Abstract
Metastasis is the leading cause of cancer patient mortality. Metastasis suppressors are genes that, upon reexpression in metastatic tumor cells to levels observed in their nonmetastatic counterparts, significantly reduce metastasis without affecting the growth of the primary tumor. Analysis of > 30 metastasis suppressors revealed complex mechanisms of action that include multiple signaling pathways, transcriptional patterns, posttranscriptional regulatory mechanisms, and potential contributions of genomic stability. Clinical testing of strategies to re-establish a validated metastasis suppressor pathway in tumors is best directed to the adjuvant setting, with the goal of inhibiting the outgrowth of occult micrometastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
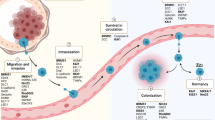
The overwhelming majority of cancer deaths in patients with solid tumors are due to metastatic disease, either by direct organ compromise or consequences of treatment. Intuitively, it makes sense that gaining the ability to invade, travel, and colonize foreign tissues would lead to organ compromise by tumor cells, but the interplay of metastasis and other aspects of lethal cancer such as drug resistance remains incompletely understood. Therapeutic advances directed towards signaling pathways operative in metastatic cancers have provided improvements in progression free and overall survival. These pathways were largely operative in initial tumorigenesis and then tested in the metastatic setting. Few therapies have effectively targeted the metastatic process itself. In addition, diverse pathways can stimulate metastasis, including tumor cell mutations, direct tumor-microenvironmental interactions, formation of a premetastatic niche, extracellular vesicles, gene programs mediated by microRNAs, etc., and are a potent obstacle to therapeutic success. Given this reality, it is extraordinary that re-expression of a gene/protein/miRNAs can halt the process, even in some model systems.
The metastasis suppressor field has provided insights into metastasis signaling apart from tumorigenesis. By definition, metastasis suppressors are genes that, upon re-expression at physiologically relevant levels, inhibit metastasis in vivo without significant effects on primary tumor growth. Because the only accepted endpoint for metastasis is in vivo, screens for metastasis suppressors are difficult. The first metastasis suppressor, NM23 or NME, was found in our lab by differential RNA expression screening of low- versus high-metastatic melanoma cell lines, all derived from a single primary tumor [1, 2]. These genes can affect various parts of the metastatic cascade, from primary tumor invasion to colonization of a foreign organ, the latter of which may be most translationally accessible.
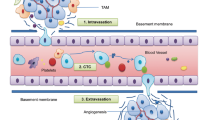
Identifying the mechanism of action of metastasis suppressors is key to basic and translational advances. Their loss of function is a major rate-limiting step in cancer progression to metastasis at distant sites. This process has proven extraordinarily complex. Often the metastasis suppressor lies at the nexus of several pathways. In addition, the protein may have biochemical functions other than those involved in metastasis suppression. Another aspect of metastasis suppressor function is coordination of the complex pathways involved in the metastatic process. In metastasis, complex programs or pathways are turned on and off in sequence to initiate invasion, survival in the circulatory system, arrest and extravasation, dormancy as opposed to cell death, and growth in the absence of tissue-of-origin microenvironmental influences. Metastasis suppressors have been reported to use transcriptional regulatory pathways that appear more specific than global changes in epigenetic markers: while many microRNAs promote metastasis, BRMS1 expression upregulated miR-146, which then altered gene transcription and suppressed breast cancer invasion and metastasis [3]. The YAP/TAZ transcriptional complex (along with tenascin C) has been implicated in NDRG1 metastasis suppressor function in pancreatic cancer-microenvironmental crosstalk [4]. Other complex regulatory mechanisms are post-translational: for NME, studies of its Drosophila counterpart AWD in normal development revealed a role for altered vesicular trafficking downstream of multiple receptors [5, 6], which has been demonstrated to be involved in metastasis suppression [7, 8]. For the NDRG1 metastasis suppressor, signaling networks were also regulated at the level of protein degradation [9]. Other potentially important avenues are incompletely studied to date: metastasis is known occur together with global genomic instability, and a role for metastasis suppressors in re-establishment of normal transcription, DNA repair and other genomic stability components are additional points of possible regulatory control. Other potentially important pathways such as tumor cell interactions with the immune system, and microenvironment remain incompletely studied from the metastasis suppressor standpoint.
While metastasis suppressors can act at any point in the metastatic cascade, it is their contributions to the final tumor colonization of a distant site that may be most therapeutically tractable. Invasion from a primary tumor can happen early in cancer, even before diagnosis. Particularly for cancers diagnosed at a more advanced stage, anti-invasives may be administered too late. Adjuvant therapy is systemic therapy based on the hypothesis that, even though metastases cannot be found in the body, isolated tumor cells, or micrometastases below the limit of detection are present in distant organs and possibly sanctuary sites such as the bone marrow. This is the likely window of opportunity for metastasis suppressors. Several approaches have been reported in the preclinical literature. Antagonists to miR-10b, a metastasis promoting microRNA, significantly reduced metastasis without effects on primary tumor size [10]. We preclinically explored increasing the transcription of NME using high-dose medroxyprogesterone acetate as an unusual glucocorticoid receptor agonist [11]; a phase II trial was conducted in breast cancer but drug levels never achieved an effective concentration, although some stable disease was noted [12]. Drugs upregulating the NDRG1 metastasis suppressor have also been reported [13]. Another approach in our lab identified potentially druggable inverse correlates of NME to LPA1 [14], but poor drug potency halted these efforts [15]. Most recently, we reported that exosomes from metastatic breast cancer cells contain NME and that NME-laden liposomes were metastasis suppressive due to alteration of endocytic patterns in agreement with NME1 function [16]. The potential for this finding to be successfully translated is limited by the finding that this strategy is “obvious” and non-patentable. Other efforts have involved in silico modeling to repurpose drugs for the RKIP metastasis suppressor using a reverse causal reasoning approach [17]. Besides being effective, drugs administered in the adjuvant setting to relatively healthy patients must have very low toxicity.
As we look back on 30+ years of metastasis suppressor research, the field has grown in both the number of credentialled members and the depth of our understanding of their mechanisms of action. However, their clinical application is still in a developing stage. Future work will hopefully identify valid preclinical and clinical strategies for moving this field to an effective adjuvant therapy for metastatic disease.
References
Leone, A., Flatow, U., King, C. R., Sandeen, M. A., Margulies, I. M. K., Liotta, L. A., et al. (1991). Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell, 65, 25–35. https://doi.org/10.1016/0092-8674(91)90404-M
Steeg, P. S., Bevilacqua, G., Kopper, L., Thorgeirsson, U. P., Talmadge, J. E., Liotta, L. A., et al. (1988). Evidence for a novel gene associated with low tumor metastatic potential. J. Nat′l. Cancer Inst., 80, 200–204. https://doi.org/10.1093/jnci/80.3.200
Hurst, D. R., Edmonds, M. D., Scott, G. K., Benz, C. C., Vaidya, K. S., & Welch, D. R. (2009). Breast cancer metastasis suppressor 1 up-regulates miR-146, which suppresses breast cancer metastasis. Cancer Research, 69(4), 1279–1283. https://doi.org/10.1158/0008-5472.CAN-08-3559
Geleta, B. R., Tout, F., Lim, S. C., Sahni, S., Jansson, P. J., Apte, M. V., et al. (2022). Targeting Wnt/tenascin C-mediated cross talk between pancreatic cancer cells and stellate cells via activation of the metastasis suppressor NDRG1. Journal of Biological Chemistry, 298(3). https://doi.org/10.1016/j.jbc.2022.101608
Dammai, V., Adryan, B., Lavenburg, K. R., & Hsu, T. (2003). Drosophila awd, the homolog of human nm23, regulates FGF receptor levels and functions synergistically with shi/dynamin during tracheal development. Genes & Development, 17(22), 2812–2824. https://doi.org/10.1101/gad.1096903
Boissan, M., Montagnac, G., Shen, Q., Griparic, L., Guitton, J., Romao, M., et al. (2014). Membrane trafficking. Nucleoside diphosphate kinases fuel dynamin superfamily proteins with GTP for membrane remodeling. Science, 344(6191), 1510–1515. https://doi.org/10.1126/science.1253768
Khan, I., Gril, B., & Steeg, P. S. (2019). Metastasis suppressors NME1 and NME2 promote dynamin 2 oligomerization and regulate tumor cell endocytosis, motility, and metastasis. Cancer Research, 79(18), 4689–4702. https://doi.org/10.1158/0008-5472.CAN-19-0492
Lodillinsky, C., Fuhrmann, L., Irondelle, M., Pylypenko, O., Li, X. Y., Bonsang-Kitzis, H., et al. (2021). Metastasis-suppressor NME1 controls the invasive switch of breast cancer by regulating MT1-MMP surface clearance. Oncogene, 40(23), 4019–4032. https://doi.org/10.1038/s41388-021-01826-1
Menezes, S. V., Kovacevic, Z., & Richardson, D. R. (2019). The metastasis suppressor NDRG1 down-regulates the epidermal growth factor receptor via a lysosomal mechanism by up-regulating mitogen-inducible gene 6. Journal of Biological Chemistry, 294(11), 4045–4064. https://doi.org/10.1074/jbc.RA118.006279
Ma, L., Reinhardt, F., Pan, E., Soutschek, J., Bhat, B., Marcusson, E. G., et al. (2010). Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nature Biotechnology, 28(4), 341–U367. https://doi.org/10.1038/nbt.1618
Palmieri, D., Halverson, D. O., Ouatas, T., Horak, C. E., Salerno, M., Johnson, J., et al. (2005). Medroxyprogesterone acetate elevation of Nm23-H1 metastasis suppressor expression in hormone receptor-negative breast cancer. J Natl Cancer Inst, 97(9), 632–642. https://doi.org/10.1093/jnci/dji111
Miller, K. D., Althouse, S. K., Nabell, L., Rugo, H., Carey, L., Kimmick, G., et al. (2014). A phase II study of medroxyprogesterone acetate in patients with hormone receptor negative metastatic breast cancer: Translational breast cancer research consortium trial 007. Breast Cancer Research and Treatment, 148(1), 99–106. https://doi.org/10.1007/s10549-014-3131-3
Park, K. C., Paluncic, J., Kovacevic, Z., & Richardson, D. R. (2020). Pharmacological targeting and the diverse functions of the metastasis suppressor, NDRG1, in cancer. Free Radical Biology and Medicine, 157, 154–175. https://doi.org/10.1016/j.freeradbiomed.2019.05.020
Marshall, J. C. A., Collins, J. W., Nakayama, J., Horak, C. E., Liewehr, D. J., Steinberg, S. M., et al. (2012). Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. Journal of the National Cancer Institute, 104(17), 1306–1319. https://doi.org/10.1093/jnci/djs319
Brooks, D., Zimmer, A., Wakefield, L., Lyle, T., Difilippantonio, S., Tucci, F., et al. (2018). Limited fibrosis accompanies triple-negative breast cancer metastasis in multiple model systems and is not a preventive target. Oncotarget, 9(34). https://doi.org/10.18632/oncotarget.25231
Khan, I., Gril, B., Hoshino, A., Yang, H. H., Lee, M. P., Difilippantonio, S., et al. (2022). Metastasis suppressor NME1 in exosomes or liposomes conveys motility and migration inhibition in breast cancer model systems. Clinical & Experimental Metastasis, 39(5), 815–831. https://doi.org/10.1007/s10585-022-10182-7
Ahmed, M., Lai, T. H., Kim, W., & Kim, D. R. (2021). A functional network model of the metastasis suppressor PEBP1/RKIP and its regulators in breast cancer cells. Cancers, 13(23). https://doi.org/10.3390/cancers13236098
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, I., Steeg, P.S. A perspective on the metastasis suppressor field. Cancer Metastasis Rev 42, 1061–1063 (2023). https://doi.org/10.1007/s10555-023-10131-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-023-10131-0