catalyst
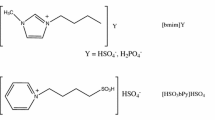
All the ILs used in the experiments including 1-sulfobutyl-3-methylimidazolium trifluoromethanesulfonate [HSO3bmim][OTF], 1-sulfobutyl-3-methylimidazolium chloride [HSO3bmim][Cl], 1-sulfobutyl-3-methylimidazolium tosylate [HSO3bmim][p-TS], N-sulfobutylpyridinium trifluoromethanesulfonate [HSO3bpy][OTF] and 1-octyl-3-methylimidazolium hexafluorophosphate [omim][PF6] were supplied by Chengjie Chemical Reagents Co., Shanghai, China, with a minimum mass fraction of 0.99 (confirmed by liquid chromatography) and the mass fraction of water in ILs was less than 0.0001 (confirmed by Karl Fischer titration). Therefore, the ILs were used without further purification.
2.2 Apparatus and Procedure
All experiments were carried out in a 100 mL round-bottom flask equipped with a reflux condenser which was utilized to avoid any loss of volatile compounds. A mechanical agitator was used to mix the solution in the flask. The flask was put in a thermostatic water bath. All the temperatures were measured by a calibrated thermometer to an accuracy of ±0.01 K.
Catalyst and n-butanol were added in the flask and heated by thermostatic water bath, while methyl acetate was preheated in a separate vessel in the same water bath. When the temperatures of methyl acetate and solution in the flask reached the desired value, methyl acetate was added to the flask, and the reaction started. Liquid samples of 1 μL were taken with a syringe at regular intervals and analyzed by gas chromatograph (GC).
For convenience in description, we set the basic reaction conditions as a catalyst concentration of 0.183 mol/L, reaction temperature 315 K, agitation speed 250 rpm and an initial molar ratio of n-butanol to methyl acetate 1:1.
2.3 Sample Analysis
All the samples were analyzed by a gas chromatograph equipped with a FID detector and a capillary column SE-30 (50 m × 0.32 mm × 0.25 μm). The temperatures of injector, detector and column were set 473.15, 493.15, 343.15 K, respectively. The flow rates of nitrogen, hydrogen and air are 30, 30, 300 mL/min, respectively. To prevent ILs from passing into the GC column, a porous ceramics trap was located in the middle section of the glass tube in injector.
2.4 UV–Vis Determination of Acidity of Ionic Liquid Catalysts
A series of ethanol solutions of 4-Nitroaniline (used as indicator) of different concentrations ranging from 0.01885 to 0.05792 mM were prepared. The absorbance of these solutions was measured by Shimadzu UV-1800 spectroscopy at 300–500 nm. Several ethanol solutions containing ILs (75 mM) and 4-Nitroaniline (0.05792 mM) were also prepared to measure the absorbance, then a Hammett function was used to calculate the acidity of these acidic ILs [25–27].
3 Results and Discussion
As all chemicals were water free, the by-products caused by side reaction hydrolysis are less than 0.01 wt % which is confirmed by gas chromatography, so the side reaction caused by hydrolysis can be neglected. Similar results were also reported in the literature [23]. Therefore, the selectivity of the products for the transesterification of methyl acetate and n-butanol is close to 1.
3.1 Acidity of ILs
Peng et al. have studied the mechanism of the transesterification of methyl acetate and ethanol catalyzed by [HSO3bmim][OTF] [19]. Cui et al. have studied the kinetics of the transesterification of methyl acetate and n-butanol with [HSO3bHim][HSO4] [24]. Rodriguez et al. have catalyzed the transesterification of methyl acetate and isobutanol using NaHSO4 [28]. Based on their work, the reaction mechanism of methyl acetate and n-butanol catalyzed by IL can be described in Fig. 1, taking IL catalyst [HSO3bmim][OTF] as example.
As shown in Fig. 1, the transesterification was catalyzed by proton produced by IL catalyst. Therefore, the ability to provide protons (acidity) is an essential property of acidic IL catalyst.
The acidity of an IL can be calculated by the Hammett function (H 0):
where pK(I)aq is the pK a value of the indicator referred to an aqueous solution (0.99 for 4-Nitroaniline); \( \left[ {IH} \right]_{S}^{ + } \) and [I]s are the molar concentrations of the protonated and unprotonated forms of the indicator in the acidic ILs.
There is a good linear relationship between the concentration of 4-Nitroaniline and absorbance (see Fig. 2), which indicates the concentration of indicator between 0.01885 and 0.05792 mM follows Beer–Lambert Law. The maximal absorbance of the unprotonated form of 4-Nitroaniline is observed at 375 nm and the results of Hammett acidity of acidic ILs were listed in Table 1. The acidity order is as follows: [HSO3bHim][HSO4] > [HSO3bmim][p-TS] > [HSO3bmim][OTF] > [HSO3bpy][OTF] > [HSO3bmim][Cl]. It is obvious that the cations and anions of ILs have significant influences on their acidity which is an important factor of catalytic performance on the transesterification reaction.
3.2 Effects of Single Ionic Liquid Catalyst Structure on Transesterification
The transesterification of n-butanol and methyl acetate with several IL catalysts were conducted to investigate the effects of IL catalyst structure. The catalysts we used are all well dissolved in the reaction mixture at basic reaction conditions except [HSO3bmim][Cl]. The results are illustrated in Fig. 3. Cui et al. has used [HSO3bHim][HSO4] to catalyze the transesterification [24] and the results are also shown in Fig. 3 for comparison. It is obvious that the reaction rates are in an increasing order of [HSO3bHim][HSO4] > [HSO3bmim][p-TS] > [HSO3bmim][OTF] ≈ [HSO3bpy][OTF] > [HSO3bmim][Cl], which is generally in line with the acidity order described above. The catalyst activity of [HSO3bmim][Cl] is the smallest, because its acidity is the lowest and it is partially dissolved in the reaction mixture. The ILs [HSO3bmim][p-TS], [HSO3bmim][OTF] and [HSO3bmim][Cl] have the same cation but different anions, and their acidity and catalyst activity are in the same order, so in such a case the acidity of acidic IL is affected by different anions, which results in different catalytic activities. However, when we compare the imidazolium ring-based IL [HSO3bmim][OTF] and pyridinium ring-based IL [HSO3bpy][OTF], their catalytic activity are nearly the same though the acidity of [HSO3bmim][OTF] is stronger than that of [HSO3bpy][OTF]. In such a case, the acidity is influenced by different heterocyclic rings in cations, but the catalyst activity is not affected by them. Therefore, the acidity characterized by Hammett function (H 0) is a rough indicator for catalytic activity of the IL catalyst.
3.3 Transesterification Catalyzed by Mixed Ionic Liquids
As mentioned above, The IL [HSO3bmim][Cl] cannot be well dissolved in the reaction mixture at basic reaction conditions, so we use a hydrophobic IL [omim][PF6] to enhance its solubility in the reaction mixture. The IL [omim][PF6] has no catalytic activity, because it cannot provide protons to catalyze the transesterification (as shown in Fig. 1). This is also confirmed by reaction tests. The IL [omim][PF6] can be well dissolved in both esters and [HSO3bmim][Cl], so it was mixed with [HSO3bmim][Cl] and added to the reaction system to enhance the solubility of [HSO3bmim][Cl]. To investigate the behavior of mixed IL catalysts, experiments were also carried out using the mixed ILs of [HSO3bmim][p-TS] + [omim][PF6] at basic reaction conditions. The molar ratios of [omim][PF6] to IL catalysts are varied from 1:1 to 3:1 and the experimental results are shown in Figs. 4 and 5. Figure 4 indicates that the reaction rate with a molar ratio of [omim][PF6] to [HSO3bmim][Cl] 1:1 is almost the same as that of without [omim][PF6]. However, the reaction rate increases obviously with the molar ratio of [omim][PF6] to [HSO3bmim][Cl] 3:1. The IL [omim][PF6] has two opposite effects: it can enhance the solubility of IL catalyst in reaction system and it can also dilute the IL catalyst. When a small amount of [omim][PF6] (molar ratio of [omim][PF6] to [HSO3bmim][Cl] 1:1) is added, only a small amount of extra catalyst [HSO3bmim][Cl] dissolves further in the reaction mixture and it is also diluted by [omim][PF6] meanwhile. The two effects are both not obvious, so the reaction rate is nearly unchanged. When a large amount of [omim][PF6] is added (molar ratio of [omim][PF6] to [HSO3bmim][Cl] 3:1), all of the catalyst [HSO3bmim][Cl] dissolves in the reaction mixture, and the extra dissolved catalyst plays the main role even though it is diluted by [omim][PF6], so the reaction rate increases. Therefore, the solubility of IL catalyst in the reaction mixture is another important factor that determines the catalytic activity except acidity for the partial dissolved catalyst. However, when we carried out the transesterification with [HSO3bmim][p-TS] + [omim][PF6] as catalyst, the reaction rate decreases with the addition of [omim][PF6] (see Fig. 5). Since catalyst [HSO3bmim][p-TS] is totally dissolved in the reaction system, when [omim][PF6] is added, the catalyst is diluted by [omim][PF6], and the reaction rate slows down.
Therefore, if a Bronsted acidic IL catalyst such as [HSO3bmim][CL] is partially dissolved in the transesterification reaction system, its solubility can be enhanced by another IL such as [omim][PF6], and the reaction rate can be improved. However, for a totally dissolved Bronsted acidic IL catalyst ([HSO3bmim][p-TS]), the reaction rate cannot be enhanced by the mixed catalyst ([HSO3bmim][p-TS] + [omim][PF6]).
In summary, among all the catalysts discussed above, the strong acidic IL [HSO3bHim][HSO4] has the best performance for the transesterification reaction, but the study of IL mixture provides a method of improving the performance of those catalysts such as [HSO3bmim][Cl] that cannot be well dissolved in reaction mixture.
3.4 Reaction Kinetics
In this section, the reaction temperatures were in a range of 310.15–325.15 K, the initial molar ratios of n-butanol to methyl acetate were 0.5–2 and the catalyst concentrations were 0.122 mol/L to 0.305 mol/L. Since the catalyst [HSO3bmim][Cl] cannot be well dissolved in the reaction mixture, it is not utilized in this section, and the catalysts used are [HSO3bmim][OTF] and [HSO3bmim][p-TS]. When we studied the effects of the operational parameters, only one parameter was varied and the other reaction conditions were the same as those of basic reaction conditions.
3.4.1 Effects of the Initial Reactant Molar Ratio
For convenience to investigate the effects of initial molar ratio of n-butanol to methyl acetate (\( R_{B/M}^{0} \)), we set the methanol concentration as the objective function. Because the transesterification is a chemical equilibrium reaction, it is obvious that the conversion of methyl acetate increases with \( R_{B/M}^{0} \), but the concentration of product such as methanol in the reaction mixture varies differently. As shown in Fig. 6a, the biggest methanol concentration is obtained at \( R_{B/M}^{0} \) = 1 for both of the catalysts, because \( R_{B/M}^{0} \) in such cases is equal to the ratio of stoichiometric coefficients of corresponding reactants. When the initial reactant molar ratio \( R_{B/M}^{0} \) is 0.5 and 2, the mole fractions of methanol in the reaction mixture are equal, but the mole density of the reaction mixture of \( R_{B/M}^{0} \) = 2 is less than that of \( R_{B/M}^{0} \) = 0.5, because the density of n-butanol is less than methyl acetate. Therefore, methanol concentration of \( R_{B/M}^{0} \) = 2 is less than that of \( R_{B/M}^{0} \) = 0.5.
3.4.2 Effects of Reaction Temperature
Experiments using [HSO3bmim][OTF] and [HSO3bmim][p-TS] as catalysts were carried out to investigate the effects of reaction temperature. Figure 6b shows obviously that for both of the two catalysts, reaction rates increase rapidly with reaction temperature. However, the equilibrium conversions of methyl acetate only change a little in the investigated temperature range. Similar results have been reported in the literature [29, 30]. The reason is that the heat of reaction for this transesterification reaction is small, the equilibrium constant depends only slightly on temperature.
3.4.3 Effects of Catalyst Concentration
Catalyst concentration is an important factor that affects the reaction rate. Figure 6c illustrates that the transesterification reaction rate increases with the catalyst concentration for both of the catalysts, but for the chemical equilibrium constant, the same value was obtained. In addition, the initial reaction rates in the presence of [HSO3bmim][OTF] and [HSO3bmim][p-TS] were calculated at the starting point of the reaction and proposed to be a linear function of the catalyst loading (see Fig. 7). It can be expressed as:
where r 0 is the initial rate of transesterification reaction, mol/(L min); v i is the stoichiometric coefficient of component i; c i is the mole concentration of component i, mol/L; c cat is the mole concentration of catalyst, mol/L; E is constant.
The constant E is obtained from the slope of the best fitted line in Fig. 7, and the values are 0.165 min−1 for [HSO3bmim][OTF] and 0.185 min−1 for [HSO3bmim][p-TS], respectively.
3.4.4 Kinetic Model
Peng et al. has studied the kinetics of the transesterification of methyl acetate and ethanol catalyzed by [HSO3bmim][OTF] and used the IH model and NIH model to describe the reaction rate [19]. The mechanisms of the two transesterification reactions are similar. Therefore, the expressions for IH model and NIH model are also used in this work:
where V is volume of the reaction mixture, L; n i is moles of component i, mol; ρ is the molar density which can be considered as constant during the reaction process, mol/L; αi(= γ i x i) is the activity of component i, while γ i and x i are the activity coefficient and molar fractions respectively. The activity coefficient γ i is calculated by the UNIQUAC model and the parameters needed are obtained from the literature reported [22, 24]. k + and k - are forward and backward reaction rate constants respectively, which can be calculated by Arrhenius equations:
where \( k_{ + }^{0} \) and \( k_{ - }^{0} \) are pre-exponential factors and E A+ and E A- are activation energies.
The kinetic parameters were estimated by minimizing the objective function of mean relative deviation (MRD) between the calculated and experimental mole fractions of methyl acetate, using all of the experimental data in the above sections.
where N is the total number of data points.
The correlated results were summarized in Table 2. It is clear that the mean relative deviations of the IH model are larger than those of the NIH model, which indicates NIH model is more accurately than IH model. Bozek-Winkler et al. [22] have used pseudohomogeneous model to describe the reaction kinetics for the transesterification of methyl acetate and n-butanol catalyzed by ion-exchange resin and the active energies E A+, E A− are 40.89 and 40.85 kJ/mol. Cui et al. [24] have applied a NIH model to describe the same reaction catalyzed by an acidic IL and the active energies E A+, E A− are 36.26 and 36.02 kJ/mol. The active energies we correlated in Table 2 are very similar, which indicates that the transesterification is catalyzed by H+ whether the catalyst is acidic ion-exchange resin or acidic IL. Therefore, the catalytic activities are mainly indicated by the pre-exponential factors of forward and backward reactions when the active energies are quite similar. So the relationship between the measured acidity and pre-exponential factors of forward and backward reactions can be applied to predict the reaction kinetics if the acidity of the catalyst is determined. As shown in Fig. 8a, b, the relationship can be fitted by quadratic functions, and the correlated equations are, respectively, \( k_{ + }^{0} = - 428{,}338 { }H_{0}^{2} + 1042{,}130H_{0} - 620{,}157 \) and \( k_{ - }^{0} = -757{,}336 { }H_{0}^{ 2} + 1815{,}940H_{0} - 1072{,}650 \).
Substitution of the values of kinetic parameters shown in Table 2 into NIH model gives the reaction rate equation:
For catalyst [HSO3bmim][OTF]:
For catalyst [HSO3bmim][p-TS]:
The conversion of methyl acetate and the concentration of methanol in the reaction mixture can be calculated by the equations:
The calculated results were plotted in Fig. 6a–c. It is obvious to find that the calculated results agree well with the experimental results, which indicates the NIH model can reliably describe the kinetics of transesterification of n-butanol and methyl acetate catalyzed by [HSO3bmim][OTF] or [HSO3bmim][p-TS].
4 Conclusions
Several acidic ILs with different cations or anions were used as catalysts for the transesterification of methyl acetate with n-butanol and the results show that the catalytic activities of these IL catalysts differ greatly. The acidity of ILs is influenced by cations and anions. The acidity of acidic ILs with same catalytic cation is affected by different anions, which results in different catalytic activities of the IL catalysts. The acidity of acidic ILs with same anion and different catalytic cations (containing imidazolium or pyridinium ring) is affected by different heterocyclic rings in cations, but the catalyst activities are hardly influenced by the heterocyclic rings. The performance of partially soluble catalyst [HSO3bmim][CL] can be enhanced by [omim][PF6] in mixed ILs. The kinetic behavior of the transesterification catalyzed by [HSO3bmim][OTF] and [HSO3bmim][p-TS] was studied experimentally. Two different kinetic models, IH and NIH, were applied to correlate the reaction kinetics. The NIH model was found to describe the reaction rate reliably and the correlated results agree well with those of experimental.
Abbreviations
- IL:
-
Ionic liquid
- MRD:
-
Mean relative deviation
- BuOH:
-
n-Butanol
- MeOAc:
-
Methyl acetate
- [HSO3bmim][Cl]:
-
1-Sulfobutyl-3-methylimidazolium chloride
- [HSO3bmim][OTF]:
-
1-Sulfobutyl-3-methylimidazolium trifluoromethanesulfonate
- [HSO3bmim][p-TS]:
-
1-Sulfobutyl -3-methylimidazolium tosylate
- [HSO3bpy][OTF]:
-
N-sulfobutylpyridinium trifluoromethanesulfonate
- [HSO3bHim][HSO4]:
-
1-Sulfobutylimidazolium hydrogensulfate
- [omim][PF6]:
-
1-Octyl-3-methylimidazolium hexafluorophosphate
- r 0 :
-
Initial reaction rate (mol/(L min))
- r :
-
Reaction rate (mol/(L min))
- c :
-
Mole concentration (mol/L)
- E :
-
Constant defined in Eq 2
- c cat :
-
Mole concentration of ionic liquid catalysts (mol/L)
- k + :
-
Forward reaction rate constant (L2/(min mol2))
- k - :
-
Backward reaction rate constant (L2/(min mol2))
- \( k_{ + }^{0} \) :
-
Arrhenius pre-exponential factors (L2/(min mol2))
- \( k_{ - }^{0} \) :
-
Arrhenius pre-exponential factors (L2/(min mol2))
- E A+ :
-
Activation energy of forward reaction (kJ/mol)
- E A− :
-
Activation energy of backward reaction (kJ/mol)
- V :
-
Volume of the reaction mixture (L)
- R :
-
General gas constant, 8.314 kJ/(mol K)
- N :
-
Total number of experimental data
- v :
-
Stoichiometric coefficient
- x :
-
Mole fraction
- ρ:
-
Molar density of reaction mixture (mol/L)
- t :
-
Time (min)
- α:
-
Activity
- γ:
-
Activity coefficient
- 0:
-
Standard state/conditions
- +:
-
Forward reaction
- −:
-
Backward reaction
- cal :
-
Catalyst
- calc :
-
Calculated value
- exp :
-
Experimental value
References
Grasa GA, Guveli T, Singh R, Nolan SP (2003) J Org Chem 68:2812–2819
Kim HJ, Kang BS, Kim MJ, Park YM, Kim DK, Lee JS, Lee KY (2004) Catal Today 93–95:315–320
Pan FY, Wang SP, Ma XB, Li ZH, Xu GH (2004) Chem Ind Eng 21:174–176
Luyben WL (2010) Ind Eng Chem Res 50:1247–1263
Steinigeweg S, Gmehling J (2004) Chem Eng Process 43:447–456
Wang QQ, Wu XL (2011) Sichuan Huagong 14:15–17
Jimenez L, Garvin A, Costa-Lopez J (2002) Ind Eng Chem Res 41:6735–6744
Sert E, Atalay FS (2012) Ind Eng Chem Res 51:6350–6355
Sharma YC, Singh B, Korstad J (2011) Biofuels Bioprod Biorefin 5:69–92
Palani A, Gokulakrishnan N, Palanichamy M, Pandurangan A (2006) Appl Catal A Gen 304:152–158
Tao DJ, Lu XM, Lu JF, Huang K, Zhou Z, Wu YT (2011) Chem Eng J 171:1333–1339
Welton T (1999) Chem Rev 99:2071–2083
Hallett JP, Welton T (2011) Chem Rev 111:3508–3576
Domingos JB, Dupont J (2007) Catal Commun 8:1383–1385
Kotadia DA, Soni SS (2013) Monatsh Chem 144:1735–1741
de los Rios AP, Hernandez-Fernandez FJ, Rubio M, Gomez D, Villora G (2010) Desalination 250:101–104
Qureshi ZS, Deshmukh KM, Bhor MD, Bhanage BM (2009) Catal Commun 10:833–837
Kuschnerow JC, Titze-Frech K, Schulz PS, Wasserscheid P, Scholl S (2013) Chem Eng Technol 36:1643–1650
Peng YM, Cui XB, Zhang Y, Feng TY, Tian Z, Xue LX (2013) Appl Catal A Gen 466:131–136
Peng YM, Cui XB, Zhang Y, Feng TY, Tian Z, Xue LX (2014) Int J Chem Kinet 46:116–125
Wang HX, Wu CM, Bu XW, Tang WL, Li L, Qiu T (2014) Chem Eng J 246:366–372
Bozek-Winkler E, Gmehling J (2006) Ind Eng Chem Res 45:6648–6654
Jimenez L, Garvin A, Costa-Lopez J (2002) Ind Eng Chem Res 41:6663–6669
Cui XB, Cai JL, Zhang Y, Li R, Feng TY (2011) Ind Eng Chem Res 50:11521–11527
Thornazeau C, Olivier-Bourbigou H, Magna L, Luts S, Gilbert B (2003) J Am Chem Soc 125:5264–5265
Zhao YW, Long JX, Deng FG, Liu XF, Li Z, Xia CG, Peng JJ (2009) Catal Commun 10:7323–7736
Elavarasan P, Kishore KVK, Upadhyayuala S (2009) Bull Catal Soc India 8:107–113
Rodriguez AV, Plesu V, Tarragona AC, Ruiz JB, Ruiz AEB, Llacuna JL (2013) Chem Eng Trans 35:1093–1098
Sanz MT, Murga R, Beltran S, Cabezas JL, Coca J (2002) Ind Eng Chem Res 41:512–517
Choi JL, Hong WH, Chang HN (1996) Int J Chem Kinet 28:37–41
Acknowledgments
Financial support from the Innovation Fund of Tianjin University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Z., Cui, X., Yu, X. et al. Transesterification of Methyl Acetate with n-Butanol Catalyzed by Single and Mixed Ionic Liquids. Catal Lett 145, 1281–1289 (2015). https://doi.org/10.1007/s10562-015-1506-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1506-7