Abstract
Cryopreservation exposes sperm to physical and chemical stresses causing cell damages and impairs sperm functions. The aim of this study was to evaluate the association between motility and sperm chromatin/DNA damage before and after cryopreservation and investigate the effects of folic acid and nicotinic acid on post-thaw sperm quality. Thirty semen samples were obtained from 30 normozoospermic men, aged between 25 and 45 years old. Each sample were divided into five aliquots to form the following groups: fresh, cryopreserved with sperm-freeze only (control), with nicotinic acid (10 mM), with folic acid (50 nM), and with a combination of folic acid (50 nM) + nicotinic acid (10 mM). Sperm viability and motility in each group were assessed by eosin-nigrosine staining and computer-aided sperm analysis respectively. Sperm chromatin quality was studied by aniline blue, toluidine blue, acridine orange staining methods and sperm chromatin dispersion test. Cryopreservation led to a significant reduction in sperm quality in comparison to fresh sample groups (p < 0.05). Sperm chromatin damage was negatively correlated with the percentage of progressively motile cells. Supplementation of the cryopreservation medium with folic acid or nicotinic acid induced a significant improvement in sperm parameters and chromatin quality, compared to control groups (p < 0.05). Meanwhile, the combination of folic acid + nicotinic acid showed a significant protective effect in post thaw sperm. In conclusion, cryopreservation generated oxidative stress, inducingsperm cryodamage, reducing progressive motility and sperm quality, as an indicator of significant chromatin/DNA damage. Folic acid and nicotinic acid exhibited a potential cryoprotective effect by enhancing sperm quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sperm freezing is a widely used method in human assisted reproductive technology (ART) setup for keeping sperm cells from being damaged or destroyed by time and chemical reactions and stored safely for a long time (Oberoi et al. 2014; Oehninger et al. 2000; Verza et al. 2009).
In spite, of many benefits of cryopreservation, this procedure causes cell damages, including changes in the composition of membrane lipid, acrosome’s condition, motility, survival rate and increase DNA damage (Amidi et al. 2016; Bateni et al. 2014; Gadea et al. 2011; Najafi et al. 2016). The levels of correlation between sperm chromatin structure and motility status range from nonsignificant up to − 0.53 (Giwercman et al. 2003). The studies reported that sperm parameters and chromatin/DNA integrity are significantly associated (Irvine et al. 2000; Zribi et al. 2012).
Mechanism of cryoinjuries are osmotic stresses, cold shock, intracellular ice crystals formation, ROS and decrease antioxidant defense capacity of sperm (Amidi et al. 2016; Bateni et al. 2014; Gadea et al. 2011; Najafi et al. 2016). ROS are active molecules derived from oxygen (Agarwal et al. 2014; Amidi et al. 2016). The lipid peroxidation is increased through loss of unsaturated fatty acids causing production of altered oxidative products, effecting DNA fragmentation and sperm function (Anghel et al. 2010; Gadea et al. 2011; Gharagozloo and Aitken 2011; Iranpour et al. 2017; Toghiani et al. 2016). Efforts are made to decrease the adverse impact of ROS on sperm DNA function by pharmacological agents, vitamins and antioxidants. Antioxidants may has a role in decreasing DNA damage, as the antioxidant capacity in sperm cells is insufficient in preventing lipid peroxidation during freeze–thawing process. Recently, there is a growing awareness about antioxidants, as a safe alternate agents that can enhance sperm functions during cryopreservation (Agarwal et al. 2014; Murphy et al. 2011; Greenberg et al. 2011).
Folic acid is a water-soluble vitamin, used to prevent peroxidation of lipids. It can regenerate the oxidized thiol forms by giving electron and oxidation (Joshi et al. 2001; Nakano et al. 2001; Pietrzik et al. 2010). Folic acid has an important role in the synthesis of RNA and DNA. It participates in the synthesis of purines and methionine–homocysteine cycle, exhibiting its role in development and reproduction (Kamen 1997).
Nicotinic acid is a water-soluble vitamin (Lee et al. 2014), participates in different biological processes by contributing to mitochondrial energy production (Kim et al. 2015). However, its effect on human sperm function, survival and DNA integrity during freezing–thawing process was not known until now. In this study, association between sperm motility and chromatin/DNA damage before and after cryopreservation was explored, with cryoprotective role of folic acid and nicotinic acid on sperm quality, as a promising enhancer of sperm in ART field.
Materials and methods
Chemicals
All chemicals were supplied by Sigma-Aldrich (St. Louis. MO. USA) and Merck company-Germany unless stated otherwise.
Experimental design
Semen collection
This study was approved by the Ethical Committee of Isfahan University of Medical Sciences for all procedures performed in this study involving human participants. Informed consent obtained from all individual included in this study. In this experimental study, thirty fresh semen samples were collected from 30 normozoospermic men (age range between 25 and 45 years old) who were referred to Andrology Unit of Saint Maryam Fertility and Infertility Center of Shahid Beheshti Hospital, Isfahan, Iran for a routine semen analysis. Semen samples were classified as normozoospermic according to the World Health Organization (WHO) guidelines (Cooper et al. 2010).
Freshly collected ejaculates were obtained in sterile containers after 3–4 days of sexual abstinence. Each sample was first evaluated for semen volume, appearance, PH, and viscosity. Then, sperm concentration and motility was evaluated (Cooper et al. 2010).
Sperm preparation and cryopreservation
After liquefaction of the samples at room temperature for 30 min at 37 °C, routine semen analysis was performed using computer-assisted sperm analysis system (CASA system, VT-Sperm Test.2.3 model-company of Video Test-Finland) according to the WHO guidelines (2010). The semen samples were washed twice and re-suspended in modified Hams F10 with 5% human serum albumin (Irvine Scientific, Santa Ana. California).
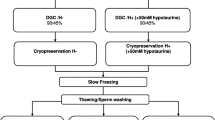
Each sample were divided into five aliquots to form the following groups: fresh, cryopreserved with spermfreeze only (control), with nicotinic acid (10 mM), with folic acid (50 nM), and with a combination of folic acid (50 nM) + nicotinic acid (10 mM).
In each fresh semen samples, sperm motility, viability, chromatin condensation, DNA fragmentation, and DNA denaturation were evaluated. Sperm cryopreservation of other four groups, was performed using an egg yolk-free cryoprotectant medium, Sperm Freeze Solution™ (SFS) (Sperm Freeze Solution™ 10137, Vitrolife, Goteborg, Sweden). Each frozen semen sample was slowly mixed with SFS (1:1) at room temperature and was drawn into a cryovial. Except the control group, the remaining three frozen groups of semen samples of cryovials were loaded separately with concentration of antioxidants as follows: nicotinic acid (10 mM), folic acid (50 nM), and with a combination of folic acid (50 nM) + nicotinic acid (10 mM). Each sealed cryovials containing semen samples were loaded on cryocane and exposed to liquid nitrogen (LN2) vapor for 30 min at 1–3 cm above the LN2 level, then plunged into liquid nitrogen and stored at − 196 °C until their use for evaluation of sperm parameters.
Thawing of each sample was done at room temperature by placing it in 35 ± 2 °C water bath for 20 min. Immediately after thawing, all semen samples of each group were evaluated for motility, viability and chromatin/DNA quality (Bateni et al. 2014).
Assessment of sperm viability and motility
Sperm viability was studied by using eosin-nigrosin staining. A drop of the sample mixed with two drops of Eosin (1%, prepared in distilled water, Merck company, Germany), and 3 drops of nigrosin (10% in distilled water, Merck company, Germany). Then, smears from each sample, was placed on warm glass slides (37 °C) and covered with a coverslip (22 mm × 22 mm). Each sample was analyzed by using the light microscope (magnification 1000 ×) and at least 200 spermatozoa were evaluated. Sperm with red or dark pink heads was considered nonviable (membrane-damaged), whereas spermatozoa showing no color were considered to be alive (membrane-intact) (Banihani et al. 2014; Khamsuk et al. 2014).
The percentage of total sperm motility of each group sample was evaluated by a light microscope (Olympus, BX41, Tokyo, Japan) equipped with CASA system. The percentage of the progressive, nonprogressive, total motility and immotile sperm were calculated by scanning several microscopic fields under light microscope. At least 200 sperms were classified for each sample in this way (Cooper et al. 2010).
Aniline blue (AB) staining
Aniline blue stain determines both the quality and quantity of sperm nuclear chromatin. From each sample, air-dried smears were prepared and fixed in 3% buffered glutaraldehyde in 0.2 M phosphate buffer (pH = 7.2) for 30 min at room temperature. Each smear was placed in the aqueous solution of aniline blue stain in 4% acetic acid (PH = 3.5), for 7 min. Then, at least 200 spermatozoa were counted in a different field of each slide by using a light microscope at 1000 × magnification normal spermatozoa (AB−) showed to be Unstained or pale blue stain and abnormal spermatozoa (AB+) showed to be dark blue stain and were reported as a percentage (Erenpreiss et al. 2001; Zribi et al. 2012).
Aniline blue stain identify chromatin condensation and selectively stains sperm with residual histones. From each sample, air-dried smears were fixed in 3% buffered glutaraldehyde in 0.2 M phosphate buffer (pH = 7.2) for 30 min at room temperature. Each smear was placed in aqueous solution of aniline blue stain in 4% acetic acid (PH = 3.5), for 7 min. Then, by using a light microscope at 1000 × magnification, at least 200 spermatozoa were counted in different field of each slide. Unstained or pale blue stained (normal spermatozoa or AB−) and dark blue stained (abnormal spermatozoa, AB+) cells were reported as percentage (Erenpreiss et al. 2001; Zribi et al. 2012).
Toluidine blue (TB) staining
Toluidine Blue stain investigate sperm nuclear chromatin condensation. Sperm smears were dried and fixed with fresh ethanol (96%) acetone (1:1) at 4 °C for 30 min then hydrolyzed with 0.1 N hydrochloric acids at 4 °C for 5 min and the sperm slides were washed 3 times in distilled water for 2 min then stained with 0.05% TB in 50% McIlvain’s citrate phosphate Buffer (pH 3.5) for 10 min at room temperature. Each slide was evaluated, 200 spermatozoa were examined under light microscopy (magnification 1000 ×), and sperm heads were classified with following scores, 0: light blue (good chromatin), 1: dark blue (mild abnormal chromatin), 2: violet and purple (severe chromatin abnormality). Then the sperm with score 0 was considered as normal (TB−) and spermatozoa in scores 1 and 2 were considered as abnormal cells (TB +) (Rahiminia et al. 2017; Taghizabet et al. 2016).
Sperm chromatin dispersion (SCD) test
Sperm samples of each group were evaluated for DNA fragmentation by using the Halosperm kit (Dayan Zist Azma Co.). This kit provides detection of sperm chromatin dispersion. An aliquot of sperm of each sample (50 μl) was mixed with low melting point agarose, then pipetted onto a glass slide and covered with a coverslip. The slides were transferred on a cold surface with a temperature of 2 °C to 8 °C and left to solidify at 4 °C for 5 min. This slides were immersed into denaturation and lysis solution. The sperm samples were washed in distilled water and then dehydrated in ethanol (70%, 90%, and 100%) for 2 min at room temperature and air dried. The stained cells were then washed with water and air dried. For each sample Slides, minimum of 200 sperms were examined under a light microscope with oil immersion objective (1000 × magnification) for presences of halos surrounding the sperm. Sperm with large/medium sized halos was considered to have intact DNA. Sperm with small or without halos was considered to have fragmented DNA (Gianaroli et al. 2012; Kotdawala et al. 2012).
Acridine orange (AO) test
AO stain is used to determine the rate of DNA denaturation (Zribi et al. 2012). The smears were prepared and exposed to air for 20 min and then fixed in a Carnoy’s solution (methanol/glacial acetic acid at a ratio of 3:1) for at least 2 h at 4 °C. The samples were stained by freshly prepared solution of AO (at a concentration of 0.19 mg/ml in McIlvain phosphate-citrate buffer (pH = 4) for 10 min. Each smear was evaluated on the same day by using a fluorescent microscope with a filter of 460 nm. In each slide, 200 spermatozoa were examined and the percentage of sperm with healthy double-stranded DNA (normal, green fluorescent), whereas single-stranded DNA were observed as red fluorescent (abnormal cell) (Ghasemi et al. 2014; Tejada et al. 1984; Zribi et al. 2012).
Statistical analysis
In this study, statistical analyses were performed using SPSS version 20. Bivariate associations between sperm chromatin/DNA damage assay and sperm progressive motility were evaluated by Pearson’s correlation coefficient. The result of all groups was evaluated with One-Way ANOVA test and data were presented as the mean ± standard deviation (SD). p < 0.05 was considered as statistically significant.
Results
Sperm viability
According to Fig. 1, the percentage of the sperm viability in the fresh groups was 78.23 ± 3.99% in comparison to cryopreserved groups, was found to be statistically significant. The percentage of viability in the control groups in comparison to all treatment groups after cryopreservation was found to be significant (p < 0.05). While the percentage of viable sperm was higher in the folic acid in combination with nicotinic acid groups (48.51 ± 3.76) when compared with control groups (35.18 ± 3.00%), folic acid groups (40.78 ± 2.41%) and nicotinic acid groups (37.96 ± 3.57%).
Sperm motility assessed by CASA
At the beginning of the experiment, the mean percentage of total sperm motility and progressive motility for fresh groups was 48.2 ± 5.35% and 35.16 ± 1.87%. After cryopreservation, the rate of total and progressive sperm motility in other groups was found to be significantly decreased in comparison to fresh groups (p < 0.05).
The rate of total and progressive motility after cryopreservation in control groups when compared to treatment groups was found to be statistically significant (p < 0.05). After cryopreservation, there was no significant difference between the groups receiving folic acid and nicotinic acid (p > 0.05). The groups receiving folic acid in combination with nicotinic acid showed to be statistically significant in comparison to groups receiving only folic acid and nicotinic acid and showed to have the best effect on progressive motility in the cryopreserved sperm (p < 0.05; Fig. 2).
Sperm chromatin/DNA assessment
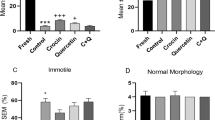
In the present study Table 1 and Fig. 3 shows the sperm chromatin condensation analysis results and DNA integrity status by using different assays. Results of AB and TB staining showed that there was a significant increase in the percentage of sperm with abnormal chromatin condensation after cryopreservation in among cryopreserved groups in comparison to the fresh groups (p < 0.05; Fig. 4). In addition, regarding the SCD and AO tests, in all cryopreserved groups, there was a significant increase in DNA damage compared to the fresh group (p < 0.05; Figs. 5, 6).
Chromatin/DNA evaluation of the fresh and frozen-thawed groups in different assays (mean ± standard deviation). a Aniline blue staining, b toluidine blue staining, c sperm chromatin dispersion test, d acridine orange staining. F, fresh group; C, control group; FA, folic acid group; NA, nicotinic acid groups; FA and NA, folic acid and nicotinic acid group. (Color figure online)
SCD test. Spermatozoa with big halo (a), medium-sized halo (b), small halo (c) and no halo (d). Sperm cells with large/medium sized halos were considered to have normal DNA. Sperm with small/absent halos was considered to have abnormal DNA (Light microscopy, magnification 1000 ×). A fresh group; B control group; C folic acid group; D nicotinic acid group; E nicotinic acid + folic acid group
Acridine orange test. Sperm with healthy double-stranded DNA (normal, green fluorescent) and single-stranded DNA (abnormal, yellow and red fluorescent). a fresh group, b control group, c folic acid group, d nicotinic acid group, e nicotinic acid + folic acid group (Fluorescent microscopy with a filter of 460 nm). (Color figure online)
The percentage of sperm with abnormal chromatin condensation in AB and TB staining in control groups was 36.33 ± 4.19% and 49.68 ± 3.6% respectively. The changes in the percentage of sperm with abnormal chromatin condensation in other groups in comparison with control groups were significant (p < 0.05). Our findings revealed folic acid had a better effect on sperm chromatin integrity in comparison to groups receiving nicotinic acid. Sperm chromatin condensation in the groups receiving folic acid and nicotinic acid showed a significant difference. The percentage of fragmented and denatured sperm within the control groups was statistically different in comparison to other groups. The changes in the percentage of DNA fragmentation and denaturation in the folic acid groups in combination with nicotinic acid show a significant difference in comparison to the groups receiving separately folic acid and nicotinic acid. In AO test the groups receiving the folic acid revealed less significant in comparison to the groups receiving nicotinic acid. In the SCD test the groups receiving folic acid and nicotinic acid separately was observed to be statistically significant (p < 0.05).
Table 2 shows the correlation results in the fresh and thawed samples. In fresh samples, a negative correlation was observed between sperm with abnormal chromatin condensation and progressive motility in both AB and TB stainings (r = − 0.394, p < 0.05 and r = 0.375, p < 0.05, respectively). In addition, the percentage of progressive motile cells in fresh sperm samples was found to show a negative correlation with sperm DNA fragmentation and denaturation (r = − 0.491, p < 0.001 and r = 0.428, p < 0.05, respectively). After cryopreservation/thawed, similar significant correlations was found between the studied parameters.
Discussion
Sperm freezing process is a useful technique in human assisted reproductive technology (ART) setup, but it is inadequate for evaluation of sperm survival, including morphology, motility, viability and chromatin/DNA integrity. The sperm cryopreservation process is a real challenge in assisted reproduction for improving motility, chromatin/DNA integrity and maintaining their survival till fertilization. In the present investigation, we observed the sperm quality, before and after cryopreservation and indicate new technical measures to avoid or minimize sperm cryoinjury by antioxidants, thereby enhancing fertilizing ability of sperm.
Several studies reported that freezing process induces DNA and chromatin damage and reduces the percentage of sperm viability and motility (Gianaroli et al. 2012; Keshtgar et al. 2016). Meanwhile, a correlation between sperm chromatin and DNA structure and semen quality was reported (Giwercman et al. 2003; Irvine et al. 2000).
In the present study, the level of correlation between the sperm chromatin/DNA integrity and percentage of progressive motility was evaluated by CASA, before and after freezing. The results revealed that there was a statistically significant difference between sperm parameters and chromatin/DNA structure before and after cryopreservation (p < 0.05; Table 2). Sperm DNA fragmentation, denaturation and chromatin abnormal condensation after cryopreservation was increased. The sperm motility was decrease and thereby a negative correlation between sperm motility and DNA fragmentation, denaturation, and chromatin abnormal condensation status was found for both fresh and thawed sperm (Table 1).
The hypothesis of present study corroborated with the study of Peris et al. who reported that motility was negatively related with the sperm chromatin structure before and after cryopreservation (Peris et al. 2004). Another study showed a negative correlation between Sperm chromatin structure assay parameters and the percentage of motile sperm (Giwercman et al. 2003). Irvine et al. found that motile sperm with normal morphology, negatively correlated with DNA damage, detected by a single-cell gel electrophoresis and in situ nick translation with prior chemical decondensation (ISNT-decondensed) assays. However, they did not observe statistically significant similar relationship by using the ISNT-condensed assay (Irvine et al. 2000). In relation to earlier research work, the present study suggested that progressive motility is directly related to normal chromatin/DNA integrity.
Cryopreservation increases the concentration of oxygen radicals in the sperm samples and thereby exposing them to high ROS concentrations which can cause cryodamage (Amidi et al. 2016; Khamsuk et al. 2014).
Antioxidants of vitamin E, vitamin C, melatonin, selenium, carnitine and etc. has been found to improve the treatment by reversing the adverse impact of high ROS concentrations (Amidi et al. 2016). In another study, the cryoprotective effects of vitamin E on rams sperm was reported to improve sperm progressive motility and viability (Pour et al. 2013). Branco et al. reported that ascorbic acid or vitamin C before cryopreservation prevented DNA damage in human sperm. In addition, melatonin, selenium, and carnitine as an antioxidant can protect spermatozoa against cryopreservation injuries and ameliorated sperm parameters and lower DNA damaged sperms (Amidi et al. 2016).
It is worthwhile to mention that to our knowledge, there is no research study that indicated the compensatory role of folic acid and nicotinic acid and in combination on sperm chromatin/DNA integrity in human sperm during cryopreservation. The results of the present study showed that folic acid or nicotinic acid added to the cryopreservation media could improve the sperm motility and viability in comparison to the control group. There was no significant difference in sperm parameters of motility in groups receiving folic acid and nicotinic acid. While sperm viability, showed a significant difference in antioxidant groups. But folic acid showed a better effect on the sperm in the present study in comparison to the nicotinic acid.
In line with our results, a study reported that the use of folic acid was found to improve sperm parameters such as motility and viability after freezing (Khamsuk et al. 2014). It was reported that the addition of nicotinic acid prior to cryopreservation had a positive effect on viability, compared with the control group. In addition, nicotinic acid was simultaneously used with the L-Carnitine to improves the sperm viability in miniature pigs (Lee et al. 2016).
In the present study on human sperm, revealed that addition of folic acid in cryopreservation medium was found to significantly improve sperm DNA and chromatin quality after thawing in comparison to control groups. Up to now, there have been no research studies investigating the direct impact of folic acid supplementation on human sperm DNA and chromatin integrity during cryopreservation. Isolated lymphocytes from human blood were studied in medium containing folic acid or medium without folic acid for a period of 10 days. They reported that poor folic acid status in human lymphocytes media, studied in vitro, was found to increase DNA strand breakage (Duthie and Hawdon 1998). Another study reported that folic acid concentration in the culture medium containing lymphocytes was correlated significantly and negatively with all markers of chromosome damage (Crott et al. 2001). It was reported that folic acid support DNA stability by donating one-carbon units and contribute to DNA synthesis, methylation, and repair (Duthie 2011).
Nicotinic acid reported to have a positive effect on survival, acrosomal intact and mitochondrial integrity in frozen-thawed boar semen and improve quality of embryo and blastocyst formation (Kim et al. 2015). Another study, reported that nicotinic acid in pig has a protective effect in fresh semen stored in a liquid state (Lee et al. 2014). Our results are in agreement with Lee et al. (2014) about the adverse effect of cryopreservation which was improved with the addition of nicotinic acid. According to previous studies, nicotinic acid is reported to be a precursor of nicotinic amide adenine nucleotide (NAD) and nicotinic amide adenine nucleotide phosphate (NADP) coenzyme. NADPH maintain ROS in the physiological rate of cells (Fleury et al. 2002). Moreover, mitochondria in spermatozoa play a very important role in maintaining sperm motility, which needs energy and metabolites such as NAD (NADH) and NADP (NADPH) participating in ATP signaling pathway (Lee et al. 2014; Verdin et al. 2010). It was reported that nicotinic acid affects ROS compound production in blood leukocytes of dairy cows (Bühler et al. 2017).
The current research revealed that the percentage of chromatin/DNA damage between folic acid group and nicotinic acid group was to be statistically significant difference (p < 0.05). The present study found that folic acid is more effective in maintaining chromatin and DNA integrity than nicotinic acid. Although an interesting point in the analysis of present study results, showed that the chromatin condensation and DNA integrity was maintained and were significantly reduced in groups receiving a combination of folic and nicotinic acid in comparison to the folic acid group (p < 0.05) and nicotinic acid group (p < 0.001).
According to earlier studies, folic acid is indicated, not to be metabolically active (Greenberg et al. 2011; Pietrzik et al. 2010). Moreover, we propose that nicotinic acid may increase cell energy level by producing NADP coenzyme which may enhance and help to activate folic acid. Moreover, folic acid and nicotinic acid are reported to have antioxidant activity (Kim et al. 2015). So, the alterations revealed in the present study, can be effective in the enhancement of folic acid by nicotinic acid leading to increase cell energy level by producing NADP coenzyme, which could be the major cause of maintaining sperm chromatin condensation and DNA integrity in the groups receiving combination of folic and nicotinic acid. Therefore, folic acid and nicotinic acid can be a safe alternative agents in protecting the sperms against cryopreservation injuries and significantly ameliorate sperm parameters, lower chromatin/DNA damage and enhance the post-thaw sperm quality.
Conclusion
This study, demonstrated that antioxidant supplementation of folic acid and nicotinic acid in the human sperm cryopreservation medium ameliorate sperm viability, motility and chromatin/DNA quality. The use of antioxidants could prevent severe and deleterious effects of cryopreservation on sperm fertility potential. Enhancing sperm quality during cryopreservation can improve functional activity of sperm for a successful fertilization in the in vitro fertilization and intracytoplasmic sperm injection procedures. It is critical for a normal embryo development and birth of healthy offspring. Further investigation is suggested in induced damage of sperm such as oligospermia and azoospermia etc. Therefore, antioxidant supplementation of folic acid and nicotinic acid may be a promising alternative agent in maintaining the post-thaw sperm quality and DNA integrity to enhance the fertilization rate.
References
Agarwal A, Durairajanayagam D, Du Plessis SS (2014) Utility of antioxidants during assisted reproductive techniques: an evidence-based review. Reprod Biol Endocrinol 12:112
Amidi F, Pazhohan A, Nashtaei MS, Khodarahmian M, Nekoonam S (2016) The role of antioxidants in sperm freezing: a review. Cell Tissue Bank 17:745–756
Anghel A, Zamfirescu S, Dragomir C, Nadolu D, Elena S, Florica B (2010) The effects of antioxidants on the cytological parameters of cryopreserved buck semen. Rom Biotechnol Lett 15:26–32
Banihani S, Agarwal A, Sharma R, Bayachou M (2014) Cryoprotective effect of L-carnitine on motility, vitality and DNA oxidation of human spermatozoa. Andrologia 46:637–641
Bateni Z, Azadi L, Tavalaee M, Kiani-Esfahani A, Fazilati M, Nasr-Esfahani MH (2014) Addition of Tempol in semen cryopreservation medium improves the post-thaw sperm function. Syst Biol Reprod Med 60:245–250
Bühler S et al (2017) Effects of energy supply and nicotinic acid supplementation on phagocytosis and ROS production of blood immune cells of periparturient primi-and pluriparous dairy cows. Res Vet Sci 116:62–71. https://doi.org/10.1016/j.rvsc.2017.09.012
Cooper TG et al (2010) World Health Organization reference values for human semen characteristics. Hum Reprod Update 16:231–245
Crott JW, Mashiyama ST, Ames BN, Fenech M (2001) The effect of folic acid deficiency and MTHFR C677T polymorphism on chromosome damage in human lymphocytes in vitro. Cancer Epidemiol Prevent Biomark 10:1089–1096
Duthie SJ (2011) Folate and cancer: how DNA damage, repair and methylation impact on colon carcinogenesis. J Inherit Metab Dis 34:101–109
Duthie SJ, Hawdon A (1998) DNA instability (strand breakage, uracil misincorporation, and defective repair) is increased by folic acid depletion in human lymphocytes in vitro. FASEB J 12:1491–1497
Erenpreiss J, Bars J, Lipatnikova V, Erenpreisa J (2001) Comparative study of cytochemical tests for sperm chromatin integrity. J Androl 22:45–53
Fleury C, Mignotte B, Vayssière J-L (2002) Mitochondrial reactive oxygen species in cell death signaling. Biochimie 84:131–141
Gadea J, Molla M, Selles E, Marco M, Garcia-Vazquez F, Gardon J (2011) Reduced glutathione content in human sperm is decreased after cryopreservation: effect of the addition of reduced glutathione to the freezing and thawing extenders. Cryobiology 62:40–46
Gharagozloo P, Aitken RJ (2011) The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod 26:1628–1640
Ghasemi N, Dashti G-R, Amoozgar F, Vaez S-A (2014) Effect of cholesterol, iron and vitamin E on protamine deficiency and DNA fragmentation of male rabbit sperm. J Isfahan Med Sch 31(259):1769–1778
Gianaroli L, Magli MC, Stanghellini I, Crippa A, Crivello AM, Pescatori ES, Ferraretti AP (2012) DNA integrity is maintained after freeze-drying of human spermatozoa. Fertil Steril 97(1067–1073):e1061
Giwercman A, Richthoff J, Hjøllund H, Bonde JP, Jepson K, Frohm B, Spano M (2003) Correlation between sperm motility and sperm chromatin structure assay parameters. Fertil Steril 80:1404–1412
Greenberg JA, Bell SJ, Guan Y, Yu Y-H (2011) Folic acid supplementation and pregnancy: more than just neural tube defect prevention. Rev Obstet Gynecol 4:52–59
Iranpour FG, Fazelian K, Dashti GR (2017) Thymoquinone as a natural spermostatic substance in reproductive medicine: an experimental study. Int J Reprod Biomed 15:641
Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken RJ (2000) DNA integrity in human spermatozoa: relationships with semen quality. J Androl 21:33–44
Joshi R, Adhikari S, Patro B, Chattopadhyay S, Mukherjee T (2001) Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radic Biol Med 30:1390–1399
Kamen B (1997) Folate and antifolate pharmacology. Semin Oncol 24:18–39
Keshtgar S, Iravanpour F, Gharesi-Fard B, Kazerooni M (2016) Combined effect of Trolox and EDTA on frozen-thawed sperm quality. Iran J Med Sci 41:230
Khamsuk K, Sinawat S, Seejorn K, Pongsritasana T, Sukkasame S (2014) The effect of folic acid on post-thaw quality of human spermatozoa. Srinagarind Med J 29:141–145
Kim Y-J et al (2015) Effect of nicotinic acid on sperm characteristic and oocyte development after in vitro fertilization using cryopreserved boar semen. J Embryo Transf 30:7–15
Kotdawala AP et al (2012) Addition of zinc to human ejaculate prior to cryopreservation prevents freeze–thaw-induced DNA damage and preserves sperm function. J Assist Reprod Genet 29:1447–1453
Lee Y-J et al (2014) Effect of nicotinic acid on fresh semen characteristics in miniature pigs. J Embryo Transf 29:385–391
Lee Y-J et al (2016) Effects of L-carnitine and nicotinic acid on sperm characteristics in miniature pigs. Reprod Dev Biol 40:1–5
Murphy LE et al (2011) Folate and vitamin B12 in idiopathic male infertility Asian. J Androl 13:856
Najafi A et al (2016) Supplementation of freezing and thawing media with brain-derived neurotrophic factor protects human sperm from freeze–thaw-induced damage. Fertil Steril 106(1658–1665):e1654
Nakano E, Higgins JA, Powers HJ (2001) Folate protects against oxidative modification of human LDL. Br J Nutr 86:637–639
Oberoi B, Kumar S, Talwar P (2014) Study of human sperm motility post-cryopreservation. Med J Armed Forces India 70:349–353
Oehninger S, Duru NK, Srisombut C, Morshedi M (2000) Assessment of sperm cryodamage and strategies to improve outcome. Mol Cell Endocrinol 169:3–10
Peris SI, Morrier A, Dufour M, Bailey JL (2004) Cryopreservation of ram semen facilitates sperm DNA damage: the relationship between sperm andrological parameters and the sperm chromatin structure assay. J Androl 25:224–233
Pietrzik K, Bailey L, Shane B (2010) Folic acid and L-5-methyltetrahydrofolate. Clin Pharmacokinet 49:535–548
Pour H, Tahbasbi A, Naserain A-A (2013) The influence of vitamin E on semen characteristics of ghezel rams in during cooling and frozen process. Eur J Med Res 2:94–99
Rahiminia T, Hosseini A, Anvari M, Ghasemi-Esmailabad S, Talebi AR (2017) Modern human sperm freezing: effect on DNA, chromatin and acrosome integrity Taiwanese. J Obstet Gynecol 56:472–476
Taghizabet N, Mangoli E, Anbari F, Masoodi SA, Talebi AR, Mazrooei M (2016) The effect of heracleum persicum (Golpar) oil and alcoholic extracts on sperm parameters and chromatin quality in mice. Int J Reprod Biomed 14:365
Tejada RI, Mitchell JC, Norman A, Marik JJ, Friedman S (1984) A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil Steril 42:87–91
Toghiani S, Dashti GR, Roudbari NH, Roozbehani S (2016) Effect of ascorbic acid and menthone on the caspase 3 in the sperm cells of acyclovir treated rats. Acta Medica 32:1213
Verdin E, Hirschey MD, Finley LW, Haigis MC (2010) Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci 35:669–675
Verza S Jr, Feijo CM, Esteves SC (2009) Resistance of human spermatozoa to cryoinjury in repeated cycles of thaw-refreezing. Int Braz J Urol 35:581–591
Zribi N et al (2012) Effect of freezing–thawing process and quercetin on human sperm survival and DNA integrity. Cryobiology 65:326–331
Acknowledgements
This study was funded by Isfahan University of Medical Sciences, Isfahan, Iran. The authors would like to thank Mrs. Fatemeh Amozegar and Mrs. Maryam Aliakbary for their technical support. The authors would like to thank the participating and express their gratitude to Isfahan University of medical sciences for its financial support.
Funding
This study was funded by Isfahan University of Medical Sciences, Isfahan, Iran (Grant No. 396378).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
The procedures in this studies involving human participants was performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants attended in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rarani, F.Z., Golshan-Iranpour, F. & Dashti, G.R. Correlation between sperm motility and sperm chromatin/DNA damage before and after cryopreservation and the effect of folic acid and nicotinic acid on post-thaw sperm quality in normozoospermic men. Cell Tissue Bank 20, 367–378 (2019). https://doi.org/10.1007/s10561-019-09775-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-019-09775-6