Abstract
Background
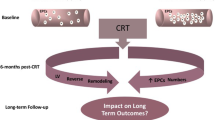
Pretreatment with high-dose statins given before percutaneous coronary intervention (PCI) has been shown to have beneficial effects, in particular by reducing peri-procedural myocardial infarction. The mechanism of these lipid-independent beneficial statin effects is unclear. Circulating endothelial progenitor cells (EPCs) have an important role in the process of vascular repair, by promoting re-endothelization following injury. We hypothesized that statins can limit the extent of endothelial injury induced by PCI and promote re-endothelization by a positive effect on EPCs. We, therefore, aimed to examine the effect of high-dose statins given prior to PCI on EPCs profile.
Methods
Included were patients, either statin naïve or treated chronically with low-dose statins, with stable or unstable angina who underwent PCI. Patients were randomized to receive either high-dose atorvastatin (80 mg the day before PCI and 40 mg 2–4 h before PCI) or low- dose statin. EPCs profile was examined before PCI and 24 h after it. Circulating EPCs levels were assessed by flow cytometry as the proportion of peripheral mononuclear cells co-expressing VEGFR-2+ CD133+ and VEGFR-2+ CD34+. The capacity of the cells to form colony forming units (CFUs) was quantified after 7 days of culture.
Results
Twenty three patients (mean age 61.4 ± 7.4 years, 87.0 % men) were included in the study, of which 12 received high-dose atorvastatin prior to PCI. The mean number of EPC-CFUs before PCI was higher in patients treated with high-dose atorvastatin vs. low-dose statins (165.8 ± 58.8 vs. 111.7 ± 38.2 CFUs/plate, respectively, p < 0.001). However, 24 h after the PCI, the number of EPC-CFUs was similar (188.0 ± 85.3 vs. 192.9 ± 66.5 CFUs/plate in patients treated with high-dose atorvastatin vs. low- dose statins, respectively, p = 0.15). There were no statistical significant differences in FACS analyses between the 2 groups.
Conclusions
The current study showed higher EPC- CFUs levels in patients treated with high-dose atorvastatin before PCI and a lower increment in EPC-CFUs after PCI. These findings could account for the beneficial effects of statins given prior to PCI, yet further investigation is required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The equilibrium between endothelial injury and repair is of particular importance in cardiovascular disease. Prior studies indicate that circulating endothelial progenitor cells (EPCs), a population of bone marrow-derived cells, have an important role in the process of vascular repair, by promoting re-endothelization following injury [1–3]. Furthermore, levels of EPCs increase following vascular injury and/or tissue ischemia, for example after percutaneous coronary intervention (PCI) [4–7]. A major goal of adjunctive therapy given during and after PCI is to minimize the extent of vascular injury, and promote the repair process of the damaged endothelial layer. Treatment with hydroxymethyl glutaryl coenzyme A reductase inhibitors (statins) before PCI has been shown to have beneficial clinical effects, in particular by a reduction in the risk of myocardial infarction after the procedure [8–11]. Statins appear to have an important role in minimizing the extent of endothelial injury caused by PCI [12–14], yet the mechanism of these short-term, lipid-independent, beneficial statin effects in patients undergoing PCI is unclear. We hypothesized that the beneficial effects of high-dose statins given before PCI may be mediated by EPCs mobilization, and EPCs-related processes which can limit the extent of endothelial injury induced by the procedure. We, therefore, aimed to examine the effect of high-dose statin treatment given before PCI, on the profile of EPCs in patients with coronary artery disease (CAD) without troponin elevation.
Methods
The HIPOCRATES trial (Effect of High Dose Statin Pretreatment on Endothelial Progenitor Cells after Percutaneous Coronary Intervention) is a single center open-labeled prospective randomized trial that examined EPCs profile in patients treated by high-dose statin prior to PCI as compared to low- dose statin treatment. The study protocol was approved by the Rabin Medical Center Helsinki committee and each patient enrolled in the study signed an informed consent prior to enrollment. The study was performed in a pre-defined period from December 2011 till June 2013.
Patients
The study included statin naïve patients or those treated chronically with low-dose statins (20 mg pravastatin or 20 mg simvastatin) who were planned to undergo coronary angiography due to stable or unstable angina pectoris. Patients with several conditions that influence EPCs level and function were excluded from this study: patients with either ST or non-ST segment elevation myocardial infarction as the indication for the procedure as well as other causes of troponin elevation, history of a myocardial infarction or revascularization in the past 3 months or current treatment with high-dose statins. In addition, patients were excluded if they had a history of muscle or liver disease, hemoglobin level < 10 g/dl or platelets count <100,000/l or renal failure (creatinine 2.5 mg/dl). Patients with malignancy or hematologic disease were also excluded.
Procedure
Patients were recruited to participate in the study about 24 h before the planned angiography. Patients were either hospitalized or were recruited in an ambulatory cardiology clinic. If the patient did not undergo PCI following the diagnostic angiography (rather he was treated conservatively or referred to coronary artery bypass graft surgery), he was withdrawn from the study. After recruitment, the patients were randomized to receive high-dose statin treatment or low- dose statins (simvastatin 20 mg). Patients who were already receiving low- dose statins and were randomized to the low-dose statins group, continued their current medication. Patients in the high- dose statin group received 80 mg of atorvastatin (one dose) 18–24 h prior to the angiography and 40 mg of atorvastatin within 2–4 h before the procedure. The rationale for these doses and time points was based mainly on the ARMYDA study [8]. In both groups from the day following the procedure the patients were treated with long-term atorvastatin 20 mg per day, unless a different statin (or statin dose) was preferred by the patient’s attending physician. All patients were pre-treated the day before the procedure with aspirin 100 mg and a loading dose of clopidogrel 600 mg (followed by aspirin 100 mg per day and clopidogrel 75 mg per day). PCI was performed according to standard criteria and operator’s choices of balloons, stents etc. Heparin was given during angiography to achieve and maintain an activated clotting time of 200–250 s. Glycoprotein IIb/IIIa inhibitors was used at the operator’s discretion. Periprocedural Myocardial Infarction was defined as an elevation of cardiac troponin concentrations of X5 of 99th percentile URL occurring within 48 h of the procedure, plus clinical, electrocardiographic, imaging or angiographic findings.
Blood Sampling
Two blood samples were taken in this study: the first was taken at baseline, just before angiography (18–24 h following loading in the high-dose statin group) and the second about 24 h following PCI. Blood was evaluated for EPCs in the cardiology laboratory in the Felsenstein medical research institute in Rabin Medical Center.
Evaluation of EPCs
Quantification of Circulating EPCs was performed by measurement of surface markers by flow cytometry (VEGFR-2+ CD34 and VEGFR-2+ CD133). Functional aspects of EPCs were obtained by measurement of colony forming units (CFUs).
EPCs Isolation From Peripheral Blood Mononuclear Cells
Mononuclear cells in peripheral blood (PMNCs) were isolated by Ficoll density-gradient centrifugation and were washed with phosphate-buffered saline after red cell lysis. PMNCs were re-suspended with a supplemented Medium 199 (Invitrogen, Carlsbad, CA, USA) with 20 % fetal calf serum (Gibco BRL Life Tech, Gaithersburg, MD, USA) and placed on 6-well plates at a concentration of 5 * 106 cells per well. These plates were coated with human fibronectin.
Flow Cytometry
Aliquots of PMNCs were incubated together with monoclonal antibodies against VEGFR-2 (FITC labeled; R&D, Minneapolis, USA), CD133 (PE- labeled, Miltenyi Biotech, Auburn, CA, USA), CD34 (PE-labled, Miltenyi Biotech) and isotype-identical antibodies which were used as controls. After washing the incubated cells with phosphate-buffered saline, they were analyzed by a flow cytometer (FACSCalibur, Becton Dickinson). Each analysis included 100,000 events, after the inclusion of CD-45 positive cells and the exclusion of platelets and debris. Next, gated CD34 or CD133 positive cells were examined for VEGFR-2 expression. Results are presented as the percentage of cells co-expressing either VEGFR-2 and CD133, or VEGFR-2 and CD34.
CFU Quantification
PMNCs were cultured for 5–7 days to obtain EPCs colonies. EPCs colonies were counted using an inverted microscope after 7 days of culture. An EPC colony was defined as a cluster of at least 100 flat cells surrounding a cluster of rounded cells, as previously described [11]. A single central cluster without associated emerging cells was not defined as a colony. In order to confirm endothelial cell lineage, indirect immunostaining of randomly selected colonies was performed using antibodies directed against VEGFR-2, CD31 (Becton Dickinson, NJ, USA), and Tie-2 (Santa Cruz, Biotechnology, CA, USA). Results are shown as the mean number of CFUs per well.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation. Continuous variables were compared by the Mann–Whitney U test for non-normally distributed variables. Inter- and intra- group comparisons were performed by the ANOVA test for repeated measures. p < 0.05 was considered statistically significant.
Results
A total of 48 patients who underwent coronary angiography were screened and signed an informed consent. Of these, 22 patients did not undergo PCI and they were withdrawn from further analysis. For 3 patients, blood samples for EPCs analysis at both time points were not available. Thus, 23 patients (mean age 61.4 ± 7.4 years, 87.0 % men) were included in the study, of which 12 received high-dose atorvastatin prior to PCI and 11 patients had received low- dose statins (Fig. 1). Most patients had undergone PCI due to unstable angina pectoris (95.6 %). Eight patients were treated with low-dose statins prior to enrollment (34.8 %) and the number of stents implanted in PCI in both groups was similar. Patient characteristics are depicted in Table 1. There were no statistically significant inter-group differences in any of the characteristics between patients who received high- dose statins and those who received low- dose statins.
The mean number of EPC- CFUs before PCI was 165.8 ± 58.8 vs. 111.7 ± 38.2 CFUs/plate in patients treated with high-dose atorvastatin vs. low- dose statins, respectively (p < 0.001). The number of EPC- CFUs after 24 h was 188.0 ± 85.3 vs. 192.9 ± 66.5 CFUs/plate in patients treated with high-dose atorvastatin vs. low- dose statins (p = 0.15) (Fig. 2). The mean number of EPCs/plate increased numerically in both groups 24 h after PCI as compared to baseline level, yet in patients treated with high- dose statins, the increase was non- significant (1.1 fold, p = 0.31) as compared to a significant 1.7 fold increase in the low- dose statin group (p = 0.002, Fig. 2). The between group difference of the change in EPC-CFUs before and after PCI was only marginally significant (p = 0.055). Examples of EPC- CFUs analyses are depicted in Figs. 3 and 4.
On flow cytometry analysis with dual staining for EPC markers VEGFR-2+ CD34+ and VEGFR-2+ CD133+, there were no significant differences between the percent of positive cells in the high- dose vs. low- dose statin groups, either before or 24 h after PCI (Fig. 5). In patients treated with high- dose statins, there was a trend towards higher relative increment of EPCs level after PCI as compared to those treated with low- dose statins, yet this was not statistically significant.
Two patients, 1 in each group, had a slight elevation in cardiac troponin after the PCI but this did not meet the criteria of periprocedural myocardial infarction.
Discussion
The current study attempted to seek a mechanism for the apparently beneficial effects of high- dose statins given prior to PCI. Although the study was small in size, we found a higher EPC- CFUs levels in patients pre-treated with high-dose atorvastatin before PCI. However, following PCI the two groups exhibited similar levels of EPCs.
EPCs have an important role in vascular repair by promoting re-endothelization following injury [1]. The level of circulating EPCs in patients with chronic stable CAD or with cardiovascular risk factors has been showed to be reduced [3, 4]. In this context, low levels of EPCs are predictive of the occurrence of cardiovascular events and death from cardiovascular causes [4]. In contrast to the reduced levels of EPCs in chronic vascular conditions, levels of EPCs increase following acute vascular injury and/or tissue ischemia [1, 5, 6]. Prior studies have demonstrated that even a focal and limited endothelial injury, such as that caused by PCI with stent placement, induces a rapid rise in the level of circulating EPCs, peaking at 6–12 h after the procedure [5–7].
An important goal of adjunctive therapy given during and after PCI is to minimize the extent of vascular injury on one hand, and promote the repair process of the damaged endothelial layer on the other. In this respect statins may have an important role. Stable CAD patients who were treated long-term (4 weeks) with 40 mg of atorvastatin per day, a 3-fold increase in the level of circulating EPCs and improvement in their functional properties has been observed [14, 15]. Thus, it appears that in patients with cardiovascular disease statin treatment may limit endothelial injury and induce repair. In general, beyond lipid-lowering effects statins have favorable “pleiotropic” effects which may contribute to atherosclerotic plaque stabilization [16]. These effects include reduction in vascular inflammation, decrease in platelet adhesion and activation as well as thrombus formation, and increase in endothelium derived nitric oxide production [16]. The positive effects of statins on EPCs may also contribute to plaque stabilization by augmenting endothelial repair following atherosclerotic injury. In the context of PCI, several clinical studies have demonstrated an important and beneficial role for high-dose statin therapy given before the procedure [8–10]. These studies have shown that in statin “naïve” patients with CAD a single high-dose of atorvastatin 80 mg given within 24 h before the PCI (followed by 40 mg just prior to the procedure in the study of Patti et al.) was associated with a reduction in periprocedural MI rates and other adverse cardiac events. Furthermore, high-dose statin pretreatment given on admission to statin-naïve patients with ACS who were scheduled for an early invasive procedure decreased the rate of contrast induced nephropathy and improved short-term clinical outcome in the PRATO-ACS trial [11]. In addition, even in patients who receive chronic statin treatment, a “reloading” dose of 80 mg of atrovastatin given 12 h before PCI followed by 40 mg just before the procedure was associated with a significant reduction in major adverse cardiac events [8].
In the current study we aimed to evaluate the role of EPCs as possible mediators of these beneficial effects of high-dose statins given prior to PCI. It was previously demonstrated that treatment with high- dose statins causes recruitment of EPCs to the circulation even without an endothelial injury [14]. Indeed, in our study, we have also demonstrated that patients that were pretreated with high- dose of atorvastatin had higher levels of EPC- CFUs in the circulation prior to PCI. However, the difference between the groups in EPC-CFUs was eliminated following the procedure. We assume that higher levels of EPCs colonies prior to the PCI may have positive effects on the endothelium and might reduce the endothelial injury during the subsequent PCI. As demonstrated in former trials, we have also showed that after the endothelial injury caused by PCI, EPCs colonies levels increase numerically by both low-dose and high- dose statin pretreatment. However, the novelty of our study is that although EPCs levels were higher after statins pretreatment, the relative increase of EPC- CFUs in patients who were pretreated with high- dose statins was significantly lower as compared to the control group. These results can be explained by the hypothesis that high- dose statins protect the endothelium from injury, thus the degree of injury and secondary EPC recruitment is lower as compared to patients who were not pre-treated with high dose statins and had prominent endothelial injury. It is also possible that the EPCs function in patients treated with high dose statins, prior to PCI, reaches a relative “plato” and, therefore, after PCI, less EPCs differentiate to endothelial like cells.
Studies that examined the association between PCI and EPCs largely examined the behavior of EPC- CFUs and their findings are consistent with an increase in EPC- CFUs 1.3–3.0- fold within the first 24 h after PCI [5]. However, only few studies have used flow cytometry to identify circulating EPC levels after PCI and these studies demonstrated conflicting results and not uniformly concordant with EPC- CFUs [5]. Indeed, our study demonstrated a trend towards higher EPCs surface markers after PCI in patients treated with high- dose statins, but given the small size of the groups the differences were not significant. Thus, as previously shown, the correlation between EPCs level in the circulation and their functional capability is complex and not direct. Our findings, therefore, need to be validated in a larger trial in order to draw any decisive conclusions.
Our study has several limitations. First, as mentioned, the sample size is too small to draw any decisive conclusions regarding the association between EPCs and statins given prior to PCI. This is a hypothesis generating pilot study that requires further validation in future studies. Second, baseline EPC levels before statin treatment, which are influenced by several patient characteristics, were not available and were not routinely measured during the study. Therefore, although it is very likely that the higher EPCs level observed in the high-statin dose represents a statin-effect, this is only a hypothesis. It should be emphasized however, that all the blood samples in the study were taken in a unified technique and in a single laboratory prior to and after PCI in all patients. The study cohort was too small to account for any difference in periprocedural myocardial infarction between the groups. Finally, CRP levels were not routinely assessed in the trial.
Conclusion
Pretreatment with high-dose statins prior to PCI is associated with higher levels of EPC- CFUs before the PCI. This difference is eliminated 24 h following the procedure. These findings could account for the beneficial effects of statins given prior to PCI but require further research in larger studies.
References
Urbich C, Dimmeler S. Endothelial progenitor cells characterization and role in vascular biology. Circ Res. 2004;95:343–53.
Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8.
Hill JM, Zalos G, Halcox JP, Schenke WH, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600.
Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007.
Padfield GJ, Newby DE, Mills NL. Understanding the role of endothelial progenitor cells in percutaneous coronary intervention. J Am Coll Cardiol. 2010;55:1553–65.
Blann AD, Woywodt A, Bertolini F, et al. Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost. 2005;93:228–35.
Bonello L, Harhouri K, Sabatier F, et al. Level of adenosine diphosphate receptor P2Y12 blockade during percutaneous coronary intervention predicts the extent of endothelial injury, assessed by circulating endothelial cell measurement. J Am Coll Cardiol. 2010;56:1024–31.
Pasceri V, Patti G, Nusca A, Pristipino C, Richichi G, Di Sciascio G. ARMYDA Investigators. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of Myocardial Damage During Angioplasty study). Circulation. 2004;110:674–8.
Briguori C, Colombo A, Airoldi F, et al. Statin administration before percutaneous coronary intervention: impact on periprocedural myocardial infarction. Eur Heart J. 2004;25(20):1822–8.
Briguori C, Visconti G, Focaccio A, et al. Novel approaches for preventing or limiting events (Naples) II trial: impact of a single high loading dose of atorvastatin on periprocedural myocardial infarction. 1. J Am Coll Cardiol. 2009;54(23):2157–63.
Leoncini M, Toso A, Maioli M, Tropeano F, Villani S, Bellandi F. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acutecoronary syndrome: Results from the PRATO-ACS Study (Protective Effect ofRosuvastatin and Antiplatelet Therapy On contrast-induced acute kidney injury andmyocardial damage in patients with Acute Coronary Syndrome). J Am Coll Cardiol. 2014;63(1):71–9.
Boos CJ, Balakrishnan B, Jessani S, Blann AD, Lip GYH. Effects of percutaneous coronary intervention on peripheral venous blood circulating endothelial cells and plasma indices of endothelial damage/dysfunction. Chest. 2007;132:1920–6.
Banerjee S, Brilakis E, Zhang S, et al. Endothelial progenitor cell mobilization after percutaneous coronary intervention. Atherosclerosis. 2006;189:70–5.
Vasa M, Fichtlscherer S, Adler K, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–90.
Schmidt-Lucke C, Fichtlscherer S, Rössig L, Kämper U, Dimmeler S. Improvement of endothelial damage and regeneration indexes in patients with coronary artery disease after 4 weeks of statin therapy. Atherosclerosis. 2010;211:249–54.
Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering—are they clinically relevant? Eur Heart J. 2003;24:225–48.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eisen, A., Leshem-Lev, D., Yavin, H. et al. Effect of High Dose Statin Pretreatment on Endothelial Progenitor Cells After Percutaneous Coronary Intervention (HIPOCRATES Study). Cardiovasc Drugs Ther 29, 129–135 (2015). https://doi.org/10.1007/s10557-015-6575-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-015-6575-8