Abstract
Purpose
While statins and antiplatelet therapies are largely prescribed together worldwide, limited information is available on the safety of their association regarding rhabdomyolysis occurrence. We aimed to assess the reporting of rhabdomyolysis in patients treated with a combination of statin and antiplatelet therapy, compared to statin alone.
Methods
We used the World Health Organization pharmacovigilance database (VigiBase®) to compare the rhabdomyolysis reporting between statin (atorvastatin, fluvastatin, pravastatin, rosuvastatin, and simvastatin) plus antiplatelet therapy (acetylsalicylic acid, clopidogrel, prasugrel and ticagrelor) groups versus statin alone groups, for each statin and antiplatelet therapy. Study setting was restricted to patients aged 45 or older, including reports up until 1st September, 2021. We computed reporting Odds-Ratio (ROR) and their 95% confidence interval (CI) to quantify the disproportionality between groups, adjusted on age and sex.
Results
Among the 11,431,708 reports of adverse reactions, we extracted 9,489 cases of rhabdomyolysis in patients treated with statins, of whom 2,464 (26%) were also treated with antiplatelet therapy. The reporting of rhabdomyolysis was increased when ticagrelor was associated with atorvastatin (ROR 1.30 [1.02–1.65]) or rosuvastatin (ROR 1.90 [1.42–2.54]) compared to the respective statin alone but did not change when aspirin, clopidogrel or prasugrel were considered.
Conclusion
Rhabdomyolysis reporting was increased when ticagrelor -but not other antiplatelet agents- was notified with the most prescribed statins in practice. This finding needs to be considered by physicians especially in high-risk patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Statins are the cornerstone for prevention and treatment of cardiovascular disease and amongst the most widely prescribed medications worldwide [1]. In patients with coronary artery disease, especially following acute coronary syndrome, medical treatment combining statins with antiplatelet agents is recommended by international guidelines [2,3,4]. A large meta-analysis of randomized controlled trials showed that statin therapy reduced the risks of myocardial infarction, coronary revascularization and ischemic stroke by about one-fifth for each mmol/L of low-density lipoprotein cholesterol reduction [5].
Although well tolerated overall, statins have been associated with muscle symptoms that remain the most prevalent adverse drug reaction [6]. Among them, rhabdomyolysis is the most serious but rare complication. About 60% of cases of statin-related rhabdomyolysis were related to drug-drug interactions in randomized clinical trials [7]. While some case reports pointed out a possible association [8,9,10], no study specifically investigated rhabdomyolysis as a possible consequence of a statin-antiplatelet therapy interaction.
Our study aimed to assess the reporting of rhabdomyolysis in patients treated with a combination of statin and antiplatelet therapy compared to statin alone, using the World Health Organization pharmacovigilance database.
Methods
Study design and data source
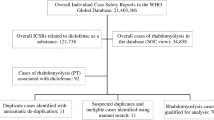
An international retrospective pharmacovigilance disproportionality analysis was performed in the WHO pharmacovigilance database, VigiBase®. Reports were extracted from inception up until 1st September 2021. VigiBase® gathers individual case safety reports (hereafter, the reports) from more than 140 countries of the World Health Organization Programme for International Drug Monitoring, and is maintained by the Uppsala Monitoring Center, Uppsala, Sweden. The dataset was deduplicated using the automated VigiGrade algorithm developed by Uppsala Monitoring Centre. To improve comparability between cases and non-cases, we selected reports occurring in patients aged 45 or older, as most statins and antiplatelet therapies are prescribed in this population [11].
Variables
Exposure variables were statins (atorvastatin, fluvastatin, pravastatin, rosuvastatin, and simvastatin) and antiplatelet therapies (acetylsalicylic acid, clopidogrel, prasugrel, and ticagrelor), identified with their international non-proprietary name in the reports. The studied statins were considered as they remain the most prescribed worldwide. Although pitavastatin could also be informative, there were not enough reports to allow comparisons (only 2 cases with the association of pitavastatin and clopidogrel; and none with other antiplatelet therapies). Drug liability status could be “suspect”, “interacting” or “concomitant”, so as to increase detection of co-administration. Quantitative variable age was accessed as a categorical variable (45–64, 65–74, > 75). The main outcome variable was rhabdomyolysis, identified with the Medical dictionary for regulatory activities query Preferred Term “Rhabdomyolysis”. We additionally identified renal disease with a standardized Medical dictionary for regulatory activities query “Chronic kidney disease” [12]. A serious reaction was defined according to the worldwide accepted definition, endorsed by Vigibase and the European Medical Agency as an adverse reaction that results in death, is life-threatening, requires hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity, or is a birth defect. We chose to report the data associated with antiplatelet therapies reported as alone and/or in combination in the main analysis to minimize the information bias as most reports with antiplatelet therapies in such situations mentioned one antiplatelet therapy although P2Y12 inhibitors such as ticagrelor or prasugrel are almost systematically prescribed in association with aspirin regarding international guidelines [2,3,4]. Consequently, this analysis appeared as the most powerful to study P2Y12 inhibitors when using the VigiBase®. Sensitivity analyses including cases with only single antiplatelet therapy (SAPT) or only dual antiplatelet therapy (DAPT) reports associated with statins were provided in the Supplementary appendix.
Objectives
The primary objective was to evaluate the reporting of rhabdomyolysis in statin plus antiplatelet therapy groups versus statin alone groups, for each statin and each antiplatelet therapy. An additional analysis considering ezetimibe plus statins versus statins alone was also performed. Secondary objectives were to evaluate the reporting of rhabdomyolysis in statin plus antiplatelet therapy groups (with an additional post hoc analysis considering antiplatelet therapies alone) versus all other reports in the database. The latter allows for comparison of an absolute risk of reporting between all combinations of statin and antiplatelet therapy. Also, for statins with an increased reporting of rhabdomyolysis when associated to an antiplatelet therapy as compared to statin alone, we investigated the association between rhabdomyolysis and age to further characterize the association. We also investigated the existence of a dose–effect considering all statins. To this end, the reference group is the one who received a dose strictly lower than the median dose for each statin. Doses for each statin were first standardized to the maximum recommended dose (i.e. 80 mg for atorvastatin, simvastatin and fluvastatin, 40 mg for pravastatin and 20 mg for rosuvastatin), then reports were classified in 3 groups as receiving: 1/ less than the median dose (« low dose»), 2/ a range from the median dose to less than the maximum dose (« medium dose»), and 3/ the maximum dose (« high dose»).
Statistical methods
Disproportionality analysis was used to assess the effect of drugs combination on the rhabdomyolysis reporting. This method is the reference to search for safety signals in pharmacovigilance databases and was described elsewhere [13, 14]. The rational is that adverse drug reaction associated with drug intake(s) will be over-represented (or disproportionality reported) in the base. The use of contingency tables makes it possible to identify which combinations of adverse reactions and drugs significantly deviate from a baseline reporting rate, assumed to be related to random independent reporting. When such case occurs between one or more drugs and an adverse reaction, it is called a disproportionality and a safety signal is raised. The reporting Odds-Ratio (ROR) quantifies the disproportion. It is a standard Odds-Ratio measure in the setting of a reporting database. Interactions can be identified by comparing the reporting of an adverse reaction between a combination of drugs versus one of these drugs alone. One important point is to check that the drug alone is also associated with a higher risk of said adverse reaction. We computed additive interactions from contingency tables in the univariate analysis, and from logistic regression models in the multivariate analysis which was adjusted on age and sex. We did not add multiplicative interaction parameters. Additive interaction provides a clinically understandable way of quantifying the difference in reporting risk between two groups. Statistical testing was performed through Chi2 test for the univariate analysis and logistic regression model for the multivariate analysis; a 95% confidence interval (CI) was computed for each analysis. A lower-end of the 95% CI > 1 was deemed significant. Statistical analyses were performed with R, version 4.0.1 for Windows (R Foundation for Statistical Computing, Vienna, Austria).
Results
We extracted 9,489 cases of rhabdomyolysis in patients treated with statins, of whom 2,464 (26%) were also treated with antiplatelet therapy. Clinical characteristics of rhabdomyolysis cases reported with a combination of antiplatelet and statin therapies are shown in Table 1, Supplementary Tables S1 and S2. Most cases occurred in males (62.5%) over 65 years of age (66.5%). The majority of cases occurred within the first 6 months regardless of the drug used (Fig. 1/graphical abstract). When available, seriousness and death were declared in 97.4% and 5.4% of the cases, respectively.
(Graphical abstract) Reporting Odds-Ratio (ROR) of rhabdomyolysis when combining antiplatelet therapy with statins (compared to respective statin without antiplatelet therapy) and timing of rhabdomyolysis onset according the antiplatelet agent or statin prescribed. Results are presented as ROR with 95% confidence intervals
When statins were associated with acetylsalicylic acid, clopidogrel or prasugrel, the risk of rhabdomyolysis reporting did not significantly differ compared to the one with the respective statin alone (Table 2 for multivariate analysis and Table S3 for univariate analysis; Fig. 1). In the univariate analysis, the risk of rhabdomyolysis was increased when ticagrelor was associated with atorvastatin (ROR 1.48 [1.17–1.88]), simvastatin (ROR 1.81 [1.19–2.75]) or rosuvastatin (ROR 2.18 [1.63–2.91]). In the multivariate analysis, the risk of rhabdomyolysis was increased when ticagrelor was associated with atorvastatin (ROR 1.30 [1.02–1.65]) or rosuvastatin (ROR 1.90 [1.42–2.54]). Sensitivity analyses were concordant and even showed higher associations when considering cases reporting only SAPT (ROR between 2.20 and 7.19 with ticagrelor in the multivariate analysis) but lacked power when considering those reporting only DAPT (Supplementary Table S5A and S5B). In the multivariate analysis compared to statins alone, the risk of rhabdomyolysis was increased with ezetimibe (ROR 1.29 [1.15–1.45]) but cases were too rare to permit subgroup analyses with each statin.
When compared to 11,431,708 other adverse reactions reported with other drugs in Vigibase, the absolute risk of rhabdomyolysis reporting with statins without antiplatelet therapy was significantly increased, with a ROR ranging from 5.52 [4.99–6.12] for pravastatin to 15.85 [15.25–16.46] for simvastatin (Table 3 for multivariate analysis and Table S4 for univariate analysis). When considering statins plus antiplatelet therapies, the strongest signals were observed with the association of ticagrelor and simvastatin (ROR 18.48 [12.05–28.34]), and ticagrelor and rosuvastatin (ROR 16.04 [12.09–21.28]). Sensitivity analyses considering only SAPT or only DAPT (in association with statins) were concordant (Supplementary Table S6A and S6B). Post hoc analysis considering antiplatelet therapies alone was detailed in Supplementary Table S7.
In patients treated with ticagrelor, risk of rhabdomyolysis reporting increased over age, with patients ≥ 75 years-old having a 2.48 [1.69–3.65] ROR versus those aged 45–64 years-old, and in those with chronic kidney disease (ROR 7.15 [3.87–13.20].
We found a dose–effect risk of rhabdomyolysis reporting, with cases with higher doses of statins more likely to report rhabdomyolysis than others: ROR 1.77 [1.66–1.89] for a medium dose as compared to a low dose and ROR 3.19 [2.97–6.42] for a high dose as compared to a low dose.
Discussion
Rhabdomyolysis remains a rare complication, strongly reported with statins. Co-administration of statin and antiplatelet therapy accounts for one quarter of these cases. Mostly, we found that the risk of rhabdomyolysis reporting was 1.3 to almost twofold higher when ticagrelor –but not other antiplatelet agents- was associated with the most prescribed statins in practice.
Worldwide, ischaemic heart disease is the leading cause of death and its frequency is increasing, although mortality has considerably decreased over the past 20 years [15, 16]. This is in line with the growing use of reperfusion therapy in acute myocardial infarction, percutaneous coronary interventions and medications. Appropriate use of recommended medications including statins was strongly associated with lower mortality [5, 15]. The absence of additive risk of rhabdomyolysis reporting provided in our study when combining statin and clopidogrel, prasugrel or aspirin is reassuring, as millions of patients are taking these drugs together. However, we found that the risk of rhabdomyolysis reporting was increased -up to sevenfold higher in sensitivity analyses- when ticagrelor was associated with atorvastatin, simvastatin or rosuvastatin which represent the most prescribed statins worldwide [17]. This was confirmed when rates of rhabdomyolysis with these associations were compared to other adverse reactions with other drugs in the Vigibase® (“absolute risk of reporting”), especially with rosuvastatin and simvastatin (with ROR of 16.04 and 18.48 respectively). The Drugbank database [18] shows moderate interactions between ticagrelor and atorvastatin, simvastatin or rosuvastatin; and even major interactions when combining clopidogrel and atorvastatin or simvastatin. However, the Drugbank reports comprehensive molecular information about drugs, their mechanisms and interactions contrary to our analysis which focused on case safety reports.
Ticagrelor is metabolized through the enzymes cytochrome P450 (CYP) 3A4/3A5 such as atorvastatin and simvastatine [19, 20]. Pharmacokinetic interaction studies showed that co-administration of ticagrelor with these statins increases their plasma concentration, especially with simvastatin which maximum plasma concentration was increased by 81% [21]. Of note, important interindividual variability was observed. These considerations are important because previous studies suggested that the rates of rhabdomyolysis were dose-dependent and related to statin blood levels [17, 22]. Our analysis confirmed this point. Competition on transporter level, especially organic anion transporter polypeptides (OATP1B1 specifically) involved in the metabolism of all statins, may also decrease their biliary and renal excretion. Genetic polymorphism affecting the function of metabolic enzymes and transporter can be involved as well [23] by causing a rise of statin levels. Recent large-scale DNA sequencing analyses argue in favor of the polygenic nature of rhabdomyolysis [24]. Finally, in the PLATO trial ticagrelor was associated with a larger increase of serum creatinine compared to clopidogrel [25], mostly in elderly. This can induce statin retention and potentiate the risk of rhabdomyolysis even if renal excretion of statin remains limited [26]. These potential interactions between ticagrelor and statins were previously reported by the Food and Drug Administration (FDA) and World Health Organization [27, 28].
Statin therapy remains a safe and well tolerated treatment with similar muscular adverse reaction rates reported in randomized controlled trial compared to placebo [7]. Rates were higher in registries [29, 30] but rhabdomyolysis remain rare with an estimated frequency of 0.1–8.4/100 000 patients-years [31, 32]. In higher risk patients with stable coronary artery disease and type 2 diabetes, reported rates of rhabdomyolysis were 0.06% in those treated with ticagrelor (and 0.03% with placebo) at a median follow-up of 40 months [33]. Statin safety has arisen as a major concern among patients, health practitioners and the media. Since in post-acute coronary syndrome patients receive multiple medications including statin and antiplatelet therapy, preferentially prasugrel or ticagrelor unless contraindicated [2,3,4], the interactions we found should be considered by physicians. When patients present risk factors of rhabdomyolysis such as renal impairment, hypertension, diabetes and older age [34], lower dose of statin perhaps in combination with other low density lipoprotein-lowering agents or alternative antiplatelet therapy may be used [17] along with a regular monitoring, especially during the first months. In case of rhabdomyolysis under statin therapy, association with ticagrelor should be searched. In such cases, switching ticagrelor to another P2Y12 inhibitor seems advisable if possible.
Limits
Pharmacovigilance databases have some bias, such as underreporting and missing data. All cases in VigiBase® are self-reported but as rhabdomyolysis is uncommon, this international large database allows for signal detection. In a separate analysis, we confirmed that statin dose was associated with the risk of rhabdomyolysis [17, 22, 34]. We did not include statin dose in the multivariate analysis to avoid loss of power. However, given the difference observed between prasugrel and ticagrelor which are mostly prescribed in patients with acute coronary syndromes usually requiring intensive high-dose statins, our findings with ticagrelor appear reliable. Patients taking SAPT or DAPT may differ. Patients taking DAPT mostly correspond to those with recent cardiovascular events which represent high risk situations for rhabdomyolysis because of additional concomitant medications or potential acute renal insufficiency. However as explained above, such analysis lacked power. Several risk factors for rhabdomyolysis as listed above could not be included in our analysis. Statin metabolism may be affected by race [35] which could not be included in our analysis.
Conclusion
Co-administration of statin and antiplatelet therapy is a common situation which accounts for more than one quarter of the rhabdomyolysis cases reported with statins. The risk of rhabdomyolysis reporting was increased when ticagrelor –but not other antiplatelet agents- was associated with atorvastatin or rosuvastatin. This finding needs to be considered by physicians especially in high-risk patients.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(10059):2532–61.
Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139–228.
Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–367.
Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77.
Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–81.
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment Aetiology and Management. Eur Heart J. 2015;36(17):1012–22.
Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114(25):2788–97.
Kariyanna PT, Haseeb S, Chowdhury YS, et al. Ticagrelor and Statin Interaction Induces Rhabdomyolysis and Acute Renal Failure: Case reports and Scoping Review. Am J Med Case Rep. 2019;7(12):337–41.
Mrotzek SM, Rassaf T, Totzeck M. Ticagrelor Leads to Statin-Induced Rhabdomyolysis: A Case Report. Am J Case Rep. 2017;18:1238–41.
Vrkić Kirhmajer M, Macolić Šarinić V, Šimičević L, et al. Rosuvastatin-Induced Rhabdomyolysis - Possible Role of Ticagrelor and Patients’ Pharmacogenetic Profile. Basic Clin Pharmacol Toxicol. 2018;123(4):509–18.
Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. 2023;147(8):e93–621.
Mozzicato P. Standardised MedDRA queries: their role in signal detection. Drug Saf. 2007;30(7):617–9.
Chrétien B, Lelong-Boulouard V, Chantepie S, et al. Haematologic malignancies associated with clozapine v. all other antipsychotic agents: a pharmacovigilance study in VigiBase(®). Psychol Med. 2021;51(9):1459–66.
Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579–89.
Puymirat E, Simon T, Cayla G, et al. Acute Myocardial Infarction: Changes in Patient Characteristics, Management, and 6-Month Outcomes Over a Period of 20 Years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation. 2017;136(20):1908–19.
Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232–45.
Hopewell JC, Offer A, Haynes R, et al. Independent risk factors for simvastatin-related myopathy and relevance to different types of muscle symptom. Eur Heart J. 2020;41(35):3336–42.
Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074-D82.
Sirtori CR. The pharmacology of statins. Pharmacol Res. 2014;88:3–11.
Zhou D, Andersson TB, Grimm SW. In vitro evaluation of potential drug-drug interactions with ticagrelor: cytochrome P450 reaction phenotyping, inhibition, induction, and differential kinetics. Drug Metab Dispos. 2011;39(4):703–10.
Teng R, Mitchell PD, Butler KA. Pharmacokinetic interaction studies of co-administration of ticagrelor and atorvastatin or simvastatin in healthy volunteers. Eur J Clin Pharmacol. 2013;69(3):477–87.
Holbrook A, Wright M, Sung M, Ribic C, Baker S. Statin-associated rhabdomyolysis: is there a dose-response relationship? Can J Cardiol. 2011;27(2):146–51.
Danielak D, Karaźniewicz-Łada M, Główka F. Assessment of the Risk of Rhabdomyolysis and Myopathy During Concomitant Treatment with Ticagrelor and Statins. Drugs. 2018;78(11):1105–12.
Calderon-Ospina CA, Hernández-Sómerson M, García AM, et al. A Pharmacogenomic Dissection of a Rosuvastatin-Induced Rhabdomyolysis Case Evokes the Polygenic Nature of Adverse Drug Reactions. Pharmgenomics Pers Med. 2020;13:59–70.
Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Turner RM, Pirmohamed M. Statin-Related Myotoxicity: A Comprehensive Review of Pharmacokinetic, Pharmacogenomic and Muscle Components. J Clin Med. 2019; 9(1)
Potential Signals of Serious Risks/New Safety Information Identified by the FDA Adverse Event Reporting System (FAERS). July - September 2018. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/july-september-2018-potential-signals-serious-risksnew-safety-information-identified-fda-adverse.
Macolic Sarinic, V. Interaction between rosuvastatin and ticagrelor resulting in rhabdomyolysis. WHO Pharmaceutical Newsletter. 2018. https://apps.who.int/iris/bitstream/handle/10665/272966/WPN-2018-03-eng.pdf?sequence=1&isAllowed=y
Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients–the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403–14.
Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158(7):526–34.
Alfirevic A, Neely D, Armitage J, et al. Phenotype standardization for statin-induced myotoxicity. Clin Pharmacol Ther. 2014;96(4):470–6.
Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97(8A):52C-60C.
Steg PG, Bhatt DL, Simon T, et al. Ticagrelor in Patients with Stable Coronary Disease and Diabetes. N Engl J Med. 2019;381(14):1309–20.
Nguyen KA, Li L, Lu D, et al. A comprehensive review and meta-analysis of risk factors for statin-induced myopathy. Eur J Clin Pharmacol. 2018;74(9):1099–109.
Kitzmiller JP, Mikulik EB, Dauki AM, Murkherjee C, Luzum JA. Pharmacogenomics of statins: understanding susceptibility to adverse effects. Pharmgenomics Pers Med. 2016;9:97–106.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
VR and CD: Formal analysis, Writing—Original Draft; CD: statistical analysis and methodology; AL, BC, MS and SF: Writing – Review, Validation; FB and JA: Writing—Review & Editing, Supervision.
Corresponding author
Ethics declarations
Competing Interests
Dr Roule has received research grants from Medtronic; speaker fees from Bristol-Myers Squibb, AstraZeneca.
Dr Beygui reports receiving consulting and lecture from Astrazeneca, Bristol-myers Squibb, medtronic, biosensors, Boston scientific Institutionnal research grants: Medtronic, Biosensors, Acist, Boston scientific.
Other authors have nothing to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roule, V., Alexandre, J., Lemaitre, A. et al. Rhabdomyolysis with Co-Administration of Statins and Antiplatelet Therapies—Analysis of the WHO Pharmacovigilance Database. Cardiovasc Drugs Ther (2023). https://doi.org/10.1007/s10557-023-07459-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-023-07459-8