Abstract
Background
Anticoagulant treatment in non-valvular atrial fibrillation (AF) patients with severe chronic kidney disease (CKD) or on dialysis remains a matter of debate. The object of this study was to quantify the benefit-risk profiles of rivaroxaban or apixaban versus warfarin in AF patients with stage 4–5 CKD or on dialysis.
Method
A comprehensive search of the Cochrane Library, PubMed, Ovid, and Google Scholar databases was performed for eligible studies that comparing the effect and safety of rivaroxaban or apixaban versus warfarin in AF patients with stage 4–5 CKD or on dialysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) were abstracted, and then pooled using a random-effects model.
Results
A total of seven studies, one post hoc analysis of RCT and six observational cohorts, were included in this meta-analysis. Compared with warfarin use, the use of rivaroxaban or apixaban was significantly associated with reduced risks of all-cause death (HR = 0.82, 95% CI 0.72–0.93) and gastrointestinal bleeding (HR = 0.87, 95% CI 0.80–0.95). There were no significant differences in the risks of stroke or systemic embolism (rivaroxaban, HR = 0.71, 95% CI 0.43–1.19; apixaban, HR = 0.86, 95%CI 0.68–1.09) and major bleeding (rivaroxaban, HR = 0.96, 95% CI 0.64–1.45; apixaban, HR = 0.56, 95%CI 0.28–1.12).
Conclusions
Current evidence suggests that rivaroxaban or apixaban are safe and at least as effective as warfarin in patients with AF and stage 4–5 CKD or on dialysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with non-valvular atrial fibrillation (AF) are at increased risk of recurrent stroke and other vascular events [1]. Oral anticoagulants are the cornerstone of management of patients with AF [2, 3]. The superiority of vitamin K antagonists (VKAs) such as warfarin in preventing stroke and systemic embolism in patients with AF has been well-established in clinical trials for decades [4]. Nevertheless, optimal results of warfarin depend on maintenance of international normalized ratio within the therapeutic range of 2 to 3 [1,2,3,4]. Numerous interactions with food and other drugs leads to the narrow therapeutic window and unpredictable response of warfarin [5]. Non-vitamin K oral anticoagulants (NOACs) (i.e., dabigatran [a direct thrombin inhibitor] and rivaroxaban, apixaban, and edoxaban [factor Xa inhibitors]) are alternatives for warfarin to prevent stroke in patients with AF and have emerged as the preferred choice [3, 6]. NOACs have an improved efficacy/safety ratio, a predictable anticoagulant effect without the need for routine coagulation monitoring, and fewer food and drug interactions compared with warfarin [3, 6].
Concomitant chronic kidney disease (CKD) in patients with AF presents an additional challenge to identify the optimum management that balances the risk of bleeding and protection from ischemic events. AF and CKD often co-exist because of complex bidirectional interactions, and AF facilitates the development or progression of CKD [7, 8]. Patients with AF and CKD have an increased morbidity and mortality due to a paradoxical increase in both thromboembolic and hemorrhagic risks [9]. Risk stratification and anticoagulant treatment in the clinical settings remains a matter of debate. Despite elimination of warfarin is almost entirely by hepatic metabolism, warfarin is associated with a low time in therapeutic range as creatinine clearance (CrCl) decreases, with either sub-therapeutic effect or over-anticoagulation [10, 11]. NOACs rely to some extent on renal elimination, the level of renal excretion for NOACs in ascending order is apixaban (25%), rivaroxaban (33%), edoxaban (35%), and dabigatran (80%) [3, 12].
While NOACs showed consistent efficacy and safety in patients with mild to moderate CKD compared with warfarin [6, 13], the effect of NOACs in patients with severe CKD or on renal replacement therapy remains unclear. Despite a potential for drug accumulation leading to an increased risk of bleeding, some NOACs (rivaroxaban, apixaban, and edoxaban) have been approved in Europe for anticoagulation in AF with CrCl as low as 15–29 mL/min [6, 14]. One retrospective and longitudinal study including patients with AF and stage 3b-4 CKD demonstrated that rivaroxaban was safer than warfarin, with no bleeding events in the rivaroxaban arm [14]. NOACs have not been well-studied in patients with AF on dialysis. Current AF guidelines do not recommend the use of dabigatran, rivaroxaban, and edoxaban in patients with end-stage renal disease (ESRD) [3]. Since the approval of apixaban in 2013, there has been an increased use of apixaban in patients with AF and ESRD [15, 16]. Future studies are still needed to address the benefits and risks of NOACs in AF patients with advanced CKD or ESRD. Therefore, the aim of this meta-analysis was to summarize available evidence of rivaroxaban or apixaban in order to perform decision-making regarding optimal anticoagulants in AF patients with stage 4–5 CKD or dialysis.
Methods
This study was conducted according to the guidance from the Cochrane Handbook for Systematic Reviews [17]. We used the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) directions to present the results [18]. We did not provide the ethical approval because only the published data were included. The data that support the findings of this meta-analysis are available from the corresponding author upon reasonable request.
Literature Search and Study Selection
Two reviewers (Chen C and Cao YL) independently and systematically searched the Cochrane Library, PubMed, Ovid, and Google Scholar databases with no linguistic restrictions to identify studies that compared the effect of NOACs versus warfarin in non-valvualr AF patients with stage 4–5 CKD or ESRD on dialysis. The retrieval periods were from inception to May 2020. The following index keywords and their similar terms were used in the search: (1) “atrial fibrillation” OR “atrial flutter” AND (2) “vitamin K antagonist” OR “warfarin” AND (3) “non-vitamin K antagonist oral anticoagulants” OR “direct oral anticoagulants” OR “dabigatran” OR “rivaroxaban” OR “apixaban” OR “edoxaban” AND (4) “dialysis” OR “hemodialysis” OR “end-stage kidney disease” OR “end-stage kidney disease” OR “end-stage renal disease” OR “advanced renal disease” OR “stage 4 or 5 chronic kidney disease” OR “stage 5 chronic kidney disease” (Supplemental Table 1). In the manual search, we screened the included articles of reviews published between 2019 and 2020 on anticoagulant therapy in CKD patients [19,20,21,22,23].
Two reviewers (Chen C and Cao YL) independently screened the retrieved studies by reading their titles/abstracts and full-texts, sequentially. Eligible studies were chosen according to the pre-defined inclusion criteria. If facing disagreements, they agreed on final study selection, or discussed with other reviewers (Zhu WG and Liu C).
Inclusion and Exclusion Criteria
Studies (RCTs or observational cohorts) were included if they compared at least one of our specified efficacy and safety outcomes of any NOAC (dabigatran, rivaroxaban, edoxaban, or apixaban; any dose) versus warfarin in adult non-valvular AF patients with stage 4–5 CKD or ESRD undergoing dialysis. Patients with stage 4–5 CKD were defined as patients with an estimated glomerular filtration rate (eGFR) less than 30 mL/min, whereas ESRD patients were defined as patients with an eGFR less than 15 mL/min and/or as patients undergoing dialysis. The adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were regarded as the effect estimates.
We excluded studies that were restricted to AF patients with certain interventions (e.g., cardioversion, ablation, or left-atrial appendage closure), or patients with specific diseases (e.g., coronary artery disease, hyperthyroidism, or cancer). Studies only involving the use of NOACs or warfarin in non-AF patients were excluded. Studies with a sample size of <100 or a follow-up of <1 year were excluded. In addition, cross-sectional studies, reviews, case reports, editorials, letters, or abstracts were excluded. If two or more studies used patients from the same database, the study with the longest follow-up or largest sample size was included.
Definitions of the Objectives and Outcomes
The primary efficacy outcome was a composite of stroke or systemic embolism (SSE), whereas the safety outcome was a composite of major bleeding. Our secondary efficacy outcomes included ischemic stroke and all-cause death, whereas the secondary safety outcomes were intracranial bleeding and gastrointestinal bleeding. We applied the original definitions of the efficacy and safety outcomes in the included individual studies.
Data Abstraction
Two reviewers (Chen C and Cao YL) independently abstracted the relevant data information and performed a cross-check for data accuracy. For each included study, the following basic information was collected: study information (first author, year published, design of study, study period, data source, location), patient characteristics (sample size, age, percentage of males, severity of renal function) information of NOACs (type, dosage, comparisons), follow-up duration, the studied outcomes (sample size and number of events reported at follow-up, and comparative treatment effect estimates of HRs), and adjusted variables. If the adjusted HRs were reported using multiple models, the most adjusted one was abstracted.
Bias Risk and Quality Assessment
The bias risk assessment of RCTs was performed using the Cochrane Collaboration’s tool [24], which involved a total of 7 parts including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. The bias risk of each included study was scored as ‘low’, ‘unclear’ or ‘high’ risk in each part.
For the observational studies, the Newcastle-Ottawa Scale (NOS) tool was used to assess the quality, which involved 3 domains with a total of 9 stars: the selection of cohorts (0–4 stars), the comparability of cohorts (0–2 stars), and the assessment of the outcome (0–3 stars) [24]. A NOS score ≥ 6 stars indicated a moderate-to-high quality, while a low quality if a NOS score < 6 stars [25].
Statistical Analysis
The statistical heterogeneity across the included studies was assessed using the Cochrane Q test and I2 statistic. A P value of <0.10 in the Cochrane Q test or an I2 value of >50% was considered statistically significant for heterogeneity. The natural logarithms of the HRs (Ln [HR]) and standard errors (Ln[upper CI]-Ln[lower CI])/3.92) were calculated. To draw a relatively conservative conclusion, the natural logarithms were pooled using a DerSimonian and Laird random-effects model with an inverse variance method. Heterogeneity is usually dealt with by using a random-effects (RE) model. However, the RE estimator might underestimate the statistical error, and have a high mean squared error, or make unjustifiable changes to individual study weights. The inverse variance heterogeneity (IVhet) model or the quality effects (QE) model could overcome the shortcomings of the RE estimator, and thus were used to re-perform the meta-analysis.
Subgroup analyses were planned on the basis of type of NOACs (rivaroxaban or apixaban), dose of NOACs (high or low dose), and severity of CKD (stage 4 CKD, stage 5 CKD, or dialysis). Comparative effectiveness and safety of edoxaban versus warfarin could not be assessed because of the limited data. The sensitivity analysis was performed to examine the influence of each included study on the pooled results. NOACs Publication bias was visually inspected using a funnel plot. In addition, the Egger’s and Begg’s tests assessed the publication bias statistically, where a P value of <0.10 indicated a potential publication bias.
All the analyses were performed using the Review Manager version 5.3 software (the Cochrane Collaboration 2014, Nordic Cochrane Centre Copenhagen, Denmark), the Stata software (version 15.0, Stata Corp LP, College Station, TX), and MetaXL (version 5.3). A value of P < 0.05 was considered statistically significant.
Results
Study Selection
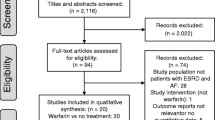
Flow chart of document retrieval is shown in Supplemental Fig. 1. A total of 209 studies were identified from the databases and the reference lists of published reviews. After the screenings of the titles and abstracts, the 14 remaining studies were assessed by full-text screenings. According to the pre-defined criteria, six studies were then excluded because they: (1) did not regard warfarin as controls (n = 2) [26, 27]; (2) focused on AF patients with venous thromboembolism (n = 4) [28,29,30,31]; and (3) included a sample size of <100 for analysis (n = 1) [32]. Finally, a total of seven studies (one post hoc analysis of RCT [33] and six observational cohorts [34,35,36,37,38,39]) were included in this meta-analysis.
Characteristics of the Included Studies
Characteristics of the seven included studies are presented in Table 1. Seven studies with a total of 45,767 AF patients with stage 4–5 CKD or ESRD on dialysis were analyzed and included in this research. The sample size ranged from 269 to 25,523 participants, and mean follow-up periods were 1.03 to 3.4 years. Of these patients, 44.45% were female and the average ages were 68.2 to 81 years. In terms of renal function, one study examined hemodialysis patients only [37], one study included patients with ESRD on dialysis [36], one study included patients with CrCl 25–30 ml/min [33], three studies included patients with stage 4–5 CKD (CrCl<30 ml/min, eGFR<30 ml/min) or on dialysis [34, 35, 39], and one study included patients with stage 4–5 CKD or undergoing hemodialysis [38]. One study used the Cockcroft–Gault equation to estimate renal function [33], two studies used the 4-variable modification of diet in renal disease (MDRD) equation [35, 38], while one study used both [34]. One study did not show how renal function was estimated [38], and another two studies included only hemodialysis or dialysis patients [36, 37]; therefore, renal function estimation was not needed. One study was conducted in Taiwan and the rest were in the USA. For the quality assessment, the post hoc analysis of RCT [33] had a low risk of bias, whereas the observational cohorts had a moderate-to-high quality with NOS scores of 6–8 out of 9 points.
Pooled Analysis of NOACs Versus Warfarin
Primary Efficacy and Safety
Data on the primary outcomes could not be extracted in some individual studies because they only presented data for individual components of the composite outcomes. For example, Makani et al. [35] only reported the outcome of ischemic stroke rather than SSE. Among the included studies, the primary outcomes of SSE and major bleeding were presented in five [33, 34, 36, 38, 39] and six [32, 33, 36,37,38,39] studies, respectively.
Our pooled results based on the RE model suggested that, compared with warfarin use, the use of NOACs was not significantly associated with reduced risks of SSE (HR = 0.71, 95% CI 0.50–1.01; Supplementary Fig. 2) and major bleeding (HR = 0.91, 95% CI 0.66–1.25; Supplementary Fig. 3) in AF patients with stage 4–5 CKD or ESRD on dialysis. Re-analyses with the IVhet (SSE [HR =0.80, 95% CI 0.54–1.19] and major bleeding [HR =1.00, 95% CI 0.69–1.44]) or QE (SSE [HR = 0.80, 95% CI 0.54–1.19] and major bleeding [HR = 1.00, 95% CI 0.69–1.44]) models showed similar results as the main analysis. Similar results were observed when patients with stage 4 CKD, stage 5 CKD, and dialysis were analyzed separately (Supplementary Fig. 4 and 5).
Secondary Efficacy and Safety
As shown in Supplementary Fig. 6 and 7, compared with warfarin use, the use of NOACs was associated with reduced risks of all-cause death (HR = 0.82, 95% CI 0.72–0.93) and gastrointestinal bleeding (HR = 0.87, 95% CI 0.80–0.95) in AF patients with stage 4–5 CKD or ESRD on dialysis. There were no significant differences in the risks of ischemic stroke (HR = 0.62, 95% CI 0.39–1.00) and intracranial bleeding (HR = 0.73, 95% CI 0.48–1.12) between groups of NOACs versus warfarin.
Sensitivity Analysis and Subgroup Analysis
For the primary outcomes, the results did not change substantially after exclusion of one included study at a time. The subgroup analysis based on the NOAC type showed that the use of rivaroxaban or apixaban compared with warfarin showed no differences in SSE (rivaroxaban, HR = 0.71, 95% CI 0.43–1.19; apixaban, HR = 0.86, 95%CI 0.68–1.09) (Fig. 1) and major bleeding (rivaroxaban, HR = 0.96, 95% CI 0.64–1.45; apixaban, HR = 0.56, 95%CI 0.28–1.12) (Fig. 2). In the subgroup analysis based on the NOAC dose, NOAC- versus warfarin-users had no differences in the risk of major bleeding in both groups of high and low dose (Supplementary Fig. 8). Summary of NOACs versus warfarin in atrial fibrillation patients with stage 4–5 chronic kidney disease or on dialysis was presented in Supplementary Table 2.
Publication Bias
As shown in Supplementary Figs. 9 and 10, there was seemingly no potential publication biases for the studied outcomes by inspecting the funnel plots. In addition, the Egger’s and Begg’s tests suggested no publication bias for the primary efficacy and safety outcomes (Supplementary Fig. 11).
Discussion
In our current meta-analysis, compared with warfarin use, the use of rivaroxaban or apixaban neither reduced the risks of SSE and ischemic stroke nor associated with the lower risks of major bleeding and intracranial bleeding in AF patients with stage 4–5 CKD or ESRD on dialysis. However, rivaroxaban or apixaban were associated with significant reductions in all-cause death and gastrointestinal bleeding compared with warfarin. These principal findings were consistent with different analytic methods and corroborate those obtained by Kuno et al. [20] and Ha et al. [22].
In patients with AF and either mild or moderate CKD, prior meta-analyses showed that NOACs are more efficacious and safer than warfarin [23, 40, 41]. However, there is limited evidence for the use of NOACs in severe renal dysfunction because all landmark NOACs trials essentially excluded patients with a CrCl of <30 mL/min (except for a few patients on apixaban with CrCl 25-30 mL/min, the ARISTOTLE trial). In addition, patients with CrCl<15 mL/min were recommended to refrain from NOACs use in European guidelines [6]. Our results showed that, for patients with AF and stage 4–5 CKD or ESRD on dialysis, the use of NOACs is a safer choice than warfarin as it remarkably reduced the risks of all-caused death and gastrointestinal bleeding, while NOACs and warfarin show no significant difference on the efficacy of reducing the risks of SSE. Previous studies have indicated that warfarin could reduce the risks of ischemic stroke, thromboembolism, and mortality in non-ESRD patients, but appeared to have increased risks of hemorrhagic stroke and major bleeding in patients with ESRD [11, 19]. Comparative studies of NOACs and warfarin revealed that, in early-stage or moderate CKD, NOACs had a benefit-risk profile superior to that of warfarin, which remarkably reduced the risks of SSE [23, 24] and major bleeding [41, 42]. Nevertheless, for advanced CKD or ESKD, there was insufficient evidence to establish benefits or harms of warfarin or NOACs [22]. Feldberg et al. [23] found that there was no difference in the stroke risk between NOACs versus warfarin in hemodialysis patients, consistent with our current findings. Furthermore, two studies [20, 21] focused on the patients on dialysis using NOACs showed that apixaban could be considered as a better choice for patients with severer renal impairment, given its favorable safety profile and similar efficacy compared to the other NOACs, which is perhaps not surprising since apixaban has the lowest renal excretion among the four NOACs. Even though there were no differences in outcomes between the four NOACs type and dose in our subgroup analysis, selecting the appropriate type of NOAC should be considered carefully as the different renal excretion of the four NOACs.
Renal insufficiency is associated with enhanced risks of thromboembolic and hemorrhagic events, and the combination of ESRD and AF confers greater risks [21]. Observational data confirm that patients with CKD and AF may benefit from full intensity anticoagulation with warfarin to prevent stroke [43]. However, the anticoagulants in the same population are poorly controlled and often overprescribed, which can lead to massive bleeding and further kidney impairment [44]. Warfarin may cause more hemorrhagic events in CKD patients mainly because warfarin is extensively metabolized by the cytoplasmic P450 (CYP) system of enzymes in the liver, mainly by CYP2C9 [5]. There is evidence that CYP2C9 is down-regulated in CKD patients, especially ESRD, resulting in impaired non-renal clearance and bioavailability of warfarin and other drugs [45]. In a large United States retrospective administrative database analysis, warfarin-related renal disease progression was seen more frequently than rivaroxaban or dabigatran [46]. The potential for warfarin to accelerate renal function decline is particularly relevant to solitary kidney, kidney transplant recipients, ESRD patients who have not yet met the dialysis requirement, and patients who are still producing urine. There are several hypothesized mechanisms by which warfarin exacerbates renal disease progression, but the precise pathophysiology remains unclear [46]. For these reasons, the choice of NOACs in CKD becomes critical and might be associated with a more favorable clinical net outcome than warfarin.
For mild or moderate CKD, the benefit of VKAs in terms of reduced stroke and mortality is well established in AF patients, while all four NOACs showed consistent efficacy and safety compared with non-CKD patients in the respective subgroup analyses of pivotal NOAC trials [13, 47,48,49]. However, there are no RCT data on the use of NOACs for stroke prevention in AF patients with severe CKD or on renal replacement therapy. Rivaroxaban, apixaban, and edoxaban (but not dabigatran) are approved in Europe for the use in patients with severe CKD, with the reduced dose regimen. In view of the individual NOACs’ pharmacokinetics, dose-reduction criteria and available evidence from RCTs, the use of either apixaban or edoxaban may be preferable in these patients. Apixaban had the lowest renal clearance rate (27%), with a 50% reduction in dose under fairly strict conditions, according to its dose reduction algorithm. Furthermore, the relative safety of apixaban versus warfarin has been demonstrated to increase with decreasing renal function [50]. Edoxaban had a renal clearance rate of 50%, but its dose was reduced to 50% more rapidly and was tested in a large subgroup [6]. The efficacy and safety of NOACs in patients with ESRD and on dialysis is unclear and subject to ongoing studies. Apixaban 5 mg BID is currently approved in the United States for patients with chronic, stable dialysis. However, plasma levels associated with apixaban 5 mg BID have recently been shown to be hypertherapeutic. Therefore, anticoagulant therapy in these patients remains a very personalized one, requiring a multidisciplinary approach that takes into account and respects the patient’s preferences. From the above, further randomized trial data are urgently required for severer CKD and ESRD patients to address this issue.
Strengths and Limitations of the Study
Our meta-analysis has several strengths. We included one post hoc analysis of RCT and six observational cohorts, and the number of participants was large enough. In addition, all the including studies adjusted the potential confounding factors of AF. The observed association was therefore less likely due to confounding biases. The sensitivity analysis showed the pooled results were stable.
Nevertheless, we should acknowledge several limitations of our meta-analysis. First, some primary outcomes and baseline characteristic could not be extracted in some studies because the provided data was a single component of the composite results. Second, the duration of follow-up was highly variable, and the methods to estimate renal function were inconsistent across the included studies (Cockcroft–Gault equation or MDRD). Third, although adjusted estimates were used for each study, potential unmeasured residual confounders will still exist. Finally, the outcomes of our study may be unilateral or segmentary, limited by the inadequateness of randomized trial data on our study population.
Conclusion
This meta-analysis presented that compared with warfarin, rivaroxaban or apixaban showed similar risks of SSE, major bleeding and intracranial bleeding, whereas they had reduced risks of all-cause death and gastrointestinal bleeding in AF patients with stage 4–5 CKD or ESRD on dialysis. Rivaroxaban or apixaban might be a reasonable alternative to warfarin in AF patients with severe renal impairment.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code Availability
Review Manager version 5.3 software (the Cochrane Collaboration 2014, Nordic Cochrane Centre Copenhagen, Denmark), the Stata software (version 15.0, Stata Corp LP, College Station, TX), and MetaXL (version 5.3).
References
Klijn CJ, Paciaroni M, Berge E, et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: a European stroke organisation guideline. Eur Stroke J. 2019;4(3):198–223.
Heidbuchel H, Verhamme P, Alings M, et al. Updated European heart rhythm association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17(10):1467–507.
January CT, Wann LS, Calkins H, et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–51.
Furie KL, Goldstein LB, Albers GW, et al. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(12):3442–53.
Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165(10):1095–106.
Steffel J, Verhamme P, Potpara TS, et al. The 2018 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–93.
Bansal N, Zelnick LR, Alonso A, et al. eGFR and albuminuria in relation to risk of incident atrial fibrillation: a meta-analysis of the Jackson heart study, the multi-ethnic study of atherosclerosis, and the cardiovascular health study. Clin J Am Soc Nephrol. 2017;12(9):1386–98.
Go AS, Fang MC, Udaltsova N, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119(10):1363–9.
Gill S, Jun M, Ravani P. Atrial fibrillation and chronic kidney disease: struggling through thick and thin. Nephrol Dial Transplant. 2017;32(7):1079–84.
Kumar S, Lim E, Covic A, et al. Anticoagulation in concomitant chronic kidney disease and atrial fibrillation: JACC review topic of the week. J Am Coll Cardiol. 2019;74(17):2204–15.
Dahal K, Kunwar S, Rijal J, Schulman P, Stroke LJ, Bleeding M. Mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: a meta-analysis of observational studies. Chest. 2016;149(4):951–9.
Weber J, Olyaei A, Shatzel J. The efficacy and safety of direct oral anticoagulants in patients with chronic renal insufficiency: a review of the literature. Eur J Haematol. 2019;102(4):312–8.
Hijazi Z, Hohnloser SH, Andersson U, et al. Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol. 2016;1(4):451–60.
Di Lullo L, Tripepi G, Ronco C, et al. Safety and effectiveness of rivaroxaban and warfarin in moderate-to-advanced CKD: real world data. J Nephrol. 2018;31(5):751–6.
Zhang L, Steckman DA, Adelstein EC, et al. Oral anticoagulation for atrial fibrillation thromboembolism prophylaxis in the chronic kidney disease population: the state of the art in 2019. Cardiovasc Drugs Ther. 2019;33(4):481–8.
Stanton BE, Barasch NS, Tellor KB. Comparison of the safety and effectiveness of apixaban versus warfarin in patients with severe renal impairment. Pharmacotherapy. 2017;37(4):412–9.
Higgins J, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. Available at: www.cochrane-handbook.org. Accessed 28 May 2020.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Randhawa MS, Vishwanath R, Rai MP, et al. Association between use of warfarin for atrial fibrillation and outcomes among patients with end-stage renal disease: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(4):e202175.
Kuno T, Takagi H, Ando T, et al. Oral anticoagulation for patients with atrial fibrillation on long-term Dialysis. J Am Coll Cardiol. 2020;75(3):273–85.
van Zyl M, Abdullah HM, Noseworthy PA, Siontis KC. Stroke prophylaxis in patients with atrial fibrillation and end-stage renal disease. J Clin Med. 2020;9(1):123.
Ha JT, Neuen BL, Cheng LP, et al. Benefits and harms of oral anticoagulant therapy in chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2019;171(3):181–9.
Feldberg J, Patel P, Farrell A, et al. A systematic review of direct oral anticoagulant use in chronic kidney disease and dialysis patients with atrial fibrillation. Nephrol Dial Transplant. 2019;34(2):265–77.
Liu X, Xu Z, Yu P, Yuan P, Zhu W. Non-vitamin K antagonist oral anticoagulants in secondary stroke prevention in atrial fibrillation patients: an updated analysis by adding observational studies. Cardiovasc Drugs Ther. 2020;34(4):569–78.
Zhu W, Wan R, Liu F, et al. Relation of body mass index with adverse outcomes among patients with atrial fibrillation: a meta-analysis and systematic review. J Am Heart Assoc. 2016;5(9):e004006.
Bhatia HS, Bailey J, Unlu O, Hoffman K, Kim RJ. Efficacy and safety of direct oral anticoagulants in patients with atrial fibrillation and chronic kidney disease. Pacing Clin Electrophysiol. 2019;42(11):1463–70.
Steuber TD, Shiltz DL, Cairns AC, Ding Q, Binger KJ, Courtney JR. A multicenter analysis of factors associated with apixaban-related bleeding in hospitalized patients with end-stage renal disease on hemodialysis. Ann Pharmacother. 2017;51(11):954–60.
Herndon K, Guidry TJ, Wassell K, Elliott W. Characterizing the safety profile of apixaban versus warfarin in moderate to severe chronic kidney disease at a veterans affairs hospital. Ann Pharmacother. 2020;54(6):554–60.
Schafer JH, Casey AL, Dupre KA, Staubes BA. Safety and efficacy of apixaban versus warfarin in patients with advanced chronic kidney disease. Ann Pharmacother. 2018;52(11):1078–84.
Reed D, Palkimas S, Hockman R, Abraham S, Le T, Maitland H. Safety and effectiveness of apixaban compared to warfarin in dialysis patients. Res Prac Thrombosis Haemostasis. 2018;2(2):291–8.
Sarratt SC, Nesbit R, Moye R. Safety outcomes of apixaban compared with warfarin in patients with end-stage renal disease. Ann Pharmacother. 2017;51(6):445–50.
De Vriese AS, Caluwé R, Pyfferoen L, et al. Multicenter randomized controlled trial of vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: the Valkyrie study. J Am Soc Nephrol. 2020;31(1):186–96.
Stanifer JW, Pokorney SD, Chertow GM, et al. Apixaban versus warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation. 2020;141(17):1384–92.
Weir MR, Ashton V, Moore KT, Shrivastava S, Peterson ED, Ammann EM. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and stage IV-V chronic kidney disease. Am Heart J. 2020;223:3–11.
Makani A, Saba S, Jain SK, et al. Safety and efficacy of direct oral anticoagulants versus warfarin in patients with chronic kidney disease and atrial fibrillation. Am J Cardiol. 2020;125(2):210–4.
Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138(15):1519–29.
Chan KE, Edelman ER, Wenger JB, Thadhani RI, Maddux FW. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. 2015;131(11):972–9.
Chang SH, Wu CCV, Yeh YH, et al. Efficacy and safety of oral anticoagulants in patients with atrial fibrillation and stages 4 or 5 chronic kidney disease. Am J Med. 2019;132(11):1335–43.
Coleman CI, Kreutz R, Sood NA, et al. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and severe kidney disease or undergoing hemodialysis. Am J Med. 2019;132(9):1078–83.
Andò G, Capranzano P. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with chronic kidney disease: a systematic review and network meta-analysis. Int J Cardiol. 2017;231:162–9.
Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–62.
Kimachi M, Furukawa TA, Kimachi K, Goto Y, Fukuma S, Fukuhara S. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017;11(11):CD011373.
Bonde AN, Lip GY, Kamper AL, et al. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol. 2014;64(23):2471–82.
Oliver T, Salman LA, Ciaudelli B, Cohen DA. Anticoagulation-related nephropathy: the most common diagnosis you've never heard of. Am J Med. 2019;132(8):e631–3.
Dreisbach AW, Japa S, Gebrekal AB, et al. Cytochrome P4502C9 activity in end-stage renal disease. Clin Pharmacol Ther. 2003;73(5):475–7.
Yao X, Tangri N, Gersh BJ, Shah ND, Nath KA, Noseworthy PA. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70(21):2621–32.
Fordyce CB, Hellkamp AS, Lokhnygina Y, et al. On-treatment outcomes in patients with worsening renal function with rivaroxaban compared with warfarin: insights from ROCKET AF. Circulation. 2016;134(1):37–47.
Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran compared with warfarin in patients with atrial fibrillation in relation to renal function over time-a RE-LY trial analysis. Am Heart J. 2018;198:169–77.
Bohula EA, Giugliano RP, Ruff CT, et al. Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation. 2016;134(1):24–36.
Hohnloser SH, Hijazi Z, Thomas L, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33(22):2821–30.
Funding
This study was funded by the National Natural Science Foundation of China (81770392, 81770394, 81700344, 81800344, 81800345), Science and Technology Program Foundation of Guangzhou (201707010124), Guangdong Natural Science Foundation (2017A030310311, 2017A030313795), Young Teachers’ Basic Scientific Research Business Expenses Project (20ykpy72), Medical Research Foundation of Guangdong Province (A2017030, A2018107, A2018082), and China Postdoctoral Science Foundation (2019 M663312, 2019TQ0380, 2019 M660229).
Author information
Authors and Affiliations
Contributions
CL and WZ developed the idea of the study. CC obtained the data and wrote the manuscript, YC performed analysis and interpreted the results. YZ participated in data analysis and results interpretation. JM and YD participated in manuscript writing and revision. All authors have read and approved the manuscript and agreed with their contributions.
Corresponding authors
Ethics declarations
Ethics Approval
We did not provide the ethical approval because only the published data were included.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 3708 kb)
Rights and permissions
About this article
Cite this article
Chen, C., Cao, Y., Zheng, Y. et al. Effect of Rivaroxaban or Apixaban in Atrial Fibrillation Patients with Stage 4–5 Chronic Kidney Disease or on Dialysis. Cardiovasc Drugs Ther 35, 273–281 (2021). https://doi.org/10.1007/s10557-021-07144-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-021-07144-8