Abstract
Beta blockers and renin-angiotensin-aldosterone-inhibitors (RAAS-i) including angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) have been a mainstay of guideline-based medical therapy for heart failure with reduced ejection fraction (HFrEF) for decades. However, initial evidence supporting each of the aforenoted class of drug for heart failure indications was largely found independently of the other two classes with the exception of the addition of BBs to ACEIs. In the initial ACEI trials for HFrEF, few participants were on BBs as BBs were seen as contraindicated in HFrEF at the time. The seminal BB in HFrEF trials had high prevalence of ACEIs use as ACEIs for HF were standard of care by then, but ARBs as a class were still in their infancy. We closely examine the evidence for combinations of BB and ACEIs versus ARBs in HFrEF. In doing so, we demonstrate the lack of evidence for consideration of ARBs to be interchangeable with ACEIs when used in combination with BB and provide evidence that calls in to question the validity of assuming benefits from each drug class are independently cumulative, widening the gap between ACEIs and ARBs when used with BBs. Modern guidelines should emphasize this lack of evidence for the combination use of ARB and BB in HFrEF, except for candesartan. Even as practice moves towards the widespread uptake of angiotensin receptor-neprilysin inhibitors (which contain the ARB valsartan) in heart failure, the distinction has important implications for the ongoing role of combination therapy with BB, which thus far has been assumed, but not proven.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Beta blockers (BBs) in combination with renin-angiotensin-aldosterone-inhibitors (RAAS-i), including angiotensin-converting enzyme inhibitors (ACEIs) and later angiotensin II receptor blocker (ARBs), have long been the mainstay of guideline-based management of reduced ejection fraction heart failure (HFrEF). The 2017 American College of Cardiology/American Heart Association/Heart Failure Society of America (ACC/AHA/HFSA) Focused Update of the 2013 ACC/AHA Guideline for the Management of Heart Failure offer a class 1 recommendation with the highest level of evidence (A) for “inhibition of RAAS with either ACEIs or ARBs in conjunction with beta blockers (BBs) for patients with chronic HFrEF to reduce morbidity and mortality.” [1] These guidelines specify that the use of ARBs is recommended in patients intolerant to ACEIs and for patients already tolerating ARBs (class of recommendation (COR) = 1, level of evidence (LOE) = A) [2]. Conversely, the 2016 European Society of Cardiology (ESC) Guidelines for the management of heart failure give a COR = 1, LOE = a recommendation for an “ACEI… in addition to BB for symptomatic patients with HFrEF” but only a COR = 1, LOE = B recommendation for “ARB…to reduce the risk of HF hospitalization and cardiovascular death in symptomatic patients unable to tolerate an ACEI.” Additionally, the ESC gives a COR = 1 indication for ARB only for HFrEF patients unable to tolerate ACEIs, and gives a COR = II, LOE = B recommendation for the use of ARBs in patients with HF who are symptomatic despite treatment with a BB and unable to tolerate mineralocorticoid receptor antagonists (MRAs) [3]. In clinical practice, ACEIs and ARBs are often treated interchangeably, with clinicians confusing hypertension and HFrEF indications [4]. The emergence of angiotensin receptor-neprilysin inhibitors (ARNIs) may seem to make the difference between ACEIs and ARBs in conjunction with BB moot for many HF patients; however, for those who cannot tolerate ARNIs, the distinction remains important. Additionally, the unproven benefit for combination use of BB and ARB may have implications for the role of BBs used in conjunction with ARB-containing ARNIs. By reviewing the seminal HF trials with specific attention paid to subgroup analysis of patients who were also on BB, we hope to drive home the fallacy in the misbelief that the combination of ARBs and BBs is equivalent to ACEIs and BBs.
Historical Context (Fig. 1)
Beta Blockers
For the first decades of their availability, BBs were contraindicated in HF as they were presumed to worsen HF due to negative inotropy; prior to the BB and RAAS-i era, a combination of digoxin and diuretics was the standard of care [5]. Waagstein first presented clinical data in 1975 challenging BBs contraindication in HF by treating 7 patients with congestive HF with alprenolol or practolol [6]. Following this, a 1980 study treated 28 patients with HFrEF with either practolol, alprenolol, or metoprolol. The treated patients showed an improvement in cardiac function, which was confirmed in a retrospective comparison to matched controls [7, 8]. In 1985, the first randomized controlled trial of metoprolol in idiopathic dilated cardiomyopathy demonstrated both the tolerability of and functional improvement with BB use in chronic congestive heart failure [9]. The larger metoprolol in Dilated Cardiomyopathy Trial in 1993 confirmed this finding [10]. In 1994, the Cardiac Insufficiency Bisoprolol Study (CIBIS) study established the role of bisoprolol in heart failure, showing improved functional status and decreased hospitalization, however, no statistically significant mortality benefit was noted [11]. By the mid-1990s, BBs still did not have widespread acceptance for the treatment of HF. The 1995 ACC/AHA heart failure guidelines still described BBs in chronic HF as investigational [6, 12]. In 1999, two seminal trials of BBs in HF were released: the Cardiac Insufficiency Bisoprolol Study-II (CIBIS-II) study [13], the first trial to establish the mortality benefit of bisoprolol in HF, and the Metoprolol CR/XL Randomized Intervention Trial in Congestive Heart Failure (MERIT-HF) trial [14], which established the mortality benefit of metoprolol succinate in heart failure. By the 2001 update of the ACC/AHA heart failure guidelines, as well as the 2001 ESC chronic heart failure guidelines, BBs in conjunction with ACEIs were made to be standard of care in heart failure management [15, 16]. Although a twentieth-century drug, it was not until the twenty-first century that BBs become the bedrock of HF management that they are today.
RAAS Inhibitors
In 1981, the first orally available ACEI, captopril, was released [17]. ACEIs’ role in HF management was more immediately pursued and clearly demonstrated by the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) [18] (1987) and Studies of Left Ventricular Dysfunction trial (SOLVD) [19] (1991) which were placebo-controlled trials showing significant reduction in HFrEF morbidity and mortality in patients treated with enalapril. The 1992 Survival and Ventricular Enlargement (SAVE) trial went on to demonstrate mortality benefit in patients with acute MI and asymptomatic LV dysfunction treated with captopril [20].
ACEIs became a cornerstone of HF treatment by the release of the 1995 ACC/AHA heart failure guidelines [12]. The 1997 ESC Working Group on Heart Failure additionally recommended ACEIs in all symptomatic heart failure patients [21]. In 1995, the first ARB, losartan, was cleared for use by the Food and Drug Administration (FDA) [22]. Given its action on RAAS inhibition, there was early interest in the use of ARBs for HFrEF. As of the early 2000s, its use in chronic HFrEF was a class IIa recommendation in ACEI-intolerant patients [15].
ACEIs were already accepted as standard of care for HF when BBs and ARBs were being investigated for use in HF. Thus, many of the participants in the studies investigating the use of BBs in HF were already on ACEIs. Only limited numbers of study subjects were on ARBs. As ARBs gained acceptance, much of the synergy with BBs was assumed based on existing ACEI data, however, this assumption would go on to be proven false [4].
Review of Evidence
Beta Blocker Trials
The 2013 ACC/AHA Guideline for the Management of Heart Failure gives a COR = I, LOE = A recommendation for the use of three different BBs in the management of HFrEF: carvedilol, metoprolol, and bisoprolol [23]. None of the trials on which these recommendations are based have studied the different effects of ACEIs versus ARBs.
The 1999 CIBIS-II study established the mortality benefits of BB therapy in heart failure [13]. Adding bisoprolol to standard HF therapy reduced mortality by 24%. A total of 96% of the patients enrolled in CIBIS-II were on ACEIs at baseline; however, there were none on ARBs. Following CIBIS-II, the MERIT-HF trial established the mortality benefits of metoprolol succinate in patients with HFrEFF [14]. Most of the patients (89%) in the MERIT-HF randomized to the intervention arm were on ACEIs prior to enrollment, compared to only 7% (n = 133) on ARBs. There was no subgroup analysis comparing these two groups. Similarly, the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study, which studied carvedilol in severe HFrEF, mandated all patients be on an ACEI or ARB (if tolerated), however, there was not any subgroup analysis on the differential outcomes of these subgroups [24]. The only other trials that studied BB use in heart failure and enrolled patients on both ACEI and ARBs were the Carvedilol or Metoprolol European Trial (COMET) [25] (2003) and the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalizations in Seniors with Heart Failure (SENIORS) [26] (2005) trials. In COMET, there was a 91–92% background ACEI use and 6–7% background ARB use. A total of 81.7% and 6.2% of patients in SENIORS were on ACEIs and ARBs respectively. Only these four trials reported the number of enrolled patients on ARBs and none of these trials included this population as a pre-defined subgroup to be analyzed. The combination of BBs and RAAS-i has become the foundation of HF management, but the number of patients on ARBs in the seminal trials for BB use has been extremely low, and as abovementioned, none of the four trials have included patients on this combination as a part of the pre-defined subgroups for analysis.
ACEI and ARB Trials
Soon after the release of ARBs, a number of trials seemed to demonstrate the interchangeability of ACEIs and ARBs for HF. However, many did not have substantial concurrent BB use, and few reported subgroup analyses stratified by BB use. In one of the earliest trials comparing ACEIs and ARBs, Dickstein et al. randomized 166 chronic HF patients to enalapril or losartan and found no significant difference in exercise capacity, dyspnea-fatigue index, or biochemical markers. A total of 30% of the ARB and 11% of the patients in the ACEI arms were on BBs, but subgroup analysis was not reported [27]. The 1997 Evaluation of Losartan in the Elderly (ELITE) trial randomized 722 ACEI naïve patients with HF to losartan versus captopril and showed a trend towards a reduction in the composite endpoint of death or HF admission in the ARB arm at 48 weeks (9.4% vs 13.2%, p = 0.075); however, this was not statistically significant. A total of 63% and 55% of the ACEI and ARB groups respectively were on BBs, but subgroup analysis was not provided [28]. In 2002, the Heart Failure Valsartan Exercise Capacity Evaluation (HEAVEN) trial randomized patients with mild to moderate HF to either enalapril or valsartan (after being on a stable dose of ACEI) and demonstrated that valsartan was as effective as enalapril in terms of improvement in exercise capacity after 12 weeks. A total of 52% of participants were on BBs; however, subgroup analysis was not reported [29]. The Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM-Alternative) trial in 2003 randomized 2028 HFrEF patients intolerant to ACEIs to candesartan versus placebo and found a reduction in composite cardiovascular death and HF admission (unadjusted HR 0.77, 95% CI 0.67–0.89, p = 0.0004). Fifty-five percent of each arm were on baseline BB therapy, subgroup analysis was not performed [30].
The Randomized Evaluation of Strategies for Left Ventricular Dysfunction Pilot Study (RESOLVD) (2003) studied the effects of metoprolol XL versus placebo on patients on either candesartan, enalapril, or a combination of candesartan plus enalapril. Ultimately, the combination of candesartan, enalapril, and metoprolol was shown to have a beneficial effect compared to either of the other combinations. However, there was no subgroup analysis comparing candesartan plus metoprolol versus enalapril plus metoprolol [31, 32].
Among studies that did offer sub-group analysis, the first indication that concomitant BB usage may negate the equivalence of ACEIs and ARBs in HF came with the results of the Losartan Heart Failure Survival Study (ELITE II) (2000) which randomized 3152 HFrEF patients to losartan versus captopril. After a mean follow-up of 555 days, there was no difference between the groups in all-cause mortality, sudden death, and resuscitated arrest. Concurrent BB use was 20% in each arm. Subgroup analysis of losartan plus BB versus captopril plus BB showed an increased risk of death (HR 1.77, CI approximately 1.1–2.9). No difference was found in the subset of patients not on BBs. It should be noted that they reported that overall patients in both arms on BBs did better than those not on BBs; however, there was no randomization to BB or not [33].
The Valsartan Heart Failure Trial (Val-HeFT trial) (2001) went on to further examine the effectiveness of various combinations of ARBs, ACEIs, and BBs. A total of 5010 HFrEF patients were randomized to valsartan or placebo. Approximately 93% of participants were on concurrent ACEI treatment and 35% had concurrent BB treatment. Overall, the valsartan group showed a treatment benefit for composite morbidity and mortality; however, subgroup analysis showed evidence of harm in the combined ACEI + ARB + BB (n = 1610) group with increased death and a trend towards increased combined morbidity and mortality endpoints. Additionally, subgroup analysis of the valsartan + BB group (n = 140) did not result in significant improvements over placebo in either morbidity or mortality but did demonstrate benefit in the absence of BB or ACEI. This is in contrast to evidence of benefit in the combination of ACEI+ARB without BB [34]. Caution must be exercised in analyzing these subgroup analyses, particularly given the small sample size of the valsartan + BB group. However, despite secondary analysis, Val-HeFT is one of a very few numbers of RCTs that analyzed combinations of ACEI, ARB, and BB in HF at all, and for lack of any large RCTs that address the question of ARB and BB as a primary goal, these findings should not be disregarded out of hand.
Seeming to contradict Val-HeFT, CHARM-Added (2003) randomized 2548 patients with HFrEF already on ACEIs to candesartan versus placebo, with 55% baseline underlying BB use and followed for a mean of 41 months. The primary outcome was a composite of cardiovascular death and hospitalization. It found a reduction in the composite outcome with the addition of ARB in the BB subset, but not the subset not on BB. However, this is in the context of ACEI use as a condition of enrollment and does not demonstrate the efficacy of ARB plus BB without ACEI [35]. The Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan (OPTIMAAL) trial (2002) randomized 5477 patients post-acute MI and HF to losartan versus captopril. The majority of the patients (79%) were on BB therapy. In both the BB and non-BB arms, there was a non-statistically significant trend favoring the ACEI over the ARB with similar effect size. This was driven by a trend towards higher rate of sudden cardiac death in the ARB arm [36].
Moreover, a 2012 Cochrane Systematic Review looking at ARBs compared to placebo for chronic heart failure analyzed 22 double-blind randomized control trials which included a total of 17,900 patients with reduced EF, and found no reduction in total mortality (RR 0.87, 95% CI 0.76–1.00) or total morbidity using hospitalization as outcome (RR 0.94, 95% CI 0.88–1.22) [37]. They did not report on concurrent BB use.
Finally, meta-analyses have used sophisticated statistical methods to attempt to compare various combinations of heart failure drugs based on data from various heart failure trials including many of the aforementioned. A network meta-analysis by Burnett et al. suggested that the combination of ARBs and BB has better outcome than ARBs alone; however, the finding was not statistically significant (HR 0.534, 95% credible interval 0.254–1.021). In contrast, the combination of ACEI and BB was demonstrated to be superior to ACEIs alone (HR 0.684, 95% credible interval 0.561–0.797) [38]. One should note that this would not be considered to be high-quality evidence as the differences in statistical significance may be due to the relatively smaller number of patients on ARBs compared to ACEIs included for analysis, and differences in baseline characteristics as patients were not randomized to BBs.
ARNIs
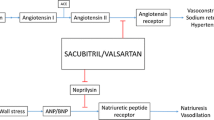
It is necessary to note that sacubitril-valsartan, the currently available ARNI, contains an ARB and not an ACEI for concern for unacceptably increased risk of angioedema with ACE inhibition as was seen with omapatrilat, an earlier abandoned agent with neprilysin inhibition properties [39]. The 2014 Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial randomized 8442 HFrEF patients already on an ACEI/ARB and a BB to sacubitril-valsartan versus enalapril. The majority (93%) of the patients in both arms were on BBs. A 2.3% absolute risk reduction of death was noted in the ARNI group (HR 0.84%, 95% CI 0.76 to 0.93) [40]. Subgroup analysis in the BB group was not performed, but high baseline BB use suggests these results are extrapolatable to simultaneous BB use in the real world.
Shortcomings and Future Steps
It must be acknowledged that there are significant limitations in the body of evidence that has directly compared HF outcomes in patients on BB with either ACEI or ARB. It remains possible that differences in ACEI and ARB groups were due to dosing effects as opposed to true class differences. For example, in ELITE I and II, participants were titrated to only 50 mg of losartan daily however from the HEAAL study, we know that high dose (150-mg losartan daily) had lower rates of composite mortality and HF hospitalization than the 50-mg losartan daily dose (HR 0.9, CI 0.82–0.99, p = 0.027) [28, 33, 41]. Beyond differences in study design, no randomized control studies have directly compared ACEIs and ARBs in context of concurrent BB use for heart failure. While there is some signal for concurrent BB use attenuating the effect of ARB in HFrEF in post hoc subgroup analyses, subgroup analysis inherently bears increased risk of false-positive findings by virtue of statistical chance. There is also substantial variance in entrance criteria, follow-up intervals, and endpoint definitions. The role of side effects and subsequent discontinuations are lost in sub-group analysis. Thus, by no means is the attenuating effect of BB when used with ARB seen in some subgroup analyses definitive proof of harm, however, they should be a cause for caution. Unfortunately for this specific question, at this point, it is unlikely that an RCT primarily comparing ACEI and ARB in HF will ever be performed and definitive evidence will likely remain elusive.
Additionally, there may be differences within classes that limit reasonable inference. Currently, evidence only supports the use of candesartan, as there is no positive data concerning efficacy and safety about combining BBs with other ARBs for the indication of heart failure. This is significant, as prescriber data suggests that real-world practices diverge from the most closely studied drugs. While some have called for the use of ARB over ACEI for HTN, we fear that indications may too easily become confounded. For example, a recent Medscape.com article was titled “Time to Ditch ACE Inhibitors for CVD?”; however, the underlying evidence was a meta-analysis by Messerli et al. specifically looking at the indication of hypertension, not the much broader label of CVD [42, 43]. In a world where providers are inundated with evidence, headlines matter and can unfortunately mislead. Careful evidence-based approaches are paramount in overcoming these forces. Providers should remain vigilant in reassessing HF patients on ARBs to assure that they were not inappropriately initiated or not held over from indications prior to HF diagnosis.
Moving forward, questions regarding the combination of ARB and BB in HF remain relevant as ARB-containing ARNIs ascend. For the ARNI intolerant, the question remains unchanged. However, given the scant evidence of positive effect and limited evidence for the benefit of BB + ARBs combination in HFrEF, it is plausible that the addition of BB to ARB-containing ARNIs provides no net additional benefit, a significant clinical question heretofore unanswered. Comparing the majority of the patients that received BB to the minority that did not receive BB could not help, as nowadays, when BB treatment is the standard of care, those who did not receive BB had different risk profile (contraindications, intolerance, etc.). Although this seems unlikely as BBs are so established as a cornerstone of HF therapy and ARNIs appear to be the future of RAAS-i in HF, such tectonic changes sparked by concerns raised in post hoc analyses are not unheard of.
As an illustrative example of the evolution of guidelines resulting in the removal of a medication from standard of care therapy as concurrent therapies advanced, one may look to the use of intravenous BB in patients presenting with ST-segment elevation myocardial infarction. For years, guidelines gave COR = I recommendation for intravenous BB in patients without hypotension, bradycardia, or heart failure. A retrospective analysis of the Global Utilization of Streptokinase and TPA (alteplase) for Occluded Coronary Arteries (GUSTO)-I trial published in 1998 showed that early intravenous atenolol increased mortality and morbidity in patient with ST-segment elevation myocardial infarction receiving thrombolytic therapy [44]. The signal found in the aforementioned post hoc analysis raised the question addressed by the clopidogrel and metoprolol in Myocardial Infarction Trial (COMMIT) randomized trial in 2005, showing that early intravenous use of beta blockers in acute myocardial infarction increased the risk of cardiogenic shock without affecting mortality, which resulted in later evolution of guidelines [45]. As RAAS-i in HF progresses to the ARNI era, more study of the interaction between ARB containing ARNIs and BB is warranted, and concerns from the ARB era should not be dismissed out of hand.
Conclusion
The 1990s and early 2000s saw a revolution in the medical management of HF with the prominence of ACEIs, discovery of ARBs, and changing role of BBs. These advances were happening concurrently, and not necessarily in a linear order. Accordingly, as each agent proved its value independently in controlled trial settings, initial studies did not necessarily take into account the concomitant rise of other agents, particularly with regard to ARBs and BBs. While early evidence emerged arguing the similarity of ACEIs and ARBs, when the simultaneous use of BB was considered, differences favoring ACEIs began to materialize.
The combination of ARBs with BBs has not been prospectively compared to ARBs alone or BBs alone. Retrospective subgroup analysis of the ELITE II and Val-HeFT trials call in to question the validity of assuming benefits from each drug class are independently cumulative.
Current ACC/AHA HF treatment guidelines give both ACEIs and ARBs in conjunction with BBs COR = 1 and LOE = A recommendation status. However, there is not high-quality RCT data demonstrating the equivalence of ARBs to ACEIs when used in conjunction with BBs. Preference for ACEI over ARB is implied in recommendations for use of ARBs for ACEI intolerant patients; however, it is not explicit as compared to current ESC heart failure guidelines [1, 3]. There is clear LOE = A evidence to support the combination of ACEI and BB in HFrEF; however, no such evidence exists for the combination of ARB plus BB. One may be tempted to write off this subtle distinction as ARNIs as a class begins to demonstrate their potential over ACEIs or ARBs, but for patients who are ARNI intolerant, this distinction remains important. For those patients who are on ARBs instead of ACEIs, providers should remain vigilant that an ACEI contraindication or intolerance truly exists. Additionally, it remains possible, given that current ARNIs contain an ARB (valsartan) that the combination of ARNI+BB is no more effective than ARNI without BB. For this question, more study is warranted.
References
Yancy CW, Mariell Jessup C, Chair Biykem Bozkurt V, et al. WRITING GROUP MEMBERS* 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure ACC/AHA/HFSA FOCUSED UPDATE Yancy et al Selection of Writing Committee Members Evidence Review and Evidence Review Committees Class of Recommendation and Level of Evidence. Circulation. 2017;136:137–61. https://doi.org/10.1161/CIR.0000000000000509.
January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. J Am Coll Cardiol. 2014;64(21):e1–e76. https://doi.org/10.1016/j.jacc.2014.03.022.
Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of. Eur Heart J. 2016;37(27):2129–200. https://doi.org/10.1093/eurheartj/ehw128.
Strauss MH, Hall AS. Angiotensin receptor blockers may increase risk of myocardial infarction: unraveling the ARB-MI paradox. Circulation. 2006;114(8):838–54. https://doi.org/10.1161/circulationaha.105.594986.
Waagstein F. The evolution of the use of β-blockers to treat heart failure. Circulation. 2017. https://doi.org/10.1161/CIRCULATIONAHA.117.029934.
Waagstein F, Hjalmarson F, Varnauskas E, Wallentin I. Effect of chronic beta-Adrenergic receptor blockade in congestive cardiomyopathy. Heart. 1975. https://doi.org/10.1136/hrt.37.10.1022.
Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. PROLONGATION OF SURVIVAL IN CONGESTIVE CARDIOMYOPATHY BY BETA-RECEPTOR BLOCKADE. Lancet. 1979;313(8131):1374–6. https://doi.org/10.1016/S0140-6736(79)92010-5.
Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Beneficial effects of long-term beta-blockade in congestive cardiomyopathy. Br Heart J. 1980;44(2):117–33. https://doi.org/10.1136/hrt.44.2.117.
Anderson JL, Lutz JR, Gilbert EM, et al. A randomized trial of low-dose beta-blockade therapy for idiopathic dilated cardiomyopathy. Am J Cardiol. 1985;55(4):471–5. https://doi.org/10.1016/0002-9149(85)90396-0.
Waagstein F, Hjalmarson A, Swedberg K, et al. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Lancet. 1993. https://doi.org/10.1016/0140-6736(93)92930-R.
CIBIS Investigators and Committees. A randomized trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS). Circulation. 1994;90(4):1765–1773. https://doi.org/10.1161/01.cir.90.4.1765.
Williams J, Bristow R, Fowler MB, et al. Guidelines for the Evaluation and Management of Heart Failure. Circulation. 1995;92(9):2764–2784. https://doi.org/10.1161/01.CIR.92.9.2764.
CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet (London, England). 1999;353(9146):9–13.
MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet (London, England). 1999;353(9169):2001–2007. https://doi.org/10.1016/S0140-6736(99)04440-2.
Hunt SA, Baker DW, Chin MH, et al. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). Circulation. 2001. https://doi.org/10.1161/hc4901.102568.
Remme W. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22(17):1527–60. https://doi.org/10.1053/euhj.2001.2783.
Williams B. Drug discovery in renin-angiotensin system intervention: Past and future. In: Therapeutic Advances in Cardiovascular Disease. Vol 10. SAGE Publications Ltd; 2016:118–125. https://doi.org/10.1177/1753944716642680
Swedberg K, Idanpaan-Heikkila U, Remes J. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316(23):1429–1435. https://doi.org/10.1056/NEJM198706043162301.
Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. https://doi.org/10.1056/NEJM199108013250501.
Pfeffer MA, Braunwald E, Moyé LA, et al. Effect of Captopril on Mortality and Morbidity in Patients with Left Ventricular Dysfunction after Myocardial Infarction: Results of the Survival and Ventricular Enlargement Trial. N Engl J Med. 1992;327(10):669–77. https://doi.org/10.1056/NEJM199209033271001.
Remme WJ, Cleland JGF, Dargie H, et al. The treatment of heart failure: The Task Force of the Working Group on Heart Failure of the European Society of Cardiology. Eur Heart J. 1997;18(5):736–753. https://doi.org/10.1093/oxfordjournals.eurheartj.a015339.
Ripley E, Hirsch A. Fifteen years of losartan: what have we learned about losartan that can benefit chronic kidney disease patients? Int J Nephrol Renovasc Dis 2010;3:93-98. http://www.ncbi.nlm.nih.gov/pubmed/21694934. Accessed Aug 7 2019.
Yancy CW, Jessup M, Bozkurt B, et al. ACCF/AHA Guideline for the Management of Heart Failure. Circulation. 2013;2013:128(16). https://doi.org/10.1161/CIR.0b013e31829e8776.
Packer M, Fowler MB, Roecker EB, et al. Effect of Carvedilol on the Morbidity of Patients With Severe Chronic Heart Failure Results of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study. 2002. doi:https://doi.org/10.1161/01.CIR.0000035653.72855.BF
Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362(9377):7–13. https://doi.org/10.1016/S0140-6736(03)13800-7.
Flather MD, Shibata MC, Coats AJS, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26(3):215–25. https://doi.org/10.1093/eurheartj/ehi118.
Dickstein K, Chang P, Willenheimer R, et al. Comparison of the effects of losartan and enalapril on clinical status and exercise performance in patients with moderate or severe chronic heart failure. J Am Coll Cardiol. 1995. https://doi.org/10.1016/0735-1097(95)80020-H.
Pitt B, Segal R, Martinez FA, et al. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet. 1997. https://doi.org/10.1016/S0140-6736(97)01187-2.
Willenheimer R, Helmers C, Pantev E, Rydberg E, Löfdahl P, Gordon A. Safety and efficacy of valsartan versus enalapril in heart failure patients. Int J Cardiol. 2002. https://doi.org/10.1016/S0167-5273(02)00154-7.
Granger CB, McMurray JJV, Yusuf S, et al. CHARM-Alternative trial (ARB CHF). Lancet. 2003;362(9386):772–6. https://doi.org/10.1016/S0140-6736(03)14284-5.
McKelvie RS, Rouleau J-L, White M, et al. Comparative impact of enalapril, candesartan or metoprolol alone or in combination on ventricular remodelling in patients with congestive heart failure. Eur Heart J. 2003;24(19):1727–34. https://doi.org/10.1016/s0195-668x(03)00477-9.
White M, Yusuf S, McKelvie RS, et al. Effects of metoprolol CR in patients with ischemic and dilated cardiomyopathy : the randomized evaluation of strategies for left ventricular dysfunction pilot study. Circulation. 2000;101(4):378–84. https://doi.org/10.1161/01.cir.101.4.378.
Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: Randomised trial - The Losartan Heart Failure Survival Study ELITE II. Lancet. 2000. https://doi.org/10.1016/S0140-6736(00)02213-3.
Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667–75. https://doi.org/10.1056/NEJMoa010713.
McMurray JJV, Ostergren J, Swedberg K, et al. CHARM-Added (2003; Lancet) ARB plus ACE-I in HF. Lancet (London, England). 2003;362(9386):767–71. https://doi.org/10.1016/S0140-6736(03)14283-3.
Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: The OPTIMAAL randomised trial. Lancet. 2002. https://doi.org/10.1016/S0140-6736(02)09895-1.
Heran BS, Musini VM, Bassett K, Taylor RS, Wright JM. Angiotensin receptor blockers for heart failure. Cochrane Database Syst Rev. 2012. https://doi.org/10.1002/14651858.CD003040.pub2.
Burnett H, Earley A, Voors AA, et al. Thirty Years of Evidence on the Efficacy of Drug Treatments for Chronic Heart Failure with Reduced Ejection Fraction: A Network Meta-Analysis. Circ Heart Fail. 2017;10(1). https://doi.org/10.1161/CIRCHEARTFAILURE.116.003529.
Shi V, Senni M, Streefkerk H, Modgill V, Zhou W, Kaplan A. Angioedema in heart failure patients treated with sacubitril/valsartan (LCZ696) or enalapril in the PARADIGM-HF study. Int J Cardiol. 2018;264:118–23. https://doi.org/10.1016/j.ijcard.2018.03.121.
John JVM, Milton P, Akshay SD, Jianjian G, Martin PL, Adel RR, et al. Angiotensin–Neprilysin Inhibition versus Enalapril in. N Engl J Med. 2014;371(11):993–1004. https://doi.org/10.1056/NEJMoa1409077.
Konstam MA, Neaton JD, Dickstein K, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374(9704):1840–8. https://doi.org/10.1016/S0140-6736(09)61913-9.
Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-Converting Enzyme Inhibitors in Hypertension: To Use or Not to Use? J Am Coll Cardiol. 2018;71(13):1474–82. https://doi.org/10.1016/j.jacc.2018.01.058.
Hughes S. Time to Ditch ACE Inhibitors for CVD? Medscape.com. https://www.medscape.com/viewarticle/894876. Published 2018. Accessed Jan 9, 2020.
Pfisterer M, Cox JL, Granger CB, et al. Atenolol use and clinical outcomes after thrombolysis for acute myocardial infarction: the GUSTO-I experience. Global Utilization of Streptokinase and TPA (alteplase) for Occluded Coronary Arteries. J Am Coll Cardiol. 1998;32(3):634–40.
Chen Z, Xie J. Early intravenous then oral metoprolol in 45 852 patients with acute myocardial infarction: Randomised placebo-controlled trial. Lancet. 2005;366(9497):1622–32. https://doi.org/10.1016/S0140-6736(05)67661-1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hyman, D.A., Siebert, V.R., Birnbaum, G.D. et al. A Modern History RAAS Inhibition and Beta Blockade for Heart Failure to Underscore the Non-equivalency of ACEIs and ARBs. Cardiovasc Drugs Ther 34, 215–221 (2020). https://doi.org/10.1007/s10557-020-06950-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-06950-w