Abstract
Treatment of angina pectoris associated with coronary microvascular dysfunction is challenging as the underlying mechanisms are often diverse and overlapping. Patients with type 1 coronary microvascular dysfunction (i.e. absence of epicardial coronary artery disease and myocardial disease) should receive strict control of their cardiovascular risk factors and thus receive statins and ACE-inhibitors in most cases. Antianginal medication consists of ß-blockers and/or calcium channel blockers. Second line drugs are ranolazine and nicorandil with limited evidence. Despite individually titrated combinations of these drugs up to 30 % of patients have refractory angina. Rho-kinase inhibitors and endothelin-receptor antagonists represent potential drugs that may prove useful in these patients in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary microvascular dysfunction (CMD) is an increasingly recognized condition that can be classified into the following 4 types. Type 1 is CMD in the absence of obstructive coronary artery disease (CAD) and myocardial diseases, type 2 is CMD in the presence of myocardial diseases, type 3 is CMD in the presence of obstructive CAD and type 4 is iatrogenic [1] (Fig. 1). Pharmacological therapy should first of all be based on guideline recommendations for the respective underlying condition (e.g. hypertrophic cardiomyopathy, coronary artery disease, etc.). There is a paucity of data regarding the efficacy of pharmacotherapy on the coronary microcirculation. Thus, treatment approaches often remain empiric. Patients with type 1 coronary microvascular dysfunction represent a specific challenge as the underlying mechanisms are diverse and overlapping, and confirmation of the diagnosis is often difficult. Frequently, invasive diagnostic tools are needed to establish the diagnosis (e.g. intracoronary acetylcholine testing, coronary flow reserve assessment) [2]. Treatment approaches should be tailored to the underlying mechanism as much as possible. However, despite close monitoring and optimization of pharmacotherapy approximately 30 % patients have refractory angina [3]. Given the large number of patients affected by this condition, randomized studies should evaluate whether newer drugs such as endothelin-receptor-antagonists or rho-kinase-inhibitors may prove beneficial in the future.
Definition and Proposed Underlying Mechanisms of Coronary Microvascular Dysfunction
Coronary microvascular dysfunction is defined as a mismatch of myocardial blood supply and oxygen consumption due to a dysfunction of the coronary microvessels with a diameter of less than 500 μm (Fig. 2). Structural abnormalities such as vascular remodeling, vascular rarefaction and perivascular fibrosis as well as functional abnormalities such as endothelial dysfunction and dysfunction of vascular smooth muscle cells have been reported in this setting. In type 1 coronary microvascular dysfunction several mechanisms have been described that may account for the clinical presentation. Among these are impaired coronary microvascular dilatation [4], enhanced vasoconstriction and spasm [5], abnormal pain perception [6] and altered adrenergic nerve function [7]. In addition, all cardiovascular risk factors can activate inflammatory pathways and thereby cause (micro) vascular dysfunction [8].
It is difficult to judge the efficacy of pharmacological treatments in this patient population. This is because the clinical studies performed so far used a variety of definitions of study endpoints and applied variable inclusion criteria [9]. This underscores the need for a thorough characterisation of patients included in clinical trials and for an accepted terminology and diagnostic assessment to make study results comparable. In 2013, COVADIS (Coronary Vasomotor Disorders International Study Group) was founded to establish internationally accepted criteria for patients with coronary microvascular dysfunction in order to improve the clinical diagnosis, standardise terminology and stimulate future research in the field [10].
Recommendations for Treatment
Pharmacotherapy in patients with CMD is challenging as there are currently no large randomized studies available. Nevertheless, various substances have been studied for the treatment of CMD. In 2013, the guideline on the management of stable CAD by the European Society of Cardiology has addressed the issue of CMD and recommended the use of aspirin, statins, ß-blockers and calcium channel blockers [11].
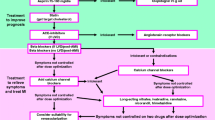
Our treatment algorithm for patients with coronary microvascular dysfunction (Fig. 3) starts with an evaluation of the cardiovascular risk factor profile. Studies have shown that all cardiovascular risk factors affect the coronary microcirculation, thus tight control of these conditions may contribute to improvement of microvascular function and thus angina pectoris. In addition to pharmacological interventions to treat the risk factors, one should not forget the importance of non-pharmacological interventions for risk factor modification such as regular physical exercise, weight loss or change of diet.
The standard approach regarding pharmacotherapy consists of an ACE-inhibitor and a statin as both drugs have shown to improve coronary microvascular dysfunction in small randomized studies [12, 13]. ACE-inhibitors can improve coronary microvascular function as assessed by coronary flow reserve. In one randomized, placebo controlled study, patients with CMD and impaired coronary flow reserve received a 16 week treatment with quinapril or placebo [14]. The treatment group showed a significant improvement of CFR which was linked to reduced angina frequency. Statins can reduce LDL-levels and thereby cardiovascular risk. Moreover, due to their pleiotropic effects a reduction of vascular inflammation and an improvement of endothelial function may occur. Small randomized studies have shown beneficial effects in terms of prolongation of exercise duration in CMD patients taking pravastatin, fluvastatin or simvastatin compared to placebo [15–17]. Based on these studies the use of statins and ACE-inhibitors is recommended for most patients with CMD unless severe side effects or contraindications are present.
On top of this baseline medication, beta-blockers should be the first option. Early studies have shown that atenolol is more effective than amlodipine in patients with CMD [18]. Moreover, studies have shown a favourable effect for nebivolol compared to metoprolol [19]. However, if patients report frequent attacks of angina pectoris at rest and acetylcholine testing reveals a diagnosis of microvascular spasm (i.e. reproduction of angina during the test without epicardial spasm but with ischemic ECG shifts) a calcium channel blocker should be initiated (e.g. amlodipine 5 mg once daily). Short acting nitrates are recommended to relieve spontaneous attacks of angina at rest. However, patients often report a limited effect of this treatment and in these cases we recommend sublingual nitrendipine for relief of symptoms.
Patients are followed up every 3–6 months in the outpatient clinic where the effect of the pharmacotherapy is evaluated. If symptoms have not improved, which is the case in approximately 30–40 % of the patients according to our experience, the first step is to increase to dose of the drugs to the maximum tolerated dose. If this does not lead to improvement of symptoms additional antianginal drugs should be given (see below). Studies on long acting nitrates have shown no positive effect and are thus not recommended in CMD patients [20]. Although in rare cases they may be effective.
In addition to the above mentioned drugs, ranolazine may be given. However, despite previous reports showing a favourable effect for ranolazine in small patient groups with CMD [21, 22] a recent randomized, double-blind, placebo-controlled, crossover trial showed no treatment differences in the outcomes. Among the 128 subjects (96 % women) ranolazine did not improve angina measured by the Seattle Angina Questionnaire and myocardial perfusion reserve index measured by cardiac magnetic resonance imaging compared to placebo [23]. This agrees with our clinical experience that ranolazine often fails to improve symptoms in these patients.
Ivabradine is a drug that selectively inhibits the funny channel in the sinus node thereby reducing the heart rate. This leads to prolongation of diastole and thus improvement of coronary perfusion. The drug has been studied in a small group of patients with CMD [21] showing an improvement in clinical symptoms but not in coronary flow reserve. It is thus of limited use in patients with CMD.
The antidiabetic drug metformin has been studied in one randomized, double-blind, placebo-controlled trial for its effects in CMD patients. In this study, an 8-week treatment with metformin significantly improved maximal ST-segment depression on exercise stress testing as well as chest pain incidence compared to placebo [24].
Data on nicorandil in patients with coronary microvascular dysfunction are sparse. Chen at el. showed that time to 1-mm ST depression and total exercise duration on treadmill exercise tests were significantly prolonged with nicorandil treatment compared with placebo suggesting that nicorandil might have a direct vasodilatory effect on the coronary microvasculature in patients with CMD [25]. Thus, nicorandil may be an option in these patients. However, the number of patients with refractory symptoms after trying several combinations of the mentioned antianginal drugs remains high.
Outlook
With advances in technology [26] diagnosis of coronary microvascular dysfunction may improve in the future. It will be especially important to better characterize the different mechanisms contributing to the clinical presentation. This may allow for a more targeted treatment approach. So far impaired vasodilatation and enhanced vasoconstriction are among the most frequent mechanisms. Moreover, there are some newer drugs that have been studied such as endothelin-1-antagonists or rho-kinase inhibitors. Preliminary data suggest that endothelin-antagonists and rho-kinase inhibitors may become useful for the treatment of coronary microvascular dysfunction [27, 28]. The rho-kinase pathway may be especially interesting as it has been shown to be substantially involved in endothelial cell dysfunction, vascular smooth muscle cell hypercontraction and spasm as well as accumulation of inflammatory cells in the adventitia of blood vessels [29]. Thus, from a pathophysiological point of view rho-kinase inhibition may also have beneficial effects in patients with CMD by enhancing vasorelaxation and by reducing vascular inflammation [Fig. 4]. This concept may be particularly interesting in patients in whom an exaggerated vasoconstrictor component is the dominant mechanism for CMD.
Interactions between endothelial cells (ECs) and vascular smooth muscle cells (VSMCs). Intracellular signaling pathways for Rho-kinase activation, ROS production, and cyclophilin A (CyPA) secretion are closely linked through VAMP2 vesicle formation. H2O2 has been reported to cause vasodilatation through several mechanisms. H2O2 rapidly reaches VSMC with subsequent VSMC hyperpolarization and relaxation. Oxidative stress promotes CyPA secretion from VSMC. Secreted CyPA promotes ROS production, contributing to the augmentation of oxidative stress. AMPK indicates AMP-activated protein kinase; CaMKK, Ca2+/calmodulin-dependent protein kinase kinase; EMMPRIN, extracellular matrix metalloproteinases inducer protein; IP3, 1,4,5-triphosphate; IRS, insulin receptor substrate; PGH2, prostaglandin H2; PGI2, prostacyclin; PI3K, phosphoinositide-3-kinase; PKG1α, protein kinase G, subunit 1α; PLC, phospholipase C; SOD, superoxide dismutase; and VAMP, vesicle-associated membrane protein (with permission from reference 29)
Clinical Implications and Limitations
Coronary microvascular dysfunction is an important clinical condition that should not be overlooked since patients often continue to have symptoms leading to repeated hospital admissions and further investigations. In addition, the condition has been shown to be prognostically relevant [30]. However, there is currently only limited evidence for pharmacotherapy in patients with CMD. Prospective, randomized clinical trials in well characterized sub-cohorts are urgently needed to evaluate new pharmacotherapies in these patients. Only then guidelines can be improved to assist the clinical community in the treatment of these patients and ultimately improve prognosis.
Conclusion
Treatment of angina pectoris associated with coronary microvascular dysfunction is challenging as the underlying mechanisms are often diverse and overlapping. Patients with type 1 coronary microvascular dysfunction (absence of epicardial coronary artery disease and myocardial disease) should receive strict control of their cardiovascular risk factors. Antianginal medication consists of ß-blockers and/or calcium channel blockers. Second line drugs are ranolazine and nicorandil with limited evidence. Despite individualized titrated combinations of these drugs up to 30 % of patients have refractory angina.
References
Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–40.
Lanza GA, Camici PG, Galiuto L, Niccoli G, Pizzi C, di Monaco a, Sestito a, novo S, Piscione F, Tritto I, Ambrosio G, Bugiardini R, Crea F, Marzilli M, Gruppo di studio di Fisiopatologia Coronarica e Microcircolazione, Società Italiana di Cardiologia. Methods to investigate coronary microvascular function in clinical practice. J Cardiovasc Med (Hagerstown). 2013;14:1–18.
Lamendola P, Lanza GA, Spinelli A, Sgueglia GA, Di Monaco A, Barone L, Sestito A, Crea F. Long-term prognosis of patients with cardiac syndrome X. Int J Cardiol. 2010;140:197–9.
Reis SE, Holubkov R, Conrad smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ. WISE investigators. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–41.
Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA study (abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol. 2012;59:655–62.
Pasceri V, Lanza GA, Buffon A, Montenero AS, Crea F, Maseri A. Role of abnormal pain sensitivity and behavioral factors in determining chest pain in syndrome X. J Am Coll Cardiol. 1998;31:62–6.
Lanza GA, Giordano A, Pristipino C, Calcagni ML, Meduri G, Trani C, Franceschini R, Crea F, Troncone L, Maseri A. Abnormal cardiac adrenergic nerve function in patients with syndrome X detected by [123I]metaiodobenzylguanidine myocardial scintigraphy. Circulation. 1997;96:821–6.
Granger DN, Rodrigues SF, Yildirim A, Senchenkova EY. Microvascular responses to cardiovascular risk factors. Microcirculation. 2010;17:192–205.
Vermeltfoort IA1, Raijmakers PG, Riphagen II, Odekerken DA, Kuijper AF, Zwijnenburg A, Teule GJ. Definitions and incidence of cardiac syndrome X: review and analysis of clinical data. Clin Res Cardiol. 2010;99:475–81.
Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Bairey Merz CN; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2015
Members TF, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, ESC Committee for Practice Guidelines, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document Reviewers, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003.
Pizzi C, Manfrini O, Fontana F, Bugiardini R. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme a reductase in cardiac syndrome X: role of superoxide dismutase activity. Circulation. 2004;109:53–8.
Chen JW1, Hsu NW, Wu TC, Lin SJ, Chang MS. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol. 2002;90:974–982.
Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM, Sopko G, Sharaf BM, Kelsey SF, Merz CN, Pepine CJ. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: a double-blind randomized study from the National Heart, lung and blood institute Women's ischemia syndrome evaluation (WISE). Am Heart J. 2011;162:678–84.
Kayikcioglu M, Payzin S, Yavuzgil O, Kultursay H, Can LH, Soydan I. Benefits of statin treatment in cardiac syndrome-X. Eur Heart J. 2003;24:1999–2005.
Fábián E, Varga A, Picano E, Vajo Z, Rónaszéki A, Csanády M. Effect of simvastatin on endothelial function in cardiac syndrome X patients. Am J Cardiol. 2004;94:652–5.
Zhang X1, Li Q, Zhao J, Li X, Sun X, Yang H, Wu Z, Yang J. Effects of combination of statin and calcium channel blocker in patients with cardiac syndrome X. Coron Artery Dis. 2014;25:40–4.
Lanza GA, Colonna G, Pasceri V, Maseri A. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am J Cardiol. 1999;84:854–6.
Sen N, Tavil Y, Erdamar H, Yazici HU, Cakir E, Akgül EO, Bilgi C, Erbil MK, Poyraz F, Okyay K, Turfan M, Cemri M. Nebivolol therapy improves endothelial function and increases exercise tolerance in patients with cardiac syndrome X. Anadolu Kardiyol Derg. 2009;9:371–9.
Russo G, Di Franco A, Lamendola P, Tarzia P, Nerla R, Stazi A, Villano A, Sestito A, Lanza GA, Crea F. Lack of effect of nitrates on exercise stress test results in patients with microvascular angina. Cardiovasc Drugs Ther. 2013;27:229–34.
Villano A, Di Franco A, Nerla R, Sestito A, Tarzia P, Lamendola P, Di Monaco A, Sarullo FM, Lanza GA, Crea F. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. 2013;112:8–13.
Mehta PK, Goykhman P, Thomson LE, Shufelt C, Wei J, Yang Y, Gill E, Minissian M, Shaw LJ, Slomka PJ, Slivka M, Berman DS, Bairey Merz CN. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging. 2011;4:514–22.
Bairey Merz CN, Handberg EM, Shufelt CL, Mehta PK, Minissian MB, Wei J, Thomson LE, Berman DS, Shaw LJ, Petersen JW, Brown GH, Anderson RD, Shuster JJ, Cook-Wiens G, Rogatko A, Pepine CJ. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): impact on angina and myocardial perfusion reserve. Eur Heart J. 2016;37:1504–13.
Jadhav S, Ferrell W, Greer IA, Petrie JR, Cobbe SM, Sattar N. Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2006;48:956–63.
Chen JW, Lee WL, Hsu NW, Lin SJ, Ting CT, Wang SP, Chang MS. Effects of short-term treatment of nicorandil on exercise-induced myocardial ischemia and abnormal cardiac autonomic activity in microvascular angina. Am J Cardiol. 1997;80:32–8.
Kühl JT, George RT, Mehra VC, Linde JJ, Chen M, Arai AE, Di Carli M, Kitagawa K, Dewey M, Lima JA, Kofoed KF. Endocardial-epicardial distribution of myocardial perfusion reserve assessed by multidetector computed tomography in symptomatic patients without significant coronary artery disease: insights from the CORE320 multicentre study. Eur Heart J Cardiovasc Imaging 2015.
Reriani M, Raichlin E, Prasad A, Mathew V, Pumper GM, Nelson RE, Lennon R, Rihal C, Lerman LO, Lerman A. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation. 2010;122:958–66.
Fukumoto Y, Mohri M, Inokuchi K, Ito A, Hirakawa Y, Masumoto A, Hirooka Y, Takeshita A, Shimokawa H. Anti-ischemic effects of fasudil, a specific rho-kinase inhibitor, in patients with stable effort angina. J Cardiovasc Pharmacol. 2007;49:117–21.
Shimokawa H, Sunamura S, Satoh K. RhoA/rho-kinase in the cardiovascular system. Circ Res. 2016;118:352–66.
Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–27.
Acknowledgments
The authors are grateful to Sabine Nägele for excellent secretarial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Ong, P., Athanasiadis, A. & Sechtem, U. Treatment of Angina Pectoris Associated with Coronary Microvascular Dysfunction. Cardiovasc Drugs Ther 30, 351–356 (2016). https://doi.org/10.1007/s10557-016-6676-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-016-6676-z