Abstract
Tendon allografts, when autograft options are limited or when obtaining an autograft is not aligned with the patients’ best interest, play an important role in tendon and ligament reconstruction. To minimize the risk of infectious disease transmission tissue banks perform screening tests and the allografts cleaned are sterilized. The current study examines and compares the initial mechanical properties and histological appearance of supercritical CO2 (SCCO2)-treated and gamma-irradiated porcine extensor tendons. Thirty intact porcine forelimb extensor tendons randomized equally into three groups: control group, gamma-irradiation group, and SCCO2-treated group. Once treated, histological assessment and histomorphologic measurements were made on the histological sections obtained from each tendon while stiffness and ultimate failure loads were evaluated from tensile testing. Histological evaluation of gamma-irradiated tendons showed significant disruption to the hierarchical morphology of the fascicle bundles, which was not evident in SCCO2-treated specimens. Histomorphologic measurements showed a significant increase for measured dead space (void) between tendon fibrils of the gamma-irradiated group comparing to both control and SCCO2 treated groups (p < 0.01). There was a significant reduction in the ultimate failure load for tendons treated by gamma-irradiation compared to the control group (p < 0.05). No statistically significant difference was detected between control and SCCO2-treated tendons in the ultimate failure load. Stiffness values were not significantly different between three-study groups. This study suggests that while gamma-irradiation has a deleterious effect on mechanical properties of tendon tissue, SCCO2 does not alter the biomechanical properties and the histological structure of porcine extensor tendons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tendon allografts, when autograft options are limited or when obtaining an autograft is not aligned with the patients’ best interest, play an important role in tendon and ligament reconstruction. They can decrease surgical time, which is ideal for both, the patient and the healthcare systems, and can help surgeons to avoid autograft harvesting site complications. Moreover, the possibility of biomechanical changes at the donor site, which can cause future complications, is eliminated by using allografts. Allograft tissue also provides the flexibility of choosing different sizes and types of grafts which is an important issue in terms of surgical decision making and planning (Baldini et al. 2014; Bhatia et al. 2012; Conrad et al. 2013; Greis et al. 2012; Guo et al. 2012; Sun et al. 2011; Yanke et al. 2013).
Allografts have been successfully used in reconstruction of the anterior cruciate ligament, in multiple ligament reconstruction of more complex knee ligamentous injuries, in the reconstruction of chronic patellar ligament rupture and in the reconstruction of the lateral ligament of the ankle (Nakata et al. 2000; Su and Healey 2003; Zhou et al. 2014; Bui et al. 2015). Allografts have also been used in the management of elbow instability, rupture of the pectoralis major tendon (Joseph et al. 2003), rupture of the biceps tendon (Sanchez-Sotelo et al. 2002a), chronic triceps insufficiency (Sanchez-Sotelo et al. 2002b), triceps deficiency in patients following total elbow arthroplasty (Celli et al. 2005) and in the reconstruction of the tendon of biceps brachii following the excision of tumoral calcinosis (Aydin et al. 2004; Robertson et al. 2006).
Using soft-tissue allografts has also been associated with certain drawbacks such as increased surgical cost, prolonged tissue incorporation, potential decrease in mechanical properties, potential host immune response, and infectious disease transmission (Baldini et al. 2014; Bhatia et al. 2012; Conrad et al. 2013; Greis et al. 2012; Guo et al. 2012; Sun et al. 2011; Yanke et al. 2013). To minimize the risk of infectious disease transmission, tissue banks perform screening tests and the allografts are subjected to rigorous cleaning and sterilization processes which can decrease the risk of a host site immune response (Conrad et al. 2013; Yanke et al. 2013; Schmidt et al. 2012).
By definition, sterilization is the process of reduction of microbial/viral contaminations to sterility assurance level (SAL) of 10-6, or SAL-6, which means that there should be less than 1 in 1,000,000 chance of a contaminating organism surviving the treatment (TGA 2011; McAllister et al. 2007; White et al. 2006; Bui et al. 2015). However, it has been shown that some of the sterilization methods, like gamma irradiation, with the methods and dosages that can achieve the sterilization goal can compromise the mechanical properties of the allograft (Conrad et al. 2013; Yanke et al. 2013; Schmidt et al. 2012; Hoburg et al. 2010; Schimizzi et al. 2007; Seto et al. 2013; Zhou et al. 2014). For example, allograft stiffness has been shown to decrease with the required dose to fully inactivate the HIV virus (Fideler et al. 1994; Hernigou et al. 2000).
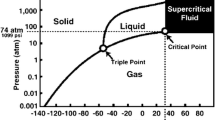
One alternative method that has been used for sterilizing allografts is supercritical CO2 (SCCO2) technology (Huang et al. 2013; Schimizzi et al. 2007; White et al. 2006). A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid (Padrela et al. 2009) (Fig. 1).
Phase diagram of carbon dioxide, demonstrating critical point (White et al. 2006)
Supercritical fluids deeply penetrate tissue and can be considered as organic solvents (Padrela et al. 2009). Supercritical carbon dioxide and water have been used in chemical and food industries; however, SCCO2 has recently been evaluated, in vitro, for processing of menisci and also bone grafts (Bui et al. 2015; Russell et al. 2012a, b). Fages et al. demonstrated that the SCCO2 process is effective in inactivating viruses on human femoral heads, and can achieve a level of inactivation similar to that obtained by traditional cleaning methods. Their study suggested that SCCO2 can replace or supplement existing sterilizing procedures of bone allografts (Fages et al. 1996). It has also been shown that SCCO2 successfully inactivates bacteria, fungi, spores and viruses when used in conjunction with sterilants such as hydrogen peroxide and peracetic acid, ensuring SAL 10−6 sterility of tissues (Christensen et al. 2004; Furukawa et al. 2009; Melo Silva et al. 2013; Zhang et al. 2006a, b; Perrut et al. 2012). In this process the allografts are sealed in their packaging and placed in a chamber with CO2 that is pressurized and heated to the critical point which is the temperature of 31 °C and the pressure of 74 bar (Fig. 2). In the study performed by Russel et al. the effect of SCCO2 treatment compared with two doses of gamma irradiation, on the mechanical properties of whole bone (Russell et al. 2012b). The aim of this study is to compare the effect of Gamma irradiation and SCCO2 as two current major allograft sterilization methods. The null hypothesis of this study is no difference in mechanical properties of non-sterilized, gamma-sterilized and SCCO2 sterilized allografts.
Methods and materials
Study design
Twenty-two porcine forelimbs, from 12 to 18-month old pigs, were obtained from the local supplier. Following trimming of excess connective tissue, the tendons were studied macroscopically for any traumatic or degenerative changes. Thirty completely intact tendons were chosen and randomized into three groups (10 per each group): control group, gamma-irradiation group and SCCO2 group. Specimens were kept moist by being wrapped in PBS-soaked gauze, and frozen for storage at − 20 °C until treatment.
Specimens in gamma-irradiated group were sent to an external laboratory for irradiation at 25 kGy using a cobalt irradiation source under well-defined operating procedures (Steritech Wetherill Park, Australia). Samples were placed on dry ice and sealed in a styrofoam box to maintain temperatures during treatment between − 20 and − 50 °C. Samples were thawed at room temperature for testing. The SCCO2 group tendons were thawed and treated whole with an in-house supercritical fluid set-up and the Novasterilis supercritical CO2 sterilisation protocol. A cellulose pad soaked with 1.04 ml peracetic acid (14.1%) and hydrogen peroxide (4.9%) placed in the bottom of the pressure vessel which loaded with tendon allografts. The system was thermally equilibrated at temperature of 37 °C and pressurized with carbon dioxide above its critical point (Pc = 73.8 bar) into the supercritical phase region using a high-pressure pump (TharSFC, MA, USA). The system was statistically isolated at 100 bars for an hour and decompressed to 0 over 45 min in a linear fashion by releasing the valve (Russell et al. 2012a, b).
Histology
Once treated, 4 mm of the end of all specimens in each group were cut and fixed for 48 h in 10% neutral buffered formalin. The axial and longitudinal sections were processed for paraffin histology using a tissue-processing machine (Shandon Excelsior ES, Thermo Fisher Scientific, Kalamazoo, USA). The segments were embedded in paraffin wax, cut into 5 μm thick serial sections using a hand-operated microtome (Leia RM2165, Leica Instrument GmbH, Nussloch, Germany) and mounted onto glass slides for histology. Sections were stained using Harris Haematoxylin and Eosin (H&E). Histology slides were assessed using an Olympus BX51 light microscope (Olympus, Sydney, Australia) with the magnification of 20 × under plain lights.
Histomorphology
Tissue organisation was analysed qualitatively and quantitatively using an in-house histomorphometry program written in MATLAB 7.0 (The Math Works Inc, Natick, Massachusetts) to specifically focus on the percentage of dead space between tendon fibrils. First, images containing the entire graft area (4 × magnification) were digitally captured for reference purpose using a camera (DP27, Olympus, Tokyo, Japan) attached to the microscope. The magnification of the microscope was increased to 20 × and a further five equivalent regions of interest were captured to measure the mean percentage of dead space between tendon fibrils.
Mechanical testing
Before using the specimens they were thawed in room temperature while they were wrapped in Phosphate Buffered Saline (PBS)-soaked gauze till they were tested. Specimens were mounted onto a material testing machine 858 Bionix (MTS System, MN, USA). Cryo-clamps were used for mechanical testing while they were set to be 50 mm apart. Mechanical testing started after confirming the presence of freeze-line at each end of the tendon close to the clamp. Each tendon was pre-tensioned to 10 N for 1 min then after tarring the displacement, cyclic loading applied from 10 to 100 N with the frequency of 0.5 Hz for a total of 150 cycles. Failure test was performed by loading the construct at a rate of 0.5 mm/s. Mechanical testing data was analyzed by using an in-house program written in MATLAB 7.0 (The Math Works Inc, Natick, Massachusetts). Load-deformation curves were generated for each specimen and load and stiffness values obtained. The maximum load required for the construct to fail, ultimate tensile failure, was defined as load-to-graft failure in Newton (N). Stiffness was defined as the linear part of the slope of the load deformation curve with units of N/mm.
Statistical analysis
All quantitative parameters (ultimate tensile failure, stiffness and percentage of dead space between fibrils) compared statistically using one-way ANOVA and Tukey’s tests.
Results
Graft measurements
Mean width and thickness of control, gamma-irradiated and SCCO2-treated groups were measured with two decimal points and compared using one-way ANOVA and Tukey’s tests. Irradiated-tendon group had the mean width of 3.03 mm and mean thickness of 1.42 mm. The mean width and thickness of SCCO2-treated group were 3.04 and 1.47 mm, respectively. The control group had the mean width of 2.98 mm and mean thickness of 1.40 mm. Using one-way ANOVA and Tukey’s tests, identified no statistically significant difference between three groups (p > 0.1) (Fig. 3).
Histology and histomorphology
Histological evaluation of gamma-irradiated tendons showed disruption to the overall hierarchical morphology of the fascicle bundles and significant changes in the orientation of collagen fibers (Fig. 4). There were regions of tight and sharp crimp in longitudinal sections in addition to increased dead space (void) between tendon fibrils in both transverse and longitudinal sections in 8 out of 10 of gamma-irradiated tendons. The fibroblast cellularity was notably different in 7 out of 10 gamma-irradiated tendon specimens in five regions of interest at 12, 3, 6 and 9 o’clock (referenced to analogous hours on a clock) and also at the central position of the visual field at 20 × magnification in qualitative assessment. These histological findings were not evident in control and SCCO2-treated specimens. This was confirmed through histomorphologic measurements which showed a significant increase for measured void between gamma-irradiated group and both control and SCCO2-treated tendons (p < 0.01) (Figs. 5, 6).
H&E stained (x20 magnification demonstrating qualitative examples), longitudinal (a) and axial (b) section slides of a control, longitudinal (c) and axial (d) section slides of 25 KGy gamma-irradiated, longitudinal (e) and axial (f) section slides of SCCO2 treated tendons. Arrow depicts example of crimp, smoothing and bundle separation
The dead spaces between the tendon fascicles are shown in red color by the software in longitudinal (a) and axial (b) section slides of a control, longitudinal (c) and axial (d) section slides of 25 KGy gamma-irradiated, longitudinal (e) and axial (f) section slides of SCCO2 treated tendons (x20 magnification demonstrating quantitative examples)
Mechanical testing
There was a significant reduction in the ultimate failure load for tendons treated by gamma irradiation compared to the control group (p < 0.05). No statistically significant difference was detected between control and SCCO2 treated tendons in the ultimate failure (Fig. 7). However, Stiffness values were not significantly different between all three study-groups (Fig. 8).
Discussion
Our study showed that there is no statistically significant difference between non-treated, gamma-irradiated and SCCO2 sterilized tendons, in terms of their stiffness. We also identified that the mean ultimate tensile failure load in gamma-irradiated group was significantly less than the other two groups and mean dead spaces in this group was significantly more than SCCO2 and control groups. There was no statistically meaningful difference in ultimate tensile failure load, stiffness and void between the control and SCCO2 groups. Our data suggest that allografts sterilized with SCCO2 have better initial biomechanical properties compared to gamma-irradiated allografts. This would be advantageous in the rehabilitation stages post-surgery.
The mechanical properties of the tendon are dependent on the collagen fiber diameter and orientation and also the presence of proteoglycans. The collagen fibrils in tendons are held together with proteoglycan components due to their multiple interactions with the surface of the fibrils in junction areas (Hulmes et al. 2002; Zhang et al. 2006a, b). While both collagen fibrils and proteoglycans allow tendons to resist tensile stress, the proteoglycans also allow them to resist compressive stress. It has been shown that the elongation and the strain of the collagen fibrils alone have been much lower than the total elongation and strain of the entire tendon under the same amount of stress, demonstrating that the proteoglycan-rich matrix must also undergo deformation, and stiffening of the matrix occurs at high strain rates. This deformation of the non-collagenous matrix occurs at all levels of the tendon hierarchy, and by modulating the organization and structure of this matrix, the different mechanical properties required by different tendons can be achieved (Screen et al. 2004; Gupta et al. 2010; Beer and Johnston 2006).
The absorption of gamma radiation in different materials increases by electron density of the materials (mass attenuation coefficient) (Meyerhof et al. 1967). Therefore, it is expected that areas where PGs are interwoven with collagen fibrils and particularly junctions between PGs and fibrils should be more susceptible to gamma irradiation due to higher electron density at those points comparing to non-junction area. Considering the cross-link effect of PGs, this fact can explain the increase in dead space between fibrils in the gamma-irradiated group that we found in our study. It can also explain why ultimate tensile failure in the gamma-group decreased significantly compared to the control and SCCO2-groups due to the mentioned role of PGs in ultimate tensile failure loads. Moreover, by definition, the strength of structures of equal cross sectional area loaded in tension is independent of the shape of the cross section, but it is sensitive to material defects or abrupt changes in geometry (Beer and Johnston 2006) which was shown in the histomorphology of the gamma-irradiated group in our study.
It has been realized that even low dose of gamma-irradiation could have significant effects on the biological properties of collagen materials such as extracellular tissue matrix (ECM) which has an important role in the biomechanical properties of connective tissue structures like tendons (Liu et al. 1989; Cheung et al. 1990; Inoue et al. 2006; Keller et al. 2001; Puxkandl et al. 2002; Ota et al. 2007; Gouk et al. 2008; McGilvray et al. 2011). Edwards et al. investigated the effects of different doses of gamma and electron beam irradiation on the biological properties of a previously developed acellular porcine superflexor tendon. Their histological observations showed clear crimp, smoothing and some bundle separation and disruption in gamma-irradiated tendons, which is quite consistent with our histological assessment. They also reported the presence of holes in one region of one sample within the gamma-irradiated tendons but this finding was identified in half of the tendons irradiated with fractioned E-beam (15 + 15 kGy) (Edwards et al. 2016). However, Zhou et al. assessed the biological properties of human flexor tendons sterilized by peracetic acid/ethanol combined with gamma irradiation. They reported that the bundles were regularly arranged with no significant bundle separation and disruption in gamma-irradiated tendons; however, they used 15 kGy irradiation dose which is significantly less than the dose used in other studies (Zhou et al. 2014).
Increase in dead space (void) between gamma-irradiated tendon fibrils, which was reported in our study and possibly is due to damage to the fascicle sheaths and to the junction area of collagen and PGs, may explain the change in initial biomechanical properties of gamma-irradiation. Lovric et al. examined the effect of introducing gaps within the tendon grafts prior to ACL reconstruction in a rodent model. Their study showed that the fenestrated grafts had less necrotic changes in the initial 2 weeks of healing process and tendon-bone incorporation process was facilitated and faster in this group of allografts (Lovric et al. 2011). It is necessary to have controlled in vitro, pre-clinical and well-designed randomized clinical trials to understand the effect of histologic and biomechanical changes of different tendon allograft sterilizing methods in final clinical outcome.
In another study that compared failure load, ultimate stress and stiffness in anterior and posterior tibialis tendons that were unprocessed, gamma-irradiated, or sterilized with SCCO2, Baldini et al. had different results from the current study (Baldini et al. 2014). They found no significant difference among the group’s failure load and the SCCO2 group was significantly less stiff than the unprocessed and gamma-irradiated allografts during cyclic testing. The significant difference in stiffness and failure load may be due to significant difference between the size of allograft groups. SCCO2 allografts were significantly smaller in cross-sectional area than the gamma-irradiated group and the unprocessed group by 28 and 18%, respectively. However, like our study they found no significant difference between the ultimate tensile failure of SCCO2-sterilised and control groups and no significant difference between stiffness of gamma-irradiated and control groups.
Our results were consistent with the result of the study (Schimizzi et al. 2007) which compared mechanical properties of anterior tibialis allografts that were fresh-frozen, gamma-irradiated, or sterilized with BioCleanse®. They found that stiffness of the fresh-frozen allograft group was lower than the other two groups in the first cycle but there was no significant difference in the stiffness after the first cycle. Our result was also compatible with the result of the study that compared failure load, ultimate stress, and stiffness of ACL allografts (Conrad et al. 2013). They tested achilles tendon allografts in three groups of control, gamma-irradiated (1.5–2.5 MRad) and BioCleanse®. They found the failure stress of the untreated allografts to be significantly greater than gamma-irradiated and BioCleanse®. In our study we did not use BioCleanse; however, the results of both studies in terms of comparing the failure stress of control and gamma-irradiated groups are similar.
Our study has limitations as well. There are three major factors that determine the success of a reconstruction procedure using tendons grafts, including allografts: first, biomechanical properties of the grafts versus the mechanical forces that they should withstand. Second, the surgical technique of the reconstruction procedure and third, graft incorporation (Canale et al. 2016). As an in vitro study, we could only assess one of the three important factors. It is important for the incorporation of the allografts sterilized with different methods to be studied by controlled in vitro, pre-clinical and then well-designed clinical trials.
Conclusion
Our study showed that there is no statistically significant difference between non-treated, gamma-radiated and SCCO2 sterilized tendons in terms of their stiffness. However, the gamma-radiated tendons have significantly less ultimate tensile failure and more dead spaces between the tendon fascicles comparing to the other two groups, while there was no meaningful difference between the control and SCCO2 groups in terms of these two items. It seems that from a biomechanical point of view, patients who are exposed to sudden and substantial forces, like professional sport players would benefit more from SCCO2-sterilized allografts rather than gamma-irradiated allografts, due to their higher ultimate tensile failure load.
References
Aydin A, Tuncer S, Eeer M, Bilgic B (2004) Tumoural calcinosis infiltrating the biceps brachii tendon: excision and reconstruction with allograft. J Hand Surg [Br] 29-B:170–172
Baldini T, Caperton Hawkins M, McCarty E (2014) Effect of novel sterilization method of biomechanical properties of soft tissue allografts. Knee Surg Sports Traumatol Arthroscop. https://doi.org/10.1007/s00167-014-3221-0
Beer FP, Johnston R (2006) Mechanics of materials. McGraw-Hill, New York. ISBN 978-0-07-352938-7
Bhatia S, Bell R, Frank RM, Rodeo SA, Bach BR Jr, Cole BJ, Chubinskaya S, Wang VM, Verma NN (2012) Bony incorporation of soft tissue anterior cruciate ligament grafts in an animal model: autograft versus allograft with low-dose gamma irradiation. Am J Sports Med 40(8):1789–1798
Bui D, Lovric V, Oliver R, Bertollo N, Broe D, Walsh WR (2015) Meniscal allograft sterilisation: effect on biomechanical and histological properties. Cell Tissue Bank 16:467–475. https://doi.org/10.1007/s10561-014-9492-3
Canale ST, Azar FM, Beaty HD (2016) Campbell’s operative orthopaedics. Elsevier, Amsterdam. ISBN 978-0323072434
Celli A, Arash A, Adams RA, Morrey BF (2005) Triceps insufficiency following total elbow arthroplasty. J Bone Joint Surg [Am] 87-A:1957–1964
Cheung DT, Perelman N, Tong D, Nimni ME (1990) The effect of gamma irradiation on collagen molecules, isolated alpha-chains and cross-linked native fibers. J Biomed Mater Res 24:581–589
Christensen TW, Burns D, White A, Ganem B, Eisenhunt A (2004) Sterilization methods and apparatus which employ additive-containing supercritical carbon dioxide sterilant. US 7108832 B2, 2006
Conrad BP, Rappe M, Horodyski M, Farmer KW, Indelicato PA (2013) The effect of sterilization on mechanical properties of soft tissue allografts. Cell Tissue Bank 14(3):359–366
Edwards JH, Herbert A, Jones GL, Manfield IW, Fisher J, Ingham E (2016) The effects of irradiation on the biological and biomechanical properties of an acellular porcine superflexor tendon graft for cruciate ligament repair. J Biomed Mater Res Part B Appl Biomater. https://doi.org/10.1002/jbm.b.33786
Fages J, Poirier B, Barbier Y, Frayssinet P, Joffret ML, Majewski W, Bonel G, Larzul D (1996) Viral inactivation of human bone tissue using supercritical fluid extraction. ASAIO J 44(4):289–293
Fideler BM, Vangsness CT Jr, Moore T, Li Z, Rasheed S (1994) Effects of gamma irradiation on the human immunodeficiency virus. A study in frozen human bone-patellar ligament-bone grafts obtained from infected cadavera. J Bone Joint Surg Am 76(7):1032–1035
Furukawa S, Watanabe T, Koyama T, Hirata J, Narisawa N, Ogihara H, Yamasaki M (2009) Inactivation of food poioning bacteria and Geobacillus stearothermophilus spores by high pressure carbon dioxide treatment. Food Control 20:53–58. https://doi.org/10.1016/j.foodcont.2008.02.002
Gouk SS, Lim TM, Teoh SH, Sun WQ (2008) Alterations of human acellular tissue matrix by gamma irradiation: histology, biomechanical property, stability, in vitro cell repopulation and remodeling. J Biomed Mat Res Part B Appl Biomater 84B:205–217
Greis PE, Koch BS, Adams B (2012) Tibialis anterior or posterior allograft anterior cruciate ligament reconstruction versus hamstring autograft reconstruction: an economic analysis in a hospital-based outpatient setting. Arthroscopy 28(11):1695–1701
Guo L, Yang L, Duan XJ, He R, Chen GX, Wang FY, Zhang Y (2012) Anterior cruciate ligament reconstruction with bonepatellar tendon-bone graft: comparison of autograft, fresh-frozen allograft, and gamma-irradiated allograft. Arthroscopy 28(2):211–217
Gupta HS, Seto J, Krauss S, Boesecke P, Screen HRC (2010) In situ multi-level analysis of viscoelastic deformation mechanisms in tendon collagen. J Struct Biol 169(2):183–191
Hernigou P, Gras G, Marinello G, Dormont D (2000) Influence of irradiation on the risk of transmission of HIV in bone grafts obtained from appropriately screened donors and followed by radiation sterilization. Cell Tissue Bank 1(4):279–289
Hoburg AT, Keshlaf S, Schmidt T, Smith M, Gohs U, Perka C, Pruss A, Scheffler S (2010) Effect of electron beam irradiation on biomechanical properties of patellar tendon allografts in anterior cruciate ligament reconstruction. Am J Sports Med 38(6):1134–1140
Huang Q, Ingham E, Rooney P, Kearney JN (2013) Production of a sterilised decellularised tendon allograft for clinical use. Cell Tissue Bank 14(4):645–654
Hulmes DJS et al (2002) Building collagen molecules, fibrils, and suprafibrillar structures. J Struct Biol 137(1–2):2–10. https://doi.org/10.1006/jsbi.2002.4450
Inoue N, Bessho M, Furuta M, Kojima T, Okuda S, Hara M (2006) A novel collagen hydrogel cross-linked by gamma-ray irradiation in acidic pH conditions. J Biomater Sci Polym Ed 17:837–858
Joseph TA, Defranco MJ, Weiker GG (2003) Delayed repair of a pectoralis major tendon rupture with allograft: a case report. J Shoulder Elbow Surg 12:101–104
Keller PF, Verin V, Ziegler T, Mermillod B, Popowski Y, Delafontaine P (2001) Gamma-irradiation markedly inhibits the hydrated collagen gel contradiction by arterial smooth muscle cells. J Invest Med 49:258–264
Liu BC, Harrell R, Davis RH, Dresden MH, Spira M (1989) The effect of gamma irradiation on injectable human amnion collagen. J Biomed Mater Res 23:833–844
Lovric V, Kanazawa T, Nakamura Y, Oliver R, Yu Y, Walsh WR (2011) Effects of gaps induced into the ACL tendon graft on tendon-bone healing in a rodent ACL reconstruction model. Muscle Tendon Ligament J 1(3):91–99
McAllister DR, Joyce MJ, Mann BJ, Vangsness CT Jr (2007) Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med 35:2148–2158
McGilvray KC, Santoni BG, Turner AS, Bogdansky S, Wheeler DL, Puttlitz CM (2011) Effects of 60Co gamma radiation dose on initial structural biomechanical properties of ovine bone-patellar tendon-bone allografts. Cell Tissue Bank 12:89–98
Melo Silva J, Rigo AA, Dalmolin IA, Debien I, Cansian RL, Oliveira JV, Mazutti MA (2013) Effect of pressure, depressurization rate and pressure cycling on the inactivation of Escherichia coli by supercritical carbon dioxide. Food Control 29:76–81. https://doi.org/10.1016/j.foodcont.2012.05.068
Meyerhof WE et al (1967) Elements of nuclear physics. McGraw-Hill, New York. ISBN 978-0070417458
Nakata K, Shino K, Horibe S et al (2000) Reconstruction of the lateral ligaments of the ankle using solvent-dried and gamma-irradiated allogeneic fascia lata. J Bone Joint Surg [Br] 82-B:579–582
Ota T, Taketani S, Iwai S, Miyagawa S, Furuta M, Hara M et al (2007) Novel method of decellularization of porcine valves using polyethylene glycol and gamma irradiation. Ann Thorac Surg 83:1501–1507
Padrela L, Rodrigues MA, Velaga SP, Matos HA, Azevedo EG (2009) Formation of indomethacin–saccharin cocrystals using supercritical fluid technology. Eur J Pharm Sci 38:9–17. https://doi.org/10.1016/j.ejps.2009.05.010
Perrut M et al (2012) Sterilization and virus inactivation by supercritical fluids (a review). J Supercrit Fluids 66:359–371. https://doi.org/10.1016/j.supflu.2011.07.007
Puxkandl R, Zizak I, Paris O, Keckes J, Tesch W, Bernstorff S, Purslow P, Fratzl P (2002) Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos Trans R Soc 357(1418):191–197. https://doi.org/10.1098/rstb.2001.1033
Robertson A, Nutton RW, Keating JF (2006) Current trends in the use of tendon allografts in orthopaedic surgery. J Bone Joint Surg [Br] 88-B:988–992
Russell NA, Pelletier MH, Bruce WJ, Walsh WR (2012a) The effect of gamma irradiation on the anisotropy of bovine cortical bone. Med Eng Phys 34:1117–1122. https://doi.org/10.1016/j.medengphy.2011.11.021
Russell NA, Rives A, Pelletier MH, Bruce WJ, Walsh WR (2012b) The effect of sterilization on the mechanical properties of intact rabbit humeri in three-point bending, four-point bending and torsion. Cell Tissue Bank. https://doi.org/10.1007/s10561-012-9318-0
Sanchez-Sotelo J, Morrey BF (2002) Surgical techniques for reconstruction of chronic insufficiency of the triceps: rotation flap using anconeus and tendo achillis allograft. J Bone Joint Surg [Br] 84-B:1116–1120
Sanchez-Sotelo J, Morrey BF, Adams RA, O’Driscoll SW (2002) Reconstruction of chronic ruptures of the distal biceps tendon with use of an achilles tendon allograft. J Bone Joint Surg [Am] 84-A:999–1005
Schimizzi A, Wedemeyer M, Odell T, Thomas W, Mahar AT, Pedowitz R (2007) Effects of a novel sterilization process on soft tissue mechanical properties for anterior cruciate ligament allografts. Am J Sports Med 35(4):612–616
Schmidt T, Hoburg A, Broziat C, Smith MD, Gohs U, Pruss A, Scheffler S (2012) Sterilization with electron beam irradiation influences the biomechanical properties and the early remodeling of tendon allografts for reconstruction of the anterior cruciate ligament (ACL). Cell Tissue Bank 13(3):387–400
Screen H, Lee DA, Bader DL, Shelton JC (2004) An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. J Eng Med 218:109–119
Seto AU, Culp BM, Gatt CJ Jr, Dunn M (2013) Radioprotection provides functional mechanics but delays healing of irradiated tendon allografts after ACL reconstruction in sheep. Cell Tissue Bank 14(4):655–665
Su EP, Healey JH (2003) Salvage reconstruction for lateral ankle instability using a tendon allograft. Clin Orthop 415:232–238
Sun K, Zhang J, Wang Y, Xia C, Zhang C, Yu T, Tian S (2011) Arthroscopic anterior cruciate ligament reconstruction with at least 2.5 years’ follow-up comparing hamstring tendon autograft and irradiated allograft. Arthroscopy 27(9):1195–1202
TGA (2011) Regulatory life cycle for biologicals that are included on the Australian Register of Therapeutic Goods, vol 1, 1st edn. Australian Government, Canberra
White A, Burns D, Christensen TW (2006) Effective terminal sterilization using supercritical carbon dioxide. J Biotechnol 123(4):504–515
Yanke AB, Bell R, Lee A, Kang RW, Mather RC III, Shewman EF, Wang VM, Bach BR Jr (2013) The biomechanical effects of 1.0 to 1.2 Mrad of gamma irradiation on human bone-patellar tendon-bone allografts. Am J Sports Med 41(4):835–840
Zhang GEY, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE (2006a) Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem 98(6):1436–1449. https://doi.org/10.1002/jcb.20776
Zhang J, Davis TA, Matthews MA, Drews MJ, LaBerge M, An YH (2006b) Sterilization using high-pressure carbon dioxide. J Supercrit Fluids 38:354–372. https://doi.org/10.1016/j.supflu.2005.05.005
Zhou M, Zhang N, Liu X, Li Y, Zhang Y, Wang X, Li B (2014) Tendon allograft sterilized by peracetic acid/ethanol combined with gamma irradiation. J Orthop Sci. https://doi.org/10.1007/s00776-014-0556-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this study clarify that they have not accepted any funds for this study and had no conflict of interest related to this study to declare.
Rights and permissions
About this article
Cite this article
Irani, M., Lovric, V. & Walsh, W.R. Effects of supercritical fluid CO2 and 25 kGy gamma irradiation on the initial mechanical properties and histological appearance of tendon allograft. Cell Tissue Bank 19, 603–612 (2018). https://doi.org/10.1007/s10561-018-9709-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-018-9709-y