Abstract
Cancer is considered a major public health concern worldwide and is characterized by an uncontrolled division of abnormal cells. The human immune system recognizes cancerous cells and induces innate immunity to destroy those cells. However, sustained tumors may protect themselves by developing immune escape mechanisms through multiple soluble and cellular mediators. Neutrophils are the most plenteous leukocytes in the human blood and are crucial for immune defense in infection and inflammation. Besides, neutrophils emancipate the antimicrobial contents, secrete different cytokines or chemokines, and interact with other immune cells to combat and successfully kill cancerous cells. Conversely, many clinical and experimental studies signpost that being a polarized and heterogeneous population with plasticity, neutrophils, particularly their subpopulations, act as a modulator of cancer development by promoting tumor metastasis, angiogenesis, and immunosuppression. Studies also suggest that tumor infiltrating macrophages, neutrophils, and other innate immune cells support tumor growth and survival. Additionally, neutrophils promote tumor cell invasion, migration and intravasation, epithelial to mesenchymal transition, survival of cancer cells in the circulation, seeding, and extravasation of tumor cells, and advanced growth and development of cancer cells to form metastases. In this manuscript, we describe and review recent studies on the mechanisms for neutrophil recruitment, activation, and their interplay with different immune cells to promote their pro-tumorigenic functions. Understanding the detailed mechanisms of neutrophil-tumor cell interactions and the concomitant roles of other immune cells will substantially improve the clinical utility of neutrophils in cancer and eventually may aid in the identification of biomarkers for cancer prognosis and the development of novel therapeutic approaches for cancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer is a non-communicable disease caused by genetic instability brought on by DNA mutations. Uncontrolled cell division, resistance to apoptosis, irregular infiltration into local tissue, and migration to distant organs are all features of malignant cells. Inflammation and resistance to immune-facilitated elimination have lately been identified as another “hallmarks of cancer” [1].
Neutrophils are the most common leukocytes and are considered as the first line of innate immune defense. They are the most abundant immune cells making up ~ 60–70% of the human body’s total population of immune cells. The well-known function of neutrophils includes healing damaged tissues and fighting infections. Being primary responders of inflammation, neutrophils save host cells from infection by eradicating pathogenic organisms and by employing a variety of mechanisms, which includes the discharge of antimicrobial components, phagocytosis, and formation of neutrophil extracellular traps (NETs) [2]. Following infections, injuries, and other types of stress, the neutrophil level in the blood and tissue is increased as induced by an active innate immune response.
Transcriptional activation and changes in the expression of surface molecules or activity cause a wide range of neutrophil functional responses. Only a subset of neutrophils shows these phenotypic changes, implying heterogeneity and plasticity in neutrophils [3]. Low-density and high-density neutrophils (LDN and HDN), polymorphonuclear neutrophils (PMNs), myeloid-derived suppressor cells (MDSCs), and tumor-associated neutrophils (TANs) are all examples of heterogeneous neutrophil subpopulations [4]. Furthermore, neutrophils go through polarization processes that determine whether they are anti- or pro-tumorigenic, depending on the environmental cues. Neutrophils can polarize into either an anticancer (N1) or a protumor (N2) phenotype after being stimulated with cytokines [5, 6]. N1 type neutrophils are characterized by increased expression of tumor necrosis factor-α (TNF-α), C–C motif chemokine ligand 3 (CCL3), intercellular adhesion molecule-1 (ICAM-1), and decreased expression of arginase. On the other hand, CCL2, CCL3, CCL4, CCL8, CCL12, and CCL17 are upregulated in N2 neutrophils, and the CXC motif chemokine ligand 1 (CXCL1), CXCL2, CXCL8 (also called interleukin-8, IL-8), and CXCL16 are downregulated in N2 neutrophils [6]. Notably, cancer is a condition in which the quantity of neutrophils in the bloodstream increases and the heterogeneity, plasticity, and polarization of neutrophils varies as the disease progresses. This suggests that the phenotypes and functions of neutrophils vary with cancer progression. Numerous subpopulations of circulating neutrophils (such as granulocytic myeloid-derived suppressor cells, G-MDSC) have been reported in advanced cancer with varying maturation, tumor cytotoxicity, and immune suppression features [4]. These diverse neutrophil subpopulations play a significant role in the genesis and progression of malignant neoplasm, especially in cancer metastasis [7].
Although neutrophils are naturally well known for their antibacterial and antifungal functions, it is obvious from various studies that neutrophils are involved in cancer biology especially in the modulation of tumor growth and metastatic progression. In recent years, neutrophils have been shown to play a plethora of roles in the development and progression of several types of cancer, including lung cancer and breast cancer, the two most prevalent cancers worldwide [8,9,10]. Furthermore, the interplay between neutrophils and other immune cells in cancer is evident from previous studies [11, 12]. However, the precise mechanistic role of neutrophils and other immune cells in the tumor microenvironment is still inconclusive. Understanding this mechanism concerning cancer growth is an emerging area of cancer research, with substantial potential for shedding light into cancer biology, leading to identifying cancer biomarkers and developing novel therapeutics.
Until now, most studies on the role of neutrophils in cancer had been performed either with animal models or circulatory human neutrophils. Therefore, little is known about the actual phenomena of tumor-associated neutrophils (TANs) in cancer patients. This review outlines the latest mechanistic understandings of the contribution of neutrophils and their interaction with other immune cells and soluble mediators in cancer. We further discuss how anti-tumorigenic or pro-tumorigenic TANs, and their polarization processes may affect cancer etiology, growth, and metastasis. The clinical utility of TANs in tumor prognosis and potential immunotherapeutics is also discussed.
2 Immunobiology and functional roles of neutrophils
Being the most abundant leukocytes, neutrophils act as the first responders to infections or injuries. The bone marrow and spleen are the organs responsible for producing neutrophils by a process called granulopoiesis [13, 14]. Neutrophil homeostasis is maintained by constitutive apoptosis, consumption in the tissues, and the response to infection or injury [2]. More than 1011 neutrophils are usually generated per day, which can be further increased by inflammation as a result of infection or induction of cancer [15]. The half-life of neutrophils is much shorter; however, they may subsist for 5–7 days in the circulation and tissues [16]. After being released into the blood, newly formed granulocytes (neutrophils) migrate to the epicenter of infection or injuries and protect the body from microbial attacks or injuries [2]. The rapid release and subsequent migration of neutrophils to infected/inflamed tissues are induced by various immunological factors, pathogen signals, and different cytokines or chemokines, including but not limited to IL-23, IL-17, G-CSF, and CXCR [17].
Granulocyte–macrophage-colony-stimulating factor (GM-CSF) and the granulocyte-colony-stimulating factor (G-CSF) are the two major growth factors involved in the maturation process of neutrophils. The stages of maturation include myeloblast, promyelocyte, myelocyte, metamyelocyte, band cell, mature neutrophil, and finally, hyper-segmented neutrophils [18]. Notably, neutrophils undergo a different kind of cell death other than apoptosis, where its surplus chromatin materials and secreted proteases form a unique NET (neutrophil extracellular traps)-web-like structure. The process is termed NETosis which traps and destroys opportunistic microbes in the body [19, 20].
The function of neutrophils depends on the granules secreted from the cytoplasm. Different granule proteins are synthesized and accumulated during various stages of neutrophil differentiation. Following neutrophil activation, these granule proteins are released during inflammatory response [21, 22]. Three distinct granule subsets of neutrophils have been identified so far: (i) primary or azurophilic granules, which store myeloperoxidase (MPO), proteolytic enzymes (e.g., elastase, proteinase 3, lysozyme), and bactericidal proteins (e.g., cathepsins, defensins); (ii) secondary or specific granules, which are rich with complement activators such as lactoferrin, cathelicidin, and enzymes, e.g., collagenases and lysozymes; and (iii) tertiary or gelatinase granules, which store matrix metalloproteinases 9 (MMP9)—an essential modulator in the development of cancer- and lysozymes [18, 23,24,25]. MMP9 is implicated in a variety of tumor developmental processes, including vascular growth, proliferation, migration, invasion, tumor-microenvironment regulation, cancer stemness, and chemotherapeutic drug resistance [26, 27].
3 Polarization of neutrophils: why do neutrophils play a dual role in cancer?
It has been a long-term belief that neutrophils are terminally differentiated cells. However, several lines of experimental evidence show that this belief is no longer appropriate for neutrophils. Instead, these cells are truly heterogeneous, multifaceted, and undergo polarization events [28]. The notion of neutrophil heterogeneity indicates that they can possess different morphologies, functions, and express discrete cell surface molecules in different conditions and maturation stages [13, 28]. TANs are neutrophils that can be activated by various cytokines and chemokines in the tumor microenvironment. Diverse TAN populations have been identified based on the expression of cell surface markers and their ability to impede or stimulate tumor growth [29]. TANs are divided into N1 and N2 types, similar to M1 and M2 macrophages [5]. Anti-tumorigenic TANs (N1) are characterized by enhanced immune-activating cytokines “TNFα, CCL3, ICAM-1,” and reduced levels of T cell suppressive enzymes Arginase1 (ARG1). Pro-tumorigenic TANs (N2), on the other hand, have higher levels of the chemokines CCL2, CCL3, CCL4, CCL8, CCL12, CCL17, and chemokine ligand CXCL1, CXCL2, CXCL8, and CXCL16 [5, 30] and increased levels of ARG1. Furthermore, N2 TANs can induce angiogenesis in the tumor microenvironment by recruiting matrix metalloproteinases (MMPs) and Bv8 [31, 32].
Mirroring the heterogeneity of TANs, circulatory neutrophils also show polarization and can be classified by two types based on their density: high-density neutrophils (HDNs) and low-density neutrophils (LDNs) [13, 33]. LDNs contain a combination of mature and immature neutrophils with diverted roles such as the production of ROS, nitric oxide and arginase, and the stimulation of regulatory T cells (Tregs) [4, 29]. It has been speculated that accumulation of LDNs hampers immunosuppressive activities and is positively correlated with increased tumor growth in mice and human cancers [4]. In murine models, Sagiv et al. reported that LDNs show antagonistic functions compared to HDNs in cancer development and progression [4].
Polarization of neutrophils is the key factor producing distinct subtypes with distinct roles in cancer growth and progression. Depending on the degree of plasticity, neutrophils can change their roles in the tissues based on the stimuli (cytokines, chemokines, or other factors) present in the tissue microenvironment. For example, a previous study showed that complete knockout/knockdown of IFN-β stimulates the production of N2 neutrophils in mice and humans, which is associated with reduced tumor killing, less NETosis, lower level of the expression of ICAM1, and TNF-α [34]. This study, therefore, suggests the direct involvement of IFN-β in the polarization of N1 neutrophils stimulating their antitumor properties. Furthermore, cytokine IL-35 has been found to polarize neutrophils toward N2 phenotypes by mediating the elevated expression of G-CSF and IL-6, and this eventually stimulates the recruitment and infiltration of neutrophils into the tumor microenvironment [35]. In addition, treatment with the TGF-β receptor inhibitor SM16 was also found to upregulate activated CD8 + T cells and N1 neutrophils, causing tumor growth in syngeneic lung cancer murine models, implying that tumor-derived TGF-β is linked to the accumulation of N2 neutrophils and promotes tumor growth and progression [5]. Another recent study found that blocking TGF-β by 1D11 (a monoclonal antibody to TGF-β) treatment reduces colorectal cancer cell proliferation partly by suppressing TGF-β/Smad signaling pathways in malignant cells and extensively by inhibiting PI3K/AKT signaling pathways in TANs [36]. Overall, IFN-β and TGF-β signaling pathways are the two major pathways contributing to the formation of N1 and N2 neutrophils, respectively, and thus, modulation of these pathways may contribute to outcomes of cancers.
4 Antitumor activities of neutrophils
There is a compelling source of evidence behind the role of neutrophils in tumor suppression. Studies performed in gastric cancer, colorectal cancer (CRC), and head and neck squamous cancers (HNSC) revealed that persistent infiltration of neutrophils is correlated with more favorable outcomes, suggesting the antitumor role of neutrophils in patients in these cancer types [37,38,39]. To date, two most common mechanisms have been identified by which neutrophils impede tumor growth and progression. One is T cell-mediated cancer suppression, and another is cancer cell destruction by cytotoxic factors.
4.1 T cell-mediated antitumor activities of neutrophils
Several lines of evidence suggest that neutrophils can present antigens to T cells. For example, hybrid neutrophils with characteristics of antigen-presenting cell (APC) were discovered in early-stage lung cancer patients and these APC-like neutrophils were found to cross-present antigens to activate anticancer T cell responses (Fig. 1A). Neutrophils can play their anticancer role as APCs or fuel other APCs to stimulate T cell-mediated immune response and induce T cells possibly by enhancing cell–cell interaction or increased secretion of a variety of immune factors [40].
Mechanisms of the antitumor activities of neutrophils. A Neutrophil-mediated immune activation. NET constituents induce priming of CD4+ T cells which inhibit growth of tumor cells. APC-like neutrophil can further enhance T cell and NK cell-mediated antitumor responses. Moreover, IL-12, secreted from macrophages, stimulates neutrophil to release IFNγ which support activation of T cells. Another way neutrophils attack cancer cells could be by antibody-dependent cellular cytotoxicity (ADCC) during antibody treatment. B Neutrophil-mediated killing of tumor cells. Inflammatory modulators, such as TNF-α, increase neutrophil recruitment by increasing the expression of the receptor tyrosine protein kinase MET. When activated by HGF, MET + neutrophils destroy cancer cells by releasing nitric oxide (NO). Furthermore, neutrophils accumulating at premetastatic locations use H2O2 to destroy invading cancer cells. Certain cancer cells can be induced to apoptosis by direct contact or the production of TRAIL and FasL by neutrophils. MMP secretion driven by neutrophils can also cause tumor cell death
Besides, the activity of dendritic cells (DCs) can also be modulated by neutrophils in the context of cancer. This is evident from the fact that upregulation of activated neutrophils was observed upon treatment with intratumoral CpG oligonucleotides-B (CpG-B), which in turn trigger the activation of DC and stimulation of anticancer immunity by T cells [41]. In agreement, the antitumor effect of CpG-B treatment was reduced upon antibody-mediated attenuation of neutrophils, resulting in DC inactivation and reduction of CD8+ T cell numbers in tumor-infiltrated tissues [41].
Activation of T cells by neutrophils has also been observed in a variety of cancer models. Depletion of neutrophils, for example, hindered CD8+ T cell penetration and was linked to colon cancer progression [42]. Furthermore, a study conducted in a mouse melanoma model identified the involvement of anti-tumorigenic neutrophils as activation of neutrophil by HVJ-E, an inactivated viral particle, enhances the priming of T cell action leading to tumor suppression [43]. It has been further observed that in comparison to isolated T cell cultures, mixed-culture of CD8+ T cells with neutrophils isolated from blood samples or tumor tissues of colon cancer patients increases T cell activation and secretion of IFN-γ [11]. In syngeneic mouse tumor models, Takeshima et al. showed prompt recruitment of neutrophils to irradiated tumors, and these recruited neutrophils exhibited an elevated ROS generation, activation of tumor-specific cytotoxic T cells, and induction of apoptosis in tumor cells [44].
T cell proliferation and activation are also affected by the expression of several co-stimulatory molecules on neutrophil surface (e.g., 4-1BBL, OX40L, CD54, CD86) [45]. The idea was tested experimentally by using inhibitors against costimulatory molecules that repressed the inductive action of TANs on T cell reactions in lung cancer. Remarkably, neutrophils frequently co-localize with CD8 + T cells in tumor areas in colon carcinoma patients. This combined infiltration of both neutrophils and CD8 + T cells into tumor tissue was correlated with an improved prognosis than CD8 + T cell infiltration alone, providing a clinical clue for the interaction of neutrophils and CD8 + T cells in colon cancer and perhaps promoting antitumor immunity [11]. Additionally, multiple pro-inflammatory cytokines and granule proteins, e.g., lactoferrin, α-defensins, and IL-37 released by neutrophils, may impact the activation of T cells. Herein, lactoferrin increases the recruitment and activation of APCs, while α-defensins stimulate monocytes, promoting DC and T cell infiltration [46,47,48]. In addition, in response to IL-12, neutrophils release IFN-γ, which may boost cross-talk with T cells and activate antitumor responses (Fig. 1A) [49]. Arginase, elastase, and myeloperoxidase (MPO), on the other hand, have a significant inhibitory influence on T cell-mediated responses, with arginase causing T cell dysfunction by reducing T cell receptor-ζ chain (TCRζ) [50] and elastase shedding IL-2 and IL-6 receptors on T cells [51]. In addition, NETs were found to activate T cells in specific cases contributing to anticancer responses. This was evident from the study performed by Tillack et al. where they showed that NET-producing neutrophils directly prime CD4+ T cells and were found to be associated with improved survival of cancer patients [52].
4.2 Cytotoxic mechanisms behind anti-tumor activities of neutrophils
In addition to activating T cell-mediated immunity, some cytotoxic mechanisms have also been associated with the antitumor activity of neutrophils. Ackermann and colleagues previously identified neutrophils showing direct cytotoxicity to cultured tumor cells [53]. Recently, the term “tumor cell sloughing” has been defined, and neutrophils have been shown to inhibit preliminary growth and metastasis of cancer cells in a mouse model of uterine epithelial cancer, which was independent of lymphocyte activation [54]. Furthermore, NETs play a direct role in neutrophil-mediated cancer suppression. Recently, NETs have been found to impede the migration, multiplication, and development of malignant cells, even though neutrophils normally generate them to trap and kill pathogenic bacteria [39]. Additionally, some MMPs can possess antitumor activities. MMPs can breakdown plasminogen, collagen XVIII, and collagen IV to create angiostatin, endostatin, and tumstatin, which are natural inhibitors of angiogenesis [55]. Finally, Met (a protein product of protooncogene MET) has been linked to antitumor neutrophil recruitment. In response to TNF-α activation, the translation of MET mRNA produces a tyrosine kinase receptor, the sole known ligand of hepatocyte growth factor (HGF) (Fig. 1B). Intriguingly, MET expression by neutrophils was found to be necessary for neutrophil-mediated restriction of tumor growth and metastasis via nitrogen oxide production as evident in an HGF-secreting cancer model of mice [56]. Another mechanism by which neutrophils destroy cancer cells during antibody therapy is antibody-dependent cellular cytotoxicity (ADCC) [57]. Fcγ receptors are expressed on neutrophils and are involved in cancer cell eradication via ADCC (Fig. 1B).
5 Pro-tumor activities of neutrophils
Several mechanisms have been postulated for neutrophil-mediated protumorigenic activities, including cytotoxicity, generation of reactive oxygen species (ROS), and reactive nitrogen species (RNS). In general, the ROS production contributes to oxidative DNA damage and mutagenesis, whereas the RNS production causes genetic instability, enticing tumor growth, and progression [58]. More importantly, neutrophil granulopoiesis and TANs play vital roles in pro-tumorigenic activities. The pro-tumorigenic mechanisms of neutrophils in several types of cancer have been structured and presented in this section (Table 1).
5.1 The role of granulopoiesis in pro-tumorigenic activities
A variety of enzymes are stored in neutrophil granules, for example, neutrophil elastase (NE), myeloperoxidase (MPO), and matrix metalloproteinase (MMP, e.g., MMP8 and MMP9) [59]. These enzymes when released by degranulation can also promote tumor progression. High MMP-9 expression has been linked to the formation of malignant cells as revealed by several studies. The inherent ability of MMPs to degrade extracellular matrix (ECM) constituents makes them capable of remodeling the ECM to stimulate angiogenesis as well as cancer invasion [60]. Bergers et al. showed that MMP2 and MMP9 are upregulated in angiogenic lesions, promoting tumor growth whereby MMP9 but not MMP2 was found to be directly involved in the induction of angiogenesis [61]. A study showed that MMPs can induce cancer cell motility and invasion by stabilizing integrins at the membrane and activating focal adhesion kinase [62]. Another important enzyme, NE, has also been demonstrated to increase tumor cell growth. NE degrades insulin receptor substrate-1 (IRS-1), causing the phosphatidylinositol 3-kinase (PI3K) to interact with platelet-derived growth factor receptor (PDGFR). This in turn activates PI3K-signaling axis leading to the promotion of cancer growth [63]. Figure 2B represents the role of granulopoiesis in pro-tumorigenic activities.
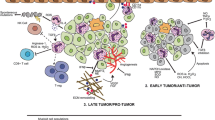
5.2 Interplay between TANs and other immune cells
Many immune cell types such as neutrophil macrophages, lymphocytes, dendritic cells, and natural killer (NK) cells have been reported to infiltrate the tumor microenvironment [64,65,66,67,68]. Residing in the tumor microenvironment, neutrophils can interplay and induce other immune cells to promote tumor growth and progression. For instance, TANs have been found to produce chemokines, such as CCL2 and CCL17, and recruit macrophages and Tregs in the tumor microenvironment, promoting growth, progression, and chemotherapeutic resistance of hepatocellular carcinoma [69]. Noteworthy, Tregs have also been linked to enhancing cancer growth by suppressing other inflammatory T cell populations. In addition, neutrophils may induce tumor cell extravasation and metastasis by secreting cytokines and growth factors. In particular, upon the secretion of IL-8, neutrophils gain the capability of recruitment and infiltration to the tumor site. Subsequent interaction between infiltrated neutrophils with intracellular adhesion molecule 1 (ICAM1) further induces tumor cell extravasation and metastasis (Fig. 3) [70]. Another potent recruiter of neutrophils is oncostatin M (OSM), an IL-6 family member, which triggers neutrophil adhesion to endothelial cells and facilitates chemotaxis [71]. Additionally, in vivo experiments suggest that OSM induces VEGF production from breast cancer cells, thereby increasing cell motility and invasive capacity [72]. Furthermore, following oncogenic kras activation, TANs can be recruited by TGF-β, a cytokine released from hepatic stellate cells, resulting in the progression of liver cancer [73]. A very recent study suggested that the interplay between TANs and macrophages (TAMs) upregulates the activation of STAT3, driving the progression of intrahepatic cholangiocarcinoma [74].
6 Neutrophils in tumor initiation
Neutrophil-mediated inflammation has direct involvement in tumor initiation. As an essential component of inflammation, neutrophils actively initiate tumorigenesis by damaging specific tissues [75]. The role of neutrophils in tumor initiation has been demonstrated in several cancer models, where neutrophils were noted to be recruited to tumor-prone tissues via the CXCR2 ligands, CXCL1, CXCL2, and CXCL5 [76, 77]. CXCR2 ligands are elevated in several genetically engineered cancer animal models, where CXCR2 deficiency or suppression reduces tumor development [77]. Furthermore, antibody (anti-Ly6G)-mediated depletion of neutrophils was found to mimic CXCR2 deficiency and inhibits carcinogenesis in various tumor models [76, 77]. Notably, in a zebrafish model of melanoma tumors, Antonio et al. reported that inflammation enhances tumor growth in a neutrophil-dependent manner [78]. All of these scientific researches in cancer models imply that neutrophils have a significant role in cancer pathogenesis.
The occurrence of genetic instability and their accumulation is also associated with the initiation of tumor. Neutrophils secrete genotoxic components and induce accumulation of DNA damage on epithelial cells resulting in the initiation of oncogenic responses and promoting cancer development (Fig. 2A) [79, 80]. Activated neutrophils, for instance, can increase the frequency of DNA replication mistakes in colon epithelial cells [81]. Wilson and colleagues also discovered that neutrophils increase the production of reactive oxygen species (ROS) and telomere DNA damage in hepatocytes, as well as exacerbate DEN-induced hepatocellular cancer (HCC) [82]. In addition, Yan et al. used a zebrafish model to demonstrate the tumor-promoting role of neutrophils in liver carcinogenesis [83]. Furthermore, another new report shows that miR-23a and miR-155 microRNAs produced by neutrophils in inflamed tissue can cause genomic instability and promote tumor formation [84].
In addition, mutational activation of oncogenic KRAS (Kirsten rat sarcoma virus) is observed in many tumors. KRAS may interplay with neutrophils to initiate tumors (Fig. 2A). For instance, expansion of neutrophils upon stimulation by cytokines IL‑17 and G‑CSF is observed in some KRAS-mediated tumor models [85]. Depletion of neutrophils or suppression of CXCR2 signaling lowers the frequency of lung tumors in these KRAS models, just as it does in chemical-induced colon and skin cancer models, demonstrating that neutrophils are essential for their survival [85, 86]. Interestingly, in humans and mice exposed to cigarette smoke, the association between KRAS and neutrophils was found to be significant. Concomitantly, it was further observed that cigarette smoke incorporated mutations in KRAS and enhanced inflammation and neutrophil accumulation [87]. These findings raise the dichotomy of whether neutrophils are required to initiate all KRAS-driven tumor types and whether KRAS orchestrates their polarization. Also, the immunosuppressive ability of neutrophils and neutrophil-derived elastase has been reported to be linked to the development of tumors [63], although the exact mechanisms are unknown. The generation of ROS, RNS, and MMP9 by neutrophils may also play a role in tumor initiation (Fig. 2A) [88].
Neutrophil function in tumor growth and development. There are several mechanisms by which neutrophils can promote tumorigenesis. Three major pathways of neutrophil-mediated tumor growth have been illustrated in this figure. A Mechanism of neutrophil-mediated cancer initiation; Neutrophils produce reactive oxygen species (ROS) and protease enzymes such as elastase, which can interfere with DNA repair mechanisms and, as a result, accelerate DNA damage and increase mutation load in non-tumor cells, thus initiating cancer formation. B Mechanism of neutrophil-mediated cancer cell proliferation; Neutrophil elastase (NE) has the ability to degrade insulin receptor substrate (IRS1) and activate PI3K/AKT signalling. Mature neutrophil and granulopoiesis further secrete costimulatory molecules, e.g., MMP9, HMGB1, G-CSF, and interleukins which trigger tumor cell proliferation. C Mechanism of N2 TAN-mediated extracellular matrix remodeling; Neutrophil-mediated release of MMP-8, MMP-9, NE, and Cathepsin G can disintegrate and destroy a variety of extracellular matrix constituents and basement membranes, thus inducing cancer growth and proliferation
7 Neutrophils in tumor growth and development
Neutrophils, in particular TANs, got increasing attention because of their dichotomous role in cancer development. The antitumor roles of neutrophils have already been discussed in earlier sections, and they also have complex roles in tumor development. Neutrophils can mediate tumor growth by different mechanisms. Firstly, neutrophils play an essential role in tumor angiogenesis by the expression of vascular endothelial growth factor (VEGF) and MMP9 (Fig. 4B) [89]. Secondly, accumulated evidence from in vivo studies suggest that neutrophils inhibit anti-tumor CD8 + T cell responses through multiple mechanisms. For example, Coffelt et al. revealed a significant role of γδ-T cells in the metastasis of breast cancer cells. Herein, γδ-T cells facilitate increased expression of IL-17, driving the G-CSF-dependent expansion and polarization of neutrophils which in turn enable neutrophil recruitment to the tumor microenvironment [90]. Furthermore, it was reported that cancer cells secrete multiple cytokines, including G-CSF, IL-3, IL-1, and IL-6, that influence granulopoiesis, leading to cancer initiation and development [91, 92]. Furthermore, as previously noted, neutrophils have tumor-promoting effects in the setting of innate immune inflammation and tumor initiation; however, they can also promote tumor development by suppressing the adaptive immune response in the tumor microenvironment. In agreement, there is an interest in optimizing T cell antitumor activity and immunotherapy efficacy against cancer by targeting the suppressive function of myeloid-derived suppressor cells (MDSC). Of note, MDSCs consist of mononuclear cells and neutrophils in different stages of maturation [18, 93]. Noteworthy, granulocytic MDSCs (G-MDSCs) accelerate suppression of CD8 + T cell responses and secretion of cytokines, promoting tumor growth in numerous murine models [94].
Moreover, chemokines affect the recruitment and infiltration of neutrophils to wounds and cancer tissues. IL-8 is one of the first chemokines produced in both wounds and cancer. IL-8 has been demonstrated to recruit neutrophils to tissue injury and malignancy in the zebrafish model via the CXCR1/CXCR2 receptors [95]. Furthermore, blocking CXCR2 lowers neutrophil recruitment to tumors and improves chemotherapeutic efficacy in various breast cancer models [96]. These findings imply that pathways that mediate neutrophil recruitment to tumor tissues can be therapeutically targeted, thereby improving the survival of patients.
The commonest mechanisms of neutrophil-mediated tumor growth are their interplay with other immune cells and are described in the following sections. Also, a structured table representing the immune factors involved in neutrophil-mediated cancer growth, development is outlined in this section (Table 2).
7.1 Neutrophil-mediated T cell inhibition promotes tumor growth
Neutrophils frequently inhibit T cell-mediated immune response through processes that are intimately linked to their antimicrobial actions. Arginase (ARG) and ROS are two of the most commonly described processes. ARG is an enzyme present in neutrophils’ gelatinase-containing granules. High ARG production in cancer cells causes l-arginine depletion, which causes activated T cells to enter the G0-G1 phase of the cell cycle, resulting in T cell malfunction [97]. l-arginine is also essential for functional TCRζ mRNA expression, protein synthesis, and cellular activities [98]. Furthermore, l-arginine-induced dephosphorylation of cofilin improves the stability of immunological synapses. As a result, ARG-mediated l-arginine depletion prevents establishing and maintaining an immunological synapse, which is required for human T cell activation [99].
The role of ROS in neutrophil-induced antimicrobial defense is also well established (Figs. 2 and 4). For example, hydrogen peroxide (H2O2) generated by NADPH oxidase can suppress T cell proliferation and activation through a variety of mechanisms, including apoptosis, reduced NF-κB activation, and TCRζ inactivation [100, 101]. Furthermore, ROS-induced T cell suppression is accompanied by cofilin oxidation [102]. Cofilin can thus be affected by both ARG and ROS, which are the two mechanisms implicated by neutrophils. Therefore, the inactivation of cofilin may prove to be a valuable therapeutic target for T cell suppression. Finally, granular elements produced by mature neutrophils, such as neutrophil elastase, can influence T cell activity, wherein IL-2 and IL-6 receptors on T cells can be cleaved and inactivated by neutrophil-secreted proteases [51].
7.2 Neutrophil-derived cytokines involved in tumor growth
Several studies have shown that neutrophils can promote tumor growth through cytokine secretion, depending on the cues available in the tumor microenvironment [103]. For instance, a proliferation-inducing ligand (APRIL) has emerged as a key neutrophil-derived tumor growth mediator [104]. In vitro studies showed that APRIL enhances antigen presentation of B cells, induces B cell proliferation, improves B cell survival, activates T cells, and drives the growth and persistence of B cell tumors and other solid cancers [105]. Also, an increased APRIL expression in neutrophils isolated from patients with oral cavity squamous cell carcinoma (OSCC) was observed and reported to be linked to tumor promotion [106]. Neutrophils have also been shown to express CD30 ligand (CD30L), a molecule that can activate CD30 and sequentially increase the proliferation of Hodgkin lymphoma (HL) cells [107]. Furthermore, neutrophils can induce prostate tumor development in a PTEN-knockout autochthonous model by counteracting tumor cell senescence via IL-1 receptor antagonist (IL-1RA), demonstrating their pro-tumorigenic effects [108]. CXCR2 ligands, on the other hand, govern neutrophil recruitment and are linked to tumor growth and progression. To elaborate, multiple studies have demonstrated the significance of CXCR2 ligands in tumor growth by preventing neutrophil recruitment to tumors, which was mainly accomplished through CXCR2 suppression. For example, mouse prostate cancer cells show elevated levels of CXCL5 stimulated by the activation of the Hippo-YAP1 (Yes-associated protein 1) pathway. Contrarily, inhibition of YAP1 and CXCR2 was found to reduce immunosuppressive neutrophil migration to tumors and suppress tumor growth in tandem [109]. Besides, in a PTEN-deficient mouse model of endometrial adenocarcinoma, inhibiting neutrophil recruitment by genetic ablation of GCSFR or CXCR2 was observed to increase uterine cancer progression [54]. Furthermore, hypoxia-mediated secretion of CXCL1, CXCL2, and CXCL5 recruits neutrophils, which slowed tumor progression by increasing cancer cell dissociation from the basement membrane through integrin modulation [54].
7.3 Neutrophils in tumor proliferation/development
Neutrophils produce and release a wide range of substances that promote tumor cell development (Figs. 2 and 4; Table 2). Particularly, COX-2-mediated prostaglandin E2 (PGE2) production by neutrophils can stimulate tumor cell growth [110]. For example, an acute wound can cause the quick recruitment of neutrophils to engage with surrounding malignant cells, resulting in enhanced cancer cell proliferation via PGE2 [78]. Liang et al. also demonstrated that the elevation of S100A4 expression by neutrophils increased the proliferation of glioblastoma-initiating cells [111]. In addition, neutrophils can increase renal tumor proliferation by curbing the androgen receptor (AR)/c-Myc signaling pathway [112]. Furthermore, neutrophils from B cell lymphoma patients activate stromal cells, promoting the growth of B cell lymphoma [113]. Moreover, by secreting soluble substances, neutrophils were found to accelerate multiple myeloma (MM) growth by evading myeloma death by chemotherapeutic drugs doxorubicin and melphalan treatment [114]. Aside from these soluble factors, the density of circulating neutrophils has a substantial impact on neoplasm caused by neutrophils. Two subgroups of neutrophils—HDNs and LDNs are detected in various cancer models [4]. In particular, increased LDN mobilization into the peripheral circulation was noted to be linked to accelerated cancer proliferation [90, 115].
7.4 NETosis in promoting tumor growth and development
Neutrophils stimulate primary tumor growth by generating NETs. NETosis is a cell death process in which chromatin structures of neutrophils are extruded and adorned with granule contents, resulting in an extracellular structure called NETs. NETosis helps neutrophils to capture and eliminate opportunistic pathogens to prevent them from spreading. NETs, on the other hand, have been reported to have a significant role in the development of a primary tumor. Neutrophilia and hyper-coagulation are noted to be linked and involved in cancer development. Herein, neutrophils express a complement receptor C3aR, which enhances NETosis and coagulation, inducing neutrophil polarization towards N2 phenotypes and prompts tumorigenesis [116]. Notably, immature LDNs favorably respond to breast cancer cell-derived C3a to elicit liver metastasis [117]. Furthermore, high G-CSF and IL-1β expression were noted to be linked to an increased number of neutrophils and tumor-associated thrombosis, both of which were dependent on NETosis (Fig. 2B) [118]. The use of pharmacological suppression of IL-1 receptor signaling, which resulted in decreased NET formation, reduced tumor-associated thrombosis, and slowed tumor development, further supported this observation [118]. NETs have also been linked to the proliferation of cancer cells. For example, neutrophil elastase (NE) found in NETs activates the TLR4-PGC1α axis in tumor cells, increasing mitochondria biogenesis and ATP generation, allowing cancer cells to grow even faster [119]. Additionally, NETs can cooperate with NK cells by promoting the interaction of NEMO with IKKα/β, activating NK cell activation, which in turn promotes breast cancer progression and metastasis [120]. Overall, although neutrophils typically form NETs to trap and kill pathogens, however, NETs generated by tumor-infiltrated neutrophils can stimulate metastasis of breast cancer cells.
8 Neutrophils in cancer metastasis
The most lethal aspect of cancer growth is the capability of tumor cells to spread beyond the primary tumor sites into other distant sites. Indeed, metastasis is the principal cause of 90% of cancer patient’s death. Neutrophils may aid tumor metastasis by increasing tumor cell migration and invasion, destroying the extracellular matrix, and facilitating tumor cell accumulation (Fig. 2C) [121]. Several lines of evidence suggest that neutrophils can stimulate metastasis in a variety of cancers. For example, neutrophils colonize in the lungs and release leukotrienes (such as LTB4), which promote the proliferation of cancer cells by increasing the activation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) (Fig. 4C) [7]. Similarly, mice with melanoma tumors those exposed to circulating neutrophils from tumor-bearing mice developed more lung metastatic nodules than the mice exposed to circulating neutrophils from healthy mice [122]. Consistently, abrogating neutrophil accumulation by neutralizing different modulators (e.g., IL-17, G-CSF) reduced lung metastasis and lymph node invasion in a mice model of mammary cancer [90]. Furthermore, a high neutrophil content and their ratio to lymphocyte (NLR) were found to be correlated to the occurrence of brain metastases in individuals with aggressive lung cancer (e.g., NSCLC) [123].
In addition, genetic loss of p53 in cancer cells augmented a high level of Wnt ligand expression, which increased IL-1β production from TAMs and, in turn, activated γδ17 T cells, thereby increasing neutrophil expansion and hastening lung cancer spread [124]. Furthermore, in an aggressive type of breast cancer (e.g., TNBC), deficiency of E74-like transcription factor (Elf5) protein resulted in the upregulation of IFN-γ signaling, leading to the recruitment of T cell suppressive neutrophils [125]. Additionally, using two mice models of melanoma, Markman et al. showed that impairment in neutrophil maturation and function resulted in elevated secretion of ROS from neutrophils and inhibition of NK cell stimulation and promoted lung metastasis [126].
Recruitment and expansion of neutrophils in the metastatic niches are also associated with multiple tumor-derived immune factors. In particular, after being released from the tumor, IL-1β induces γδT cells to secrete IL-17A and G-CSF, which triggers neutrophil enrollment to the lungs and aids in the establishment of metastases [90]. Furthermore, tumor spread was aided by GM-CSF and IL-5-induced neutrophil recruitment in the lungs of obsessive mice [127]. Also, Charan et al. reported that implantation of bone cancer cells in the tibia triggers lung epithelial cells by the secretion of angiopoietin-like-2 (ANGPTL2) protein, leading to neutrophil recruitment and increased lung metastatic load [128]. Additionally, NOTCH1 activation in colorectal tumors causes TGFβ2-dependent recruitment of T cell suppressive neutrophils in the liver, resulting in increased hepatic metastases [129]. According to Janiszewska and colleagues, few clones of breast tumors can produce IL-11, which then triggers mesenchymal stromal cells, resulting in chemokine-mediated neutrophil recruitment, dissemination, and induction of metastasis [130]. Moreover, GM-CSF generated from tumor cells has been demonstrated to induce neutrophils to produce and secrete a mitogenic protein named transferrin, which enhances lung metastasis when incorporated into the tumor cells [131].
Neutrophils can cause tumor cells to undergo epithelial-to-mesenchymal transition (EMT), which facilitates their migratory and invasive abilities (Fig. 4C) [132, 133]. Neutrophils infiltrated into the tumors are linked to enhanced EMT, increased metastatic progression, and poor survival of patients. For example, neutrophils produce tissue inhibitor of matrix metalloprotease (TIMP-1) which promotes EMT and increases tumor cell motility and invasion. Studies also showed that CD90 is expressed by tumor cells that had undergone EMT, increasing TIMP-1 release by neutrophils [134]. Also, androgen receptor (AR)/MMP13 signaling regulation by neutrophils promotes bladder cancer cell invasion [135]. Furthermore, through activating VEGF/HIF2α and estrogen receptor β signaling, neutrophils may increase renal cell carcinoma cell motility and invasion [112].
NETs also aid in the seeding of cancer cells and the colonization of metastasis. According to studies, metastatic cells utilize NETs to help them colonize more effectively. Park et al. reported that in the 4T1 breast cancer model, G-CSF produced by neoplastic cancer cells stimulates the production of NET. They also proved that targeting NETs with DNase I-coated nanoparticles reduced the progression of lung tumor cells [136]. Furthermore, the human cancer cell line AsPC-1 has been shown to directly stimulate the formation of NETs [137]. Moreover, NET stimulation by ovarian tumor-derived substances has been shown to play a role in omentum metastasis [138]. NETs have also been shown to activate cancer-associated fibroblasts in the liver, causing metastasis [139].
9 Neutrophil-derived cytokines involved in cancer metastasis
Both inherent tumor cell characteristics and stimuli generated from the tumor microenvironment are required for advanced cancer metastasis. Neutrophils release cytokines such as OSM, TGF-β, HGF, and CXCL8 that aid tumor spread (Fig. 4) [140]. Queen and colleagues reported that neutrophil produces OSM when these cells are primed with breast tumor cell-conditioned medium or co-cultured with breast cancer cells. Subsequently, this OSM not only stimulates VEGF synthesis, but also causes cancer cells to detach and boost their invasive potential [141]. Similarly, another study revealed that exogenous treatment of epithelial cells with OSM promoted induction of EMT and acquisition of cancer stem cell (CSC) properties of the transformed epithelial cells [142]. Furthermore, HGF produced from the tumor cells plays a role in oncogenesis and tumor progression in various human malignancies. In vitro studies revealed that bronchoalveolar lavage fluids (BALF) containing HGF from cancer patients caused a higher migratory capacity of bronchioloalveolar adenocarcinoma (BAC) cells than BALF from healthy participants [143]. In addition, Imai and colleagues suggested that neutrophil-derived HGF dose-dependently increases invasive potentials of both HuCC-T1 (human cholangiocarcinoma) and HepG2 (liver cancer) cells [144].
TGF-β has variable effects in regulating cancer progression depending on the stimuli present in the tumor microenvironment [145, 146]. As previously described, TGF-β has been demonstrated to be involved in the polarization of neutrophils, favoring N2 (tumor-promoting) phenotype [5]. According to Aoyagi and colleagues, TGF-β is over-expressed by neutrophils along the tumor’s invasive front, suggesting its role in neutrophil-mediated tumor invasion [147]. Moreover, the combined action of chemokines (CXCL1/2/3) and TGF-β was found to induce neutrophil recruitment to the tumor microenvironment of triple-negative breast cancer [148]. Additionally, CXCL8 generated from tumor cells has also been found to have a role in the course of human cancer by regulating cancer cell proliferation, EMT, neovascularization, and neutrophil infiltration [149, 150]. In addition to these, the roles of neutrophil-derived cytokines in cancer progression and metastasis are summarized in Table 2.
10 Neutrophils in tumor cell intravasation and extravasation
Circulating tumor cells (CTCs) are a type of cells discovered in the blood of individuals with solid tumors and that act as a seed for metastasis. CTCs adopt a variety of tactics to survive in the intravascular environment, but their capacity to rapidly extravasate into the surrounding tissue determines their metastatic potential. Although extravasation is critical for metastasis, the mechanisms that underpin this complicated process are yet poorly understood. According to growing evidence, neutrophils can control tumor cell extravasations in three ways: rolling, adhesion, and migration (Fig. 3) [151]. L-selectin (expressed on neutrophils), E-selectin, and P-selectin (expressed on endothelial cells) are involved in neutrophil rolling (Fig. 3). In addition, lymphocyte function-associated antigen 1 (LFA-1) and β2-integrin expression on neutrophils, and ICAM-1 expression on endothelial cells, are required for neutrophil adhesion and transmigration [152, 153]. In agreement, Spicer et al. revealed that CD18 mediates the interplay of neutrophils with ICAM-1-expressing CTCs, thereby enhancing neoplastic cell adhesion within hepatic sinusoids and exacerbating liver metastasis [154]. Furthermore, it has been established that interactions between cancer cells and neutrophils are dependent on VCAM-1-mediated adhesion, which promotes breast cancer cell extravasation and metastasis [155].
Neutrophil-derived substances can also influence the extravasation process by weakening the endothelium barrier and spreading cancer cells more freely. Endothelial cells have been reported to be triggered by neutrophil-derived cytokines such as IL-8, IL-1β, MMP8, and MMP9, which impair endothelial barrier function, increase transendothelial migration, and accelerate tumor cell extravasation (Fig. 3) [70, 156]. For example, CD11b + Ly6G + neutrophils promote tumor cell extravasation via secreting IL-1β and MMPs [156]. IL-1β is a cytokine produced by neutrophils and was noted to stimulate endothelial cells and promote the extravasation of leukocytes [157]. Therefore, it can be said that neutrophils aid tumor migration not only by acting directly on tumor cells, but also by activating endothelial cells.
Finally, NET components (e.g., HMGB1, Cathepsin G, MMP9, NE) can aid cancer cell extravasation by improving tumor cell trapping within metastatic sites (Fig. 3) [136, 158]. For example, NET-mediated release of Cathepsin G activates insulin-like growth factor 1 (IGF-1), increases intercellular adhesion by E-cadherin, and guides cancer cells’ entry into the blood vessels (intravasation) (Fig. 3) [159]. It has been observed that, NETosis inhibition reduces cancer cell adhesion and prevents cancer cell spread to the lungs and liver [160, 161]. Furthermore, NETosis can be triggered by a redox imbalance or ozone exposure, resulting in higher cancer cell entrapment in the lungs and acceleration of metastasis [162, 163]. Overall, these findings suggest that neutrophils are important in tumor cell intravasation and extravasation, both of which promote cancer spread.
Neutrophils promote intravasation and extravasation of tumor cells. A Intravasation; The production of HMGB1 and TNF-α by NETs promotes cancer cell motility toward the blood vessels. Cathepsin G stimulates intravasation by activating IGF-1. Besides, cancer cell survival in the peripheral circulation is promoted by neutrophil-cancer cell interaction. B Membrane tethering and rolling; Endothelial cells express E/P-selectin, which causes neutrophils to make primary contact and roll along the endothelium’s surface. Then, through interactions with LFA-1 and ICAM-1, neutrophils arrest tumor cells. C Transmigration and adhesion; IL-8 produced by tumor cells increases β2-integrin expression on neutrophils, causing them to connect with ICAM-1 on endothelial cells. As a result of β2-integrin-mediated binding, IL-8 enhances neutrophil contacts with ICAM-1-expressing tumor cells. D Extravasation; Circulating tumor cells express selectin ligands on their surface which interact with E-selectin on endothelial cells and in turn expedite tumor cell adhesion to endothelium. This cell–cell interaction promotes extravasation of the tumor cells. NETs also facilitate extravasation by releasing MMP9 and NE which stimulate the growth of latent cancer cells in distant tissues. Together, neutrophils facilitate the intravasation and extravasation mechanism of tumor cells
11 Neutrophils in tumor angiogenesis
The stimulation of angiogenesis is one strategy employed by neutrophils to enhance tumor metastasis (Fig. 4B). Angiogenesis, which is a hallmark of malignant neoplasia, is the development of new blood vessels that allow tumors to obtain sufficient oxygen and nutrients necessary for continuing growth and metastasis. The involvement of neutrophils in angiogenesis was initially implied as neutrophil ablation was reported to reduce tumor growth and microvessel density in tumor models [164, 165]. Furthermore, inhibiting CXCR2 or implanting lung cancer cells into CXCR2-deficient mice replicated these findings [166]. In other investigations, co-injection of tumor cells with Gr+CD11b+ myeloid cells taken from tumor-bearing animals increased tumor vascularization, demonstrating neutrophil’s proclivity for angiogenesis propagation [167]. Neutrophils have been demonstrated to regulate tumor angiogenesis by producing cytokines like VEGF and Prokineticin 2 (PK2) or Bv8. In addition, neutrophil elastase, MMP9, CXCL8, Angiopoietin-1 (Ang1), OSM, and FGF2 are some of the immune factors implicated in neutrophil-mediated tumor angiogenesis [63, 164, 167,168,169].
VEGF (or VEGFA), an important modulator of angiogenesis, is produced by neutrophils, monocytes, and T cells. Vascular endothelial cells and bone marrow-derived cells express two types of receptor tyrosine kinases (VEGFR1 and VEGFR2), where VEGF can bind and promotes tumor vascularization [170]. For example, tumors obtained from mouse melanoma or fibrosarcoma cell lines implanted into IFN-β-deficient mice develop more quickly and are more vascularized than tumors derived from control mice. The researchers also discovered that these neutrophils have higher levels of VEGF and MMP-9, which is linked to greater tumor infiltration [171]. VEGF levels were also found to be higher in the peripheral blood neutrophils of patients with aggressive breast neoplasm and anal malignancy as compared to healthy persons [172]. Another study found that circulatory neutrophils from OSCC patients consistently produce higher quantities of VEGF than that of healthy control neutrophils [173]. The role of Bv8 was implied by Shojaei et al. who claimed that tumor-derived G-CSF induces Bv8 expression and activates neutrophils, thereby enhancing angiogenesis [31]. It has been shown that Bv8 is involved in the recruitment of neutrophils and in the induction of tumor angiogenesis in a RIP-Tag mouse model [164, 169]. Importantly, Bv8 is also linked with neutrophils in most human tumors studied, including lung carcinomas [174], implying that neutrophil-derived Bv8 may play a role in the angiogenic switch in human cancers as well.
MMP-9 has been reported to be linked to VEGF activation to generate and maintain angiogenesis, with neutrophils being the primary source of MMP-9 [88, 164, 175]. The link between VEGF and VEGF-receptor is considerably suppressed when neutrophils are depleted [88, 176]. Furthermore, aggressive cancers, such as human fibrosarcoma and prostate cancer, have been demonstrated to recruit infiltrating MMP-9-positive neutrophils, resulting in increased angiogenesis. The reduction of angiogenesis caused by CXCL8-mediated neutralization of neutrophils was noted to be recovered by pure neutrophil proMMP-9 [165]. Importantly, human neutrophils generate TIMP-free MMP-9 that acts as a potent angiogenesis activator, as revealed by previous studies [165, 176]. Noteworthy, angiogenesis also remodels the tissue extracellular matrix (ECM) before the endothelium can sprout and rearrange for vasculogenesis. Numerous proteolytic enzymes, including MMPs and elastase, are involved in this tissue remodeling (Fig. 2C) [177]. In summary, MMP-9 has been connected to angiogenesis in general, although its direct catalytic roles and regulation in angiogenic processes in cancer are yet unclear. Furthermore, it has been discovered that neutrophils enhance angiogenesis through a novel mechanism. Neutrophils in the liver microenvironment produce and secrete fibroblast growth factor 2 (FGF2), which promotes angiogenesis and the formation of hepatic metastases (Fig. 4B) [178].
12 Neutrophil-mediated immunosuppression aggravates metastasis
One approach for cancer cells to avoid immune-mediated destruction is systemic immunosuppression. By depleting amino acids or producing certain cytokines, neutrophils can have an immunosuppressive function in cancer, promoting tumor growth and decreasing the enrollment of other immune cells to the tumor microenvironment (TME) (Fig. 4A). Neutrophils, for example, can produce ROS and arginase 1 (ARG1), both of which impair T cell responses to tumors [58]. ROS are highly oxygen-bearing compounds that produce oxidative pressure in T cells [179]. They comprise H2O2, superoxide anions (O2-), and hydroxyl radicals (HO•). ARG1 is known to catabolize arginine, an amino acid essential for the formation of T cell CD3 zeta (ζ) chains and T cell activation [180]. Intriguingly, cancer cell-induced IL-8 secretion can cause neutrophils to release ARG1 into the TME, which degrades extracellular arginine and thus suppresses the T cell-mediated immune responses [181, 182]. Reduced arginine levels also limit T cell proliferation by blocking cell cycle regulatory proteins including cyclin-dependent kinase 4 (CDK4) and cyclin D3 (Fig. 4A) [183]. Membrane-associated proteinase 3 (MAP3), a serine protease, was also found to restrict T cell proliferation in vitro by its enzymatic action on neutrophils [184].
In cancer patients, circulating neutrophils have been shown to have a negative relationship with IFN-γ producing active T cells [185]. H2O2, a key neutrophil effector molecule, was also appeared to be crucial for inhibiting IFN-γ, and other cytokines associated with T cell priming, e.g., IL-2, IL-4, and TNF-α [185]. Other researchers discovered that neutrophils isolated from glioblastoma patients’ peripheral blood suppress CD8+ T cell activation [186].
The programmed death ligand-1 (PD-L1), an immune checkpoint protein, is also involved in neutrophil-mediated T cell suppression. According to a study, neutrophils in gastric tumors suppress anticancer T cell function by overexpressing PD-L1 [187]. In human liver cancer and hepatoma-bearing mice, neutrophils were found to negatively regulate adaptive immune response via the PD-L1/PD1 signaling pathway [188]. Furthermore, tumor cell-derived GM-CSF was found to enhance the expression of PD-L1 on neutrophils via activating the JAK/STAT3 (Janus kinase/signal transducer and activator of transcription 3) pathway. Importantly, when CD3+ T cells were treated with anti-PD-L1 antibody, they were protected against the immunosuppressive effects of co-cultured tumor-conditioned neutrophils [187].
Neutrophils can inhibit NK cell activity by releasing myeloperoxidase (MPO) and H2O2, as well as by expressing CXCR4 on their membranes, which was reported to be linked to reduced secretion of IL-18. This cytokine causes NK cell activation [189] [190]. Neutrophil driven apoptosis of CD8+ T cells can also be induced in the tumor environment via TNF-α and nitric oxide contact-dependent pathways [191]. T cell activities can also be inhibited by the activation of IL-10 producing B cells, which was induced by tumor cell-released autophagosomes (TRAP) [192]. Neutrophils are engaged in TRAP-mediated immune suppression though the mechanism remains largely unknown [193]. TRAPs can be phagocytized by neutrophils quickly and effectively, and they can cause neutrophil death by releasing ROS and activating caspase-3. In addition, these TRAP phagocytized apoptotic neutrophils suppress T cell proliferation and activation in a cell contact and ROS-dependent manner (Fig. 4A) [193].
Neutrophil-mediated regulation of cancer metastasis. As illustrated in this diagram, neutrophils play a key role in promoting cancer metastasis by three important events. A Neutrophil-mediated suppression of anti-tumorigenic T cells; Neutrophils have the ability to drive the development and recruitment of regulatory T cells (Tregs) in the tumor sites while also eliminating CD8+ T cells and NK cells. Additionally, neutrophil-derived ARG1 promotes arginine depletion, resulting in the suppression of the activity of anti-tumor T cells. Other neutrophil-derived products such as ROS, iNOS, and PGE2 also contribute to the immunosuppression process. B Neutrophil-mediated tumor angiogeneisis; Neutrophils can help tumors metastasis by generating proangiogenic proteins such MMP-9, VEGF, and OSM, which stimulate tumor angiogenesis. C Cancer metastasis-induced by TANs. Moreover, NETs and immune factors released from neutrophils can entrap tumor cells and assist their translocation to distant regions, encouraging cancer metastasis
13 Clinical utility of neutrophils in cancer
A vast body of research suggests that the number of neutrophils in circulation and tumor tissues of cancer patients is related to disease’s prognosis. Furthermore, for boosting neutrophil antitumor capability while preventing pro-tumor abilities, several novel prognostic and therapeutic approaches are being studied (Fig. 5). The overall clinical utility of neutrophils along with the contribution of other immune cells has been discussed below.
13.1 Prognostic potentials of neutrophils in cancer
Tumor-associated neutrophilia has been related with a poor prognosis in a number of cancers, including lung carcinoma [194,195,196], breast cancer [197], renal carcinoma [198], head and neck squamous cell carcinoma [199], pancreatic cancer [200], colorectal carcinoma [201], hepatocellular carcinoma [188], Hodgkin lymphoma [202], and many more. While the major prognostic marker is the neutrophil to lymphocyte ratio (NLR), some other factors have emerged to be suitable for the prognosis of neutrophilia-derived cancers.
13.1.1 Neutrophil to lymphocyte ratio (NLR) as a prognostic factor
Growing research suggests that the quantity of neutrophils in the circulatory system and tumor-infiltrating neutrophils in cancer patients is connected to their prognosis. Furthermore, a high neutrophil density in malignancies is thought to be an independent predictor of poor prognosis. The NLR estimate is now considered a very accurate predictive marker in cancer patients, and various research on its clinical implications in cancer have been conducted. The presence of intra-tumor neutrophils, for example, highly predicts short recurrence-free survival (RFS), and overall survival in localized renal cell carcinoma (RCC) and can be considered an independent prognostic factor [198].
NLR has been proven to be substantially linked with the prognosis of melanoma patients treated with ipilimumab [203]. Besides, 5-fluoro uracil-treated patients, intra-tumor neutrophil density was shown to be predictively significant in stage III colorectal cancer (CRC) [204]. For patients with gastric cancer who had a radical resection and received cytokine-mediated immunotherapy and chemotherapy, an NLR of less than 2.995 can be a prognostic marker [205]. On the other hand, an NLR of less than 3 is a prognostic marker for patients with prostate cancer who have metastasis and received chemotherapy [205]. Additionally, in advanced NSCLC patients, the measurement of NLR at 2 and 4 weeks following a dose-dependent nivolumab administration correlates with treatment response [206]. Furthermore, in Hodgkin lymphoma patients, NLR has appeared as a prognostic factor for progression-free survival regardless of tumor stage (Fig. 5A) [202].
13.1.2 Other prognostic factors related to neutrophils
According to a multivariate study conducted by Zhou et al., the overexpression of CXCL5 alone or in combination with the intra-tumoral CD15 + neutrophils is associated with lymph node metastasis (Fig. 5A). It acts as an independent prognostic factor of the overall survival of gastric adenocarcinoma [207]. CXCL5-mediated neutrophil infiltration has been reported to elevate NLR levels in peritumor tissues relative to intratumor tissues in HCC patients and hepatoma-bearing mice, leading to a negative connection with the overall survival of HCC patients [188]. Trellakis and coworkers found that neutrophil infiltration into tumors is linked to shorter survival in metastatic HNSCC cancer. In this investigation, neutrophil count, NLR, and serum levels of CCL4, CCL5, and IL-8 were shown to be significantly greater in HNSCC patients’ peripheral blood compared to controls [199]. Furthermore, infiltration of dendritic cells and neutrophils has been associated with poor prognosis in melanoma patients [208]. Increased neutrophils, CD44+ lymphocytes, and WBCs have also been linked to a lower survival in lung cancer patients. For example, in stage III/IV NSCLC patients, high levels of CD44+ lymphocytes and neutrophils are associated with metastasis and a poor prognosis [195].
13.2 Therapeutic potential of neutrophils in cancer
Neutrophils (or PMNs) are considered as potential therapeutic targets for many inflammatory diseases and cancers. Tumor-promoting activities of neutrophils, like tumor growth and metastasis, render them an emerging target for anti-cancer therapy. Four key therapeutic strategies to target neutrophils have been proposed: (a) inhibiting neutrophils by targeting the CXCL8/CXCR1/CXCR2 signaling axis, (b) inhibiting tumor-derived proteins that attract and polarize neutrophils by pharmaceutical means, (c) preventing tumor growth by targeting immunological components generated by neutrophils, and (d) combining the aforementioned tactics with existing or novel anticancer medications to achieve effective therapeutic effects (Fig. 5B) [121, 209].
One example of the first strategy is the use of CXCR1 and CXCR2 inhibitors (e.g., Reparixin) to suppress the migration of TANs to tumor tissues [77, 210]. Targeting IL-23/IL-17 axis is also utilized in another treatment approach as these molecules are also involved in neutrophil recruitment [211]. Interestingly, breast cancer metastasis was induced by the expansion and polarization of neutrophils through elevated expression of IL-17 from γδ-T cells, suggesting that γδ-T cells/IL-17 axis may serve as a potential therapeutic target to suppress breast cancer metastasis [90].
Overcoming neutrophil-mediated immunosuppression could also be a strategy to combine traditional T cell checkpoint inhibitor immunotherapies. Of note, programmed death 1 (PD-1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA4) are two vital checkpoints destined for dampening the T cell immunity. Preclinical research has shown that combining an anti-PD-1 inhibitor with an anti-CXCR2 inhibitor has a synergistic impact in reducing tumor development [212]. Consistent with this notion, other experimental findings revealed that enhanced neutrophil counts in melanoma patients are linked to a lower effect to ipilimumab, a monoclonal antibody against CTLA4 [213].
Furthermore, neutrophil heterogeneity and plasticity, and their ability to polarize from anti-tumor to pro-tumor activities or vice versa, support another strategy of neutrophil targeting known as “reeducation” of TANs, which involves polarization of TANs towards N1 (anti-tumor) phenotype and modulation of tumor microenvironment components. Since TGF-β promotes N2 (pro-tumor) polarization blockade or inhibition of TGF-β could antagonize tumor formation. In support of this, blocking of TGF-β and/or TGF-β receptor has been demonstrated to have anti-tumor effects in several cancer models [214, 215]. A recent discovery also implies that anti-TGF-β inhibitor suppresses tumor development in colorectal cancer (CRC) by polarizing TANs to the N1 (anti-tumor) phenotype, offering a potential method for CRC therapy [36].
In addition, Pang et al. revealed that genetic ablation of TGFβ in neutrophils prevented tumor spread in mice, but injection of TGFβ generating neutrophils into tumor-bearing animals might restore the metastatic phenotype [216]. Moreover, deficiency of the TGFβ receptor reduces the synthesis of arginase 1 (ARG1) and iNOS in neutrophils, which promotes IFN-γ expression in CD8+ T cells and prevents tumor spread [216]. It has also been demonstrated that priming N2 TANs with TNF-α and IFN-γ changes neutrophil’s tumor-promoting potentials to tumor-suppressing capacity via NK cell activation, implying that the introduction of normal NK cells might be a feasible therapeutic strategy for cancer treatment [217]. Furthermore, it has been shown that IFN-γ treatment promotes N1 (anti-tumor) polarization of TANs both in mice and humans [218]. Another study by Shrestha et al. suggests that ACEi (angiotensin-converting enzyme inhibitors) drugs and angiotensin II type 1 receptor antagonists (AGTR1) may decrease tumor development by polarizing TANs towards the N1 phenotype [219].
Importantly, neutrophil-targeting therapeutics that are in clinical use for inflammatory and autoimmune disorders can be repurposed for cancer treatment. For example, by blocking ALOX5 (a leukotriene-producing enzyme), the anti-asthmatic medicine zileuton decreases the recruitment of pro-metastatic neutrophils and reduces lung metastasis [7]. Therefore, it would be interesting to decipher the link between zileuton treatment for asthma and the risk of lung cancer.
Recently, the role of NETs in promoting existing metastases has been described [220]. The capacity of NK cells and cytotoxic T lymphocytes to destroy cancer cells was found to be abrogated by NETs generated during cancer growth. Particularly, direct interaction between cancer cells and cytotoxic immune cells (NK cells and T cells) can be impeded by the formation of NETs. Therefore, by reducing NETosis using an inhibitor of protein arginine deiminase 4 (PAD4) in combination with immune checkpoint blockers, tumor growth, and metastasis might be managed [220].
Clinical utility of neutrophil in cancer. Clinical utility of neutrophil can be explained by to ways. A Prognostic values of neutrophil in cancer. Neutrophil numbers, NLR ratio, CD15 + neutrophils act as several potential markers for prognosis of different types of cancer. B Therapeutic approaches in targeting neutrophils and NETosis. Several neutrophil-targeting strategies have been proposed and have demonstrated anticancer activity in both experimental and clinical settings. (1) Neutrophil recruitment, growth, and polarization are inhibited by pharmacological inhibition of tumor-derived proteins and downstream signaling pathways. (2) Selective interference with neutrophils’ pro-tumoral actions could be used as an alternative cancer treatment. (3) Neutrophils can be reprogrammed from a tumor-supporting to a tumor-suppressive phenotype, which also has therapeutic promise. (4) NET-mediated release of NE and ARG1 can also be targeted for cancer therapy. (5) When these treatments are paired with traditional anticancer tactics like chemotherapy or emerging anticancer strategies like immunotherapy, the therapeutic benefits are likely to be more effective
14 Conclusion
The oncogenic functions of neutrophils in cancer pathogenesis have recently been highlighted, even though the fact that neutrophils exhibit anticancer properties depending on the environmental cues. The pro-tumorigenic activities of neutrophils are primarily driven by their ability to promote tumor cell proliferation and metastasis, increase tumor angiogenesis, and induce immunosuppression. Neutrophils are consistently recruited to local tumor sites and, in turn, they either suppress or exacerbate cancer development and metastasis depending on N1 or N2 polarization phenotypes. Several immune factors (e.g., cytokines, chemokines) and immune cells, particularly T-lymphocytes, NK cells, and dendritic cells, further regulate neutrophil-mediated cancer progression or suppression.
The roles of neutrophils in cancer, their interactions with other immune cells in tumor growth and metastasis, and their potential as a prognostic biomarker and therapeutic targets have all been discussed in this review. Most studies on the involvement of neutrophils in cancer were primarily focused on experimental animal cancer models; however, further research is needed to understand the cellular and molecular pathways that regulate neutrophil phenotypes and functions in human malignancies. Furthermore, NETosis is appeared to promote cancer progression; however, more research is needed to determine the potential role of NETs as cancer therapeutic targets, by which pharmacological interference could reduce NETs and thus cancer progression.
Despite NLR has been highlighted as a potential prognostic biomarker because its calculation is much easier from patients’ blood samples, it has not been widely embraced in clinical settings. Therefore, future research could also be focused on identifying novel biomarkers depending on neutrophil numbers or other related immune factors. Finally, new insights into the mechanisms underlying neutrophil plasticity and their pro-metastatic role would enable to design neutrophil-targeting novel therapeutics for patients with advanced cancer in the future.
References
Hanahan, D., & Robert, A. (2011). Weinberg, Hallmarks of cancer: The next generation. Cell, 144(5), 646–674.
Kolaczkowska, E., & Kubes, P. (2013). Neutrophil recruitment and function in health and inflammation. Nature Reviews Immunology, 13(3), 159–175.
Beyrau, M., J.V. Bodkin, and S. Nourshargh, Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biology, 2012. 2(11): p. 120134.
Sagiv, J. Y., et al. (2015). Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Reports, 10(4), 562–573.
Fridlender, Z. G., et al. (2009). Polarization of tumor-associated neutrophil phenotype by TGF-β: & #x201c;N1” versus & #x201c;N2”. TAN. Cancer Cell, 16(3), 183–194.
Fridlender, Z. G., & Albelda, S. M. (2012). Tumor-associated neutrophils: Friend or foe? Carcinogenesis, 33(5), 949–955.
Wculek, S. K., & Malanchi, I. (2015). Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature, 528(7582), 413–417.
ME Shaul et al 2020 Circulating neutrophil subsets in advanced lung cancer patients exhibit unique immune signature and relate to prognosis. 34 3 4204 4218
Schernberg, A., et al., Neutrophilia as prognostic biomarker in locally advanced stage III lung cancer. PLOS ONE, 2018. 13(10): p. e0204490.
Soto-Perez-de-Celis, E., et al. (2017). Tumor-associated neutrophils in breast cancer subtypes. Asian Pacific journal of cancer prevention: APJCP, 18(10), 2689–2693.
Governa, V., et al. (2017). The interplay between neutrophils and CD8<sup>+</sup> T cells improves survival in human colorectal cancer. Clinical Cancer Research, 23(14), 3847.
S Costa et al 2019 Recent advances on the crosstalk between neutrophils and B or T lymphocytes. 156 1 23 32
Rakic, A., Beaudry, P., & Mahoney, D. J. (2018). The complex interplay between neutrophils and cancer. Cell and Tissue Research, 371(3), 517–529.
Joyce, R. A., Hartmann, O., & Chervenick, P. A. (1979). Splenic granulopoiesis in mice following administration of cyclophosphamide. Cancer Research, 39(1), 215–218.
Rosales, C., Neutrophil: a cell with many roles in inflammation or several cell types? 2018. 9(113).
Pillay, J., et al., In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood, 2010. 116(4): p. 625–7.
Ocana, A., et al. (2017). Neutrophils in cancer: Prognostic role and therapeutic strategies. Molecular Cancer, 16(1), 137.
Coffelt, S. B., Wellenstein, M. D., & de Visser, K. E. (2016). Neutrophils in cancer: Neutral no more. Nature Reviews Cancer, 16(7), 431–446.
Olsson, A. K., & Cedervall, J. (2016). NETosis in cancer - platelet-neutrophil crosstalk promotes tumor-associated pathology. Frontiers in Immunology, 7, 373.
Stojkov, D., et al. (2017). ROS and glutathionylation balance cytoskeletal dynamics in neutrophil extracellular trap formation. Journal of Cell Biology, 216(12), 4073–4090.
Gullberg, U., et al., Biosynthesis, processing and sorting of neutrophil proteins: insight into neutrophil granule development. 1997. 58(3): p. 137–153.
Wu, M., et al. (2020). Neutrophil: A new player in metastatic cancers. Frontiers in immunology, 11, 565165–565165.
Borregaard, N., Sørensen, O. E., & Theilgaard-Mönch, K. (2007). Neutrophil granules: A library of innate immunity proteins. Trends in Immunology, 28(8), 340–345.
Sheshachalam, A., et al., Granule protein processing and regulated secretion in neutrophils. 2014. 5(448).
Reggiani, F., et al., Adipose progenitor cell secretion of GM-CSF and MMP9 promotes a stromal and immunological microenvironment that supports breast cancer progression. 2017. 77(18): p. 5169–5182.
Aalinkeel, R., et al. (2011). Overexpression of MMP-9 contributes to invasiveness of prostate cancer cell line LNCaP. Immunological Investigations, 40(5), 447–464.
Joseph, C., et al. (2020). Elevated MMP9 expression in breast cancer is a predictor of shorter patient survival. Breast cancer research and treatment, 182(2), 267–282.
P Scapini et al 2016 Human neutrophils in the saga of cellular heterogeneity: Insights and open questions. 273 1 48 60
Mishalian, I., Granot, Z., & Fridlender, Z. G. (2017). The diversity of circulating neutrophils in cancer. Immunobiology, 222(1), 82–88.
Masucci, M.T., M. Minopoli, and M.V. Carriero, Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. 2019. 9(1146).
Shojaei, F., et al. (2007). Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature, 450(7171), 825–831.
Kessenbrock, K., Plaks, V., & Werb, Z. (2010). Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell, 141(1), 52–67.
Scapini, P., & Cassatella, M. A. (2014). Social networking of human neutrophils within the immune system. Blood, 124(5), 710–719.
L Andzinski et al 2016 Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. 138 8 1982 1993
Zou, J.-M., et al., IL-35 induces N2 phenotype of neutrophils to promote tumor growth. 2017. 8(20).
Qin, F., et al. (2020). Anti-TGF-β attenuates tumor growth via polarization of tumor associated neutrophils towards an anti-tumor phenotype in colorectal cancer. Journal of Cancer, 11(9), 2580–2592.
Caruso, R. A., et al. (2002). Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in Northern Italy. Modern Pathology, 15(8), 831–837.
Sconocchia, G., et al., Tumor infiltration by FcγRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. 2011. 128(11): p. 2663–2672.
Millrud, C.R., et al., NET-producing CD16high CD62Ldim neutrophils migrate to tumor sites and predict improved survival in patients with HNSCC. 2017. 140(11): p. 2557–2567.
Singhal, S., et al. (2016). Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell, 30(1), 120–135.
Humbert, M., et al., Intratumoral CpG-B promotes antitumoral neutrophil, cDC, and T-cell cooperation without reprograming tolerogenic pDC. 2018. 78(12): p. 3280–3292.
Stoppacciaro, A., et al. (1993). Regression of an established tumor genetically modified to release granulocyte colony-stimulating factor requires granulocyte-T cell cooperation and T cell-produced interferon gamma. Journal of Experimental Medicine, 178(1), 151–161.
Yang Chang, C., et al., Virus-stimulated neutrophils in the tumor microenvironment enhance T cell-mediated anti-tumor immunity. 2016. 7(27).
T Takeshima et al 2016 Key role for neutrophils in radiation-induced antitumor immune responses: Potentiation with G-CSF. 113 40 11300 11305
Eruslanov, E. B., et al. (2014). Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. The Journal of Clinical Investigation, 124(12), 5466–5480.
G Rosa de la et al 2008 Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. 180 10 6868 6876
Territo, M. C., et al. (1989). Monocyte-chemotactic activity of defensins from human neutrophils. The Journal of Clinical Investigation, 84(6), 2017–2020.
Yang, D., et al. (2000). Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. Journal of Leukocyte Biology, 68(1), 9–14.
Ethuin, F., et al. (2004). Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Laboratory Investigation, 84(10), 1363–1371.
Makarenkova, V. P., et al. (2006). CD11b<sup>+</sup>/Gr-1<sup>+</sup> Myeloid suppressor cells cause T cell dysfunction after traumatic stress. The Journal of Immunology, 176(4), 2085.
Bank, U., et al. (1999). Selective proteolytic cleavage of IL-2 receptor and IL-6 receptor ligand binding chains by neutrophil-derived serine proteases at foci of inflammation. Journal of Interferon & Cytokine Research, 19(11), 1277–1287.
K Tillack et al 2012 T Lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. 188 7 3150 3159
Ackermann, M. F., et al. (1989). Antitumor activity of murine neutrophils demonstrated by cytometric analysis. Cancer Research, 49(3), 528–532.
Blaisdell, A., et al. (2015). Neutrophils oppose uterine epithelial carcinogenesis via debridement of hypoxic tumor cells. Cancer Cell, 28(6), 785–799.
Fields, G. B. (2019). Mechanisms of action of novel drugs targeting angiogenesis-promoting matrix metalloproteinases. Frontiers in Immunology, 10, 1278.
Finisguerra, V., et al. (2015). MET is required for the recruitment of anti-tumoural neutrophils. Nature, 522(7556), 349–353.
Matlung, H. L., et al. (2018). Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Reports, 23(13), 3946-3959.e6.
Güngör, N., et al. (2010). Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis, 25(2), 149–154.
Giese, M. A., Hind, L. E., & Huttenlocher, A. (2019). Neutrophil plasticity in the tumor microenvironment. Blood, 133(20), 2159–2167.
Deryugina, E. I., & Quigley, J. P. (2006). Matrix metalloproteinases and tumor metastasis. Cancer and Metastasis Reviews, 25(1), 9–34.
Bergers, G., et al. (2000). Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature Cell Biology, 2(10), 737–744.
Das, A., et al. (2017). MMP proteolytic activity regulates cancer invasiveness by modulating integrins. Scientific Reports, 7(1), 14219.
Houghton, A. M., et al. (2010). Neutrophil elastase–mediated degradation of IRS-1 accelerates lung tumor growth. Nature Medicine, 16(2), 219–223.
Hwang, W.-L., et al. (2019). Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. Journal of Hematology & Oncology, 12(1), 10.
Devalaraja, S., et al. (2020). Tumor-derived retinoic acid regulates intratumoral monocyte differentiation to promote immune suppression. Cell, 180(6), 1098-1114.e16.
Liu, B., Yan, L., & Zhou, M. (2019). Target selection of CAR T cell therapy in accordance with the TME for solid tumors. American journal of cancer research, 9(2), 228–241.
Ning, Y., et al., HDAC9 deficiency promotes tumor progression by decreasing the CD8(+) dendritic cell infiltration of the tumor microenvironment. J Immunother Cancer, 2020. 8(1).
Barry, K. C., et al. (2018). A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nature Medicine, 24(8), 1178–1191.
Zhou, S.-L., et al. (2016). Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to Sorafenib. Gastroenterology, 150(7), 1646-1658.e17.
MB Chen et al 2018 Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. 115 27 7022 7027
Kerfoot, S. M., et al. (2001). Exclusive neutrophil recruitment with oncostatin M in a human system. The American Journal of Pathology, 159(4), 1531–1539.
MM Queen et al 2005 Breast cancer cells stimulate neutrophils to produce oncostatin m: Potential implications for tumor progression. 65 19 8896 8904
Yang, Q., Yan, C., & Gong, Z. (2018). Interaction of hepatic stellate cells with neutrophils and macrophages in the liver following oncogenic kras activation in transgenic zebrafish. Scientific Reports, 8(1), 8495.
Zhou, Z., et al., Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. Journal for immunotherapy of cancer, 2021. 9(3): p. e001946.
Elinav, E., et al. (2013). Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nature Reviews Cancer, 13(11), 759–771.
Shang, K., et al., Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PLoS One, 2012. 7(12): p. e51848.
Jamieson, T., et al. (2012). Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. The Journal of Clinical Investigation, 122(9), 3127–3144.
N Antonio et al 2015 The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. 34 17 2219 2236
Haqqani, A. S., Sandhu, J. K., & Birnboim, H. C. (2000). Expression of interleukin-8 promotes neutrophil infiltration and genetic instability in mutatect tumors. Neoplasia, 2(6), 561–568.
Sandhu, J. K., et al. (2000). Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. The American Journal of Pathology, 156(2), 509–518.
Campregher, C., Luciani, M. G., & Gasche, C. (2008). Activated neutrophils induce an hMSH2-dependent G2/M checkpoint arrest and replication errors at a (CA)13-repeat in colon epithelial cells. Gut, 57(6), 780–787.
Wilson, C. L., et al. (2015). NFκB1 is a suppressor of neutrophil-driven hepatocellular carcinoma. Nature Communications, 6, 6818.
Yan, C., et al. (2015). Stimulation of hepatocarcinogenesis by neutrophils upon induction of oncogenic kras expression in transgenic zebrafish. Journal of Hepatology, 63(2), 420–428.
Butin-Israeli, V., et al. (2019). Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. The Journal of Clinical Investigation, 129(2), 712–726.
Chang, S. H., et al. (2014). T helper 17 cells play a critical pathogenic role in lung cancer. Proceedings of the National Academic Sciences U S A, 111(15), 5664–5669.
Gong, L., et al. (2013). Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Molecular Cancer, 12(1), 154.
Takahashi, H., et al. (2010). Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell, 17(1), 89–97.
Deryugina, E. I., et al. (2014). Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia, 16(10), 771–788.
Jablonska, J., et al. (2010). Neutrophils responsive to endogenous IFN-β regulate tumor angiogenesis and growth in a mouse tumor model. The Journal of Clinical Investigation, 120(4), 1151–1164.
Coffelt, S. B., et al. (2015). IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature, 522(7556), 345–348.
McGary, C. T., Miele, M. E., & Welch, D. R. (1995). Highly metastatic 13762NF rat mammary adenocarcinoma cell clones stimulate bone marrow by secretion of granulocyte-macrophage colony-stimulating factor/interleukin-3 activity. The American journal of pathology, 147(6), 1668–1681.
MG Lechner DJ Liebertz AL Epstein 2010 Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. 185 4 2273 2284
Gabrilovich, D. I., & Nagaraj, S. (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology, 9(3), 162–174.
J-I Youn et al 2008 Subsets of myeloid-derived suppressor cells in tumor-bearing mice. 181 8 5791 5802
Powell, D., et al. (2018). Cxcr1 mediates recruitment of neutrophils and supports proliferation of tumor-initiating astrocytes in vivo. Science and Reports, 8(1), 13285.
Acharyya, S., et al. (2012). A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell, 150(1), 165–178.
Rodriguez, P. C., Quiceno, D. G., & Ochoa, A. C. (2006). l-arginine availability regulates T-lymphocyte cell-cycle progression. Blood, 109(4), 1568–1573.
Rodriguez, P. C., et al. (2002). Regulation of T cell receptor CD3ζ chain expression by<span class="small">l</span>-Arginine *. Journal of Biological Chemistry, 277(24), 21123–21129.
Feldmeyer, N., et al. (2012). Arginine deficiency leads to impaired cofilin dephosphorylation in activated human T lymphocytes. International Immunology, 24(5), 303–313.
Malmberg, K.-J., et al., Inhibition of activated/memory (CD45RO<sup>+</sup>) T cells by oxidative stress associated with block of NF-κB activation. 2001. 167(5): p. 2595–2601.
KA Gelderman et al 2006 T cell surface redox levels determine T cell reactivity and arthritis susceptibility. 103 34 12831 12836
Klemke, M., et al. (2008). Oxidation of cofilin mediates T cell hyporesponsiveness under oxidative stress conditions. Immunity, 29(3), 404–413.
Mantovani, A. (2009). The Yin-Yang of tumor-associated neutrophils. Cancer Cell, 16(3), 173–174.
Scapini, P., Bazzoni, F., & Cassatella, M. A. (2008). Regulation of B-cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunology Letters, 116(1), 1–6.
Hahne, M., et al. (1998). APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. The Journal of experimental medicine, 188(6), 1185–1190.
Jabłońska, E., et al. (2012). A proliferation-inducing ligand (APRIL) in neutrophils of patients with oral cavity squamous cell carcinoma. European Cytokine Network, 23(3), 93–100.
Gruss, H. J., et al. (1994). Pleiotropic effects of the CD30 ligand on CD30-expressing cells and lymphoma cell lines. Blood, 83(8), 2045–2056.
Di Mitri, D., et al. (2014). Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature, 515(7525), 134–137.
Wang, G., et al. (2016). Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discovery, 6(1), 80–95.
Ma, X., et al. (2015). Definition of prostaglandin E2-EP2 signals in the colon tumor microenvironment that amplify inflammation and tumor growth. Cancer Research, 75(14), 2822–2832.
Liang, J., et al. (2014). Neutrophils promote the malignant glioma phenotype through S100A4. Clinical Cancer Research, 20(1), 187–198.
Song, W., et al. (2015). Infiltrating neutrophils promote renal cell carcinoma (RCC) proliferation via modulating androgen receptor (AR) → c-Myc signals. Cancer Letters, 368(1), 71–78.
Grégoire, M., et al. (2015). Neutrophils trigger a NF-κB dependent polarization of tumor-supportive stromal cells in germinal center B-cell lymphomas. Oncotarget, 6(18), 16471–16487.
Ramachandran, I. R., et al. (2016). Bone marrow PMN-MDSCs and neutrophils are functionally similar in protection of multiple myeloma from chemotherapy. Cancer Letters, 371(1), 117–124.
Hsu, B. E., et al. (2019). Immature low-density neutrophils exhibit metabolic flexibility that facilitates breast cancer liver metastasis. Cell Reports, 27(13), 3902-3915.e6.
Guglietta, S., et al. (2016). Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nature Communications, 7(1), 11037.
Hsu, B. E., et al. (2020). C3a elicits unique migratory responses in immature low-density neutrophils. Oncogene, 39(12), 2612–2623.
Gomes, T., et al., IL-1β blockade attenuates thrombosis in a neutrophil extracellular trap-dependent breast cancer model. 2019. 10(2088).
HO Yazdani et al 2019 Neutrophil extracellular traps drive mitochondrial homeostasis in tumors to augment growth. 79 21 5626 5639
Zhu, B., et al., NF-κB and neutrophil extracellular traps cooperate to promote breast cancer progression and metastasis. Experimental Cell Research, 2021. 405(2): p. 112707.
Zhang, X., et al. (2016). Neutrophils in cancer development and progression: Roles, mechanisms, and implications (Review). International Journal of Oncology, 49(3), 857–867.
Zhang, J., et al. (2016). Circulating tumor-associated neutrophils (cTAN) contribute to circulating tumor cell survival by suppressing peripheral leukocyte activation. Tumor Biology, 37(4), 5397–5404.
Koh, Y. W., et al. (2016). Baseline neutrophil-lymphocyte ratio is associated with baseline and subsequent presence of brain metastases in advanced non-small-cell lung cancer. Science and Reports, 6, 38585.
Wellenstein, M. D., et al. (2019). Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature, 572(7770), 538–542.
Singh, S., et al. (2020). Loss of ELF5-FBXW7 stabilizes IFNGR1 to promote the growth and metastasis of triple-negative breast cancer through interferon-γ signalling. Nature Cell Biology, 22(5), 591–602.
Markman, J. L., et al. (2020). Loss of testosterone impairs anti-tumor neutrophil function. Nature Communications, 11(1), 1613.
Quail, D. F., et al. (2017). Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nature Cell Biology, 19(8), 974–987.
Charan, M., et al. (2020). Tumor secreted ANGPTL2 facilitates recruitment of neutrophils to the lung to promote lung pre-metastatic niche formation and targeting ANGPTL2 signaling affects metastatic disease. Oncotarget, 11(5), 510–522.
Jackstadt, R., et al. (2019). Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell, 36(3), 319-336.e7.
Janiszewska, M., et al. (2019). Subclonal cooperation drives metastasis by modulating local and systemic immune microenvironments. Nature Cell Biology, 21(7), 879–888.
Liang, W., Li, Q., & Ferrara, N. (2018). Metastatic growth instructed by neutrophil-derived transferrin. Proceedings of the National Academic Sciences U S A, 115(43), 11060–11065.
Freisinger, C.M. and A. Huttenlocher, Live imaging and gene expression analysis in zebrafish identifies a link between neutrophils and epithelial to mesenchymal transition. PLoS One, 2014. 9(11): p. e112183.
Hu, P., et al. (2015). Intratumoral neutrophil granulocytes contribute to epithelial-mesenchymal transition in lung adenocarcinoma cells. Tumour Biology, 36(10), 7789–7796.
Wang, Y., et al. (2019). Tumor-contacted neutrophils promote metastasis by a CD90-TIMP-1 Juxtacrine-paracrine loop. Clinical Cancer Research, 25(6), 1957–1969.
Lin, C., et al. (2015). Infiltrating neutrophils increase bladder cancer cell invasion via modulation of androgen receptor (AR)/MMP13 signals. Oncotarget, 6(40), 43081–43089.
Park, J., et al., Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Science Translational Medicine, 2016. 8(361): p. 361ra138.
Abdol Razak, N., O. Elaskalani, and P. Metharom, Pancreatic cancer-induced neutrophil extracellular traps: a potential contributor to cancer-associated thrombosis. International Journal of Molecular Science, 2017. 18(3).
Lee, W., et al. (2019). Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. Journal of Experimental Medicine, 216(1), 176–194.
Takesue, S., et al. (2020). Neutrophil extracellular traps promote liver micrometastasis in pancreatic ductal adenocarcinoma via the activation of cancer-associated fibroblasts. International Journal of Oncology, 56(2), 596–605.
Tecchio, C., et al. (2013). On the cytokines produced by human neutrophils in tumors. Seminars in Cancer Biology, 23(3), 159–170.
Queen, M. M., et al. (2005). Breast cancer cells stimulate neutrophils to produce oncostatin M: Potential implications for tumor progression. Cancer Research, 65(19), 8896–8904.
Junk, D. J., et al. (2017). Oncostatin M promotes cancer cell plasticity through cooperative STAT3-SMAD3 signaling. Oncogene, 36(28), 4001–4013.
Wislez, M., et al. (2003). Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: Role in tumor progression and death. Cancer Research, 63(6), 1405–1412.
Imai, Y., et al. (2005). Neutrophils enhance invasion activity of human cholangiocellular carcinoma and hepatocellular carcinoma cells: An in vitro study. Journal of Gastroenterology and Hepatology, 20(2), 287–293.
Ikushima, H., & Miyazono, K. (2010). Cellular context-dependent “colors” of transforming growth factor-beta signaling. Cancer Science, 101(2), 306–312.
Yang, L., Pang, Y., & Moses, H. L. (2010). TGF-beta and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends in Immunology, 31(6), 220–227.
Aoyagi, Y., et al. (2004). Overexpression of TGF-beta by infiltrated granulocytes correlates with the expression of collagen mRNA in pancreatic cancer. British Journal of Cancer, 91(7), 1316–1326.
SenGupta, S., et al., Triple-negative breast cancer cells recruit neutrophils by secreting TGF-β and CXCR2 ligands. 2021. 12(973).
Waugh, D. J., & Wilson, C. (2008). The interleukin-8 pathway in cancer. Clinical Cancer Research, 14(21), 6735–6741.
Palena, C., Hamilton, D. H., & Fernando, R. I. (2012). Influence of IL-8 on the epithelial-mesenchymal transition and the tumor microenvironment. Future Oncology, 8(6), 713–722.
Konrad, F. M., et al. (2019). How adhesion molecule patterns change while neutrophils traffic through the lung during inflammation. Mediators of Inflammation, 2019, 1208086.
Gil, C. D., et al. (2006). Interaction of human neutrophils with endothelial cells regulates the expression of endogenous proteins annexin 1, galectin-1 and galectin-3. Cell Biology International, 30(4), 338–344.
Strell, C., et al. (2010). Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Experimental Cell Research, 316(1), 138–148.
Spicer, J. D., et al. (2012). Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Research, 72(16), 3919–3927.
Szczerba, B. M., et al. (2019). Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature, 566(7745), 553–557.
Spiegel, A., et al. (2016). Neutrophils suppress intraluminal nk cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discovery, 6(6), 630–649.
Nourshargh, S., et al. (1995). Interleukin-1-induced leukocyte extravasation across rat mesenteric microvessels is mediated by platelet-activating factor. Blood, 85(9), 2553–2558.
Rayes, R. F., et al. (2020). Neutrophil Extracellular trap-associated CEACAM1 as a putative therapeutic target to prevent metastatic progression of colon carcinoma. The Journal of Immunology, 204(8), 2285–2294.
S Xiong et al 2021 Neutrophils in cancer carcinogenesis and metastasis. 14 1 1 17
Rayes, R.F., et al., Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight, 2019. 5(16).
Yang, L. Y., et al. (2020). Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. Journal of Hematology & Oncology, 13(1), 3.
Rocks, N., et al. (2019). Ozone-primed neutrophils promote early steps of tumour cell metastasis to lungs by enhancing their NET production. Thorax, 74(8), 768–779.
Inoue, M., et al. (2018). Plasma redox imbalance caused by albumin oxidation promotes lung-predominant NETosis and pulmonary cancer metastasis. Nature Communications, 9(1), 5116.
Nozawa, H., Chiu, C., & Hanahan, D. (2006). Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proceedings of National Academic Sciences U S A, 103(33), 12493–12498.
Bekes, E. M., et al. (2011). Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. American Journal of Pathology, 179(3), 1455–1470.
Keane, M. P., et al. (2004). Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. The Journal of Immunology, 172(5), 2853–2860.
Yang, L., et al. (2004). Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell, 6(4), 409–421.
Coussens, L. M., et al. (2000). MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell, 103(3), 481–490.
Shojaei, F., et al. (2008). Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proceedings of National Academic Sciences U S A, 105(7), 2640–2645.
Ferrara, N., Gerber, H. P., & LeCouter, J. (2003). The biology of VEGF and its receptors. Nature Medicine, 9(6), 669–676.
Jablonska, J., et al. (2010). Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. The Journal of Clinical Investigation, 120(4), 1151–1164.
Kusumanto, Y. H., et al. (2003). Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis, 6(4), 283–287.
Jablonska, E., et al. (2002). VEGF in the culture of PMN and the serum in oral cavity cancer patients. Oral Oncology, 38(6), 605–609.
Zhong, C., et al. (2009). Characterization and regulation of bv8 in human blood cells. Clinical Cancer Research, 15(8), 2675–2684.
Kuang, D.-M., et al. (2011). Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. Journal of Hepatology, 54(5), 948–955.
Ardi, V.C., et al., Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. 2007. 104(51): p. 20262–20267.
Egeblad, M., & Werb, Z. (2002). New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer, 2(3), 161–174.
Gordon-Weeks, A. N., et al. (2017). Neutrophils promote hepatic metastasis growth through fibroblast growth factor 2-dependent angiogenesis in mice. Hepatology, 65(6), 1920–1935.
Belikov, A. V., Schraven, B., & Simeoni, L. (2015). T cells and reactive oxygen species. Journal of Biomedical Science, 22(1), 85.
Galli, S. J., Borregaard, N., & Wynn, T. A. (2011). Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nature Immunology, 12(11), 1035–1044.
Rotondo, R., et al. (2009). IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. International Journal of Cancer, 125(4), 887–893.
Yachimovich-Cohen, N., et al. (2010). Human embryonic stem cells suppress T cell responses via arginase I-dependent mechanism. The Journal of Immunology, 184(3), 1300–1308.
Waldron, T.J., et al., Myeloid derived suppressor cells: targets for therapy. Oncoimmunology, 2013. 2(4): p. e24117.
Yang, T. H., et al. (2018). Membrane-associated proteinase 3 on granulocytes and acute myeloid leukemia inhibits T cell proliferation. The Journal of Immunology, 201(5), 1389–1399.
Schmielau, J., & Finn, O. J. (2001). Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Research, 61(12), 4756–4760.
Sippel, T. R., et al. (2011). Neutrophil degranulation and immunosuppression in patients with GBM: Restoration of cellular immune function by targeting arginase I. Clinical Cancer Research, 17(22), 6992–7002.
Wang, T.-t., et al., Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. 2017. 66(11): p. 1900–1911.
He, G., et al. (2015). Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. Journal of Experimental & Clinical Cancer Research, 34(1), 141.
el-Hag, A. and R.A. Clark, Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. The Journal of Immunology, 1987. 139(7): p. 2406–13.
Yang, J., et al. (2018). Loss of CXCR4 in myeloid cells enhances antitumor immunity and reduces melanoma growth through NK cell and FASL mechanisms. Cancer Immunology Research, 6(10), 1186–1198.
Michaeli, J., et al., Tumor-associated neutrophils induce apoptosis of non-activated CD8 T-cells in a TNFα and NO-dependent mechanism, promoting a tumor-supportive environment. Oncoimmunology, 2017. 6(11): p. e1356965.
Zhou, M., et al., Tumor-released autophagosomes induce IL-10-producing B cells with suppressive activity on T lymphocytes via TLR2-MyD88-NF-κB signal pathway. Oncoimmunology, 2016. 5(7): p. e1180485.
Gao, R., et al., Tumor cell-released autophagosomes (TRAP) enhance apoptosis and immunosuppressive functions of neutrophils. Oncoimmunology, 2018. 7(6): p. e1438108.
Cedrés, S., et al. (2012). Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clinical and Translational Oncology, 14(11), 864–869.
Yang, S. Z., et al. (2015). Elevated levels of preoperative circulating CD44+ lymphocytes and neutrophils predict poor survival for non-small cell lung cancer patients. Clinica Chimica Acta, 439, 172–177.
Zhang, H., et al., Prognostic significance of combination of preoperative platelet count and neutrophil-lymphocyte ratio (COP-NLR) in patients with non-small cell lung cancer: based on a large cohort study. PLoS One, 2015. 10(5): p. e0126496.
Orditura, M., et al., Neutrophil to lymphocyte ratio (NLR) for prediction of distant metastasis-free survival (DMFS) in early breast cancer: a propensity score-matched analysis. ESMO Open, 2016. 1(2): p. e000038.
HK Jensen et al 2009 Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. 27 28 4709 4717
Trellakis, S., et al. (2011). Polymorphonuclear granulocytes in human head and neck cancer: Enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. International Journal of Cancer, 129(9), 2183–2193.
Asaoka, T., et al. (2016). Prognostic impact of preoperative NLR and CA19-9 in pancreatic cancer. Pancreatology, 16(3), 434–440.
Rao, H.L., et al., Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients' adverse prognosis. PLoS One, 2012. 7(1): p. e30806.
Romano, A., et al. (2018). Prognostic meaning of neutrophil to lymphocyte ratio (NLR) and lymphocyte to monocyte ration (LMR) in newly diagnosed Hodgkin lymphoma patients treated upfront with a PET-2 based strategy. Annals of Hematology, 97(6), 1009–1018.
Ferrucci, P. F., et al. (2015). Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. British Journal of Cancer, 112(12), 1904–1910.
Galdiero, M. R., et al. (2016). Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. International Journal of Cancer, 139(2), 446–456.
Li, Y., et al. (2017). Preoperative NLR for predicting survival rate after radical resection combined with adjuvant immunotherapy with CIK and postoperative chemotherapy in gastric cancer. Journal of Cancer Research and Clinical Oncology, 143(5), 861–871.
Nakaya, A., et al. (2018). Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. International Journal of Clinical Oncology, 23(4), 634–640.
Zhou, S. L., et al. (2012). Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology, 56(6), 2242–2254.
Jensen, T. O., et al. (2012). Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer, 118(9), 2476–2485.
AD Gregory 2011 and A McGarry Houghton, Tumor-associated neutrophils: New targets for cancer therapy. 71 7 2411 2416
Ruffini, P.A., The CXCL8-CXCR1/2 axis as a therapeutic target in breast cancer stem-like cells. 2019. 9(40).
Lemos, H. P., et al. (2009). Prostaglandin mediates IL-23/IL-17-induced neutrophil migration in inflammation by inhibiting IL-12 and IFNgamma production. Proceedings of National Academic Sciences U S A, 106(14), 5954–5959.
Highfill, S.L., et al., Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Science Translational Medicine, 2014. 6(237): p. 237ra67.
Gebhardt, C., et al. (2015). Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clinical Cancer Research, 21(24), 5453–5459.
Nam, J. S., et al. (2008). An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Research, 68(10), 3835–3843.
Bhola, N. E., et al. (2013). TGF-β inhibition enhances chemotherapy action against triple-negative breast cancer. The Journal of Clinical Investigation, 123(3), 1348–1358.
Pang, Y., et al. (2013). TGF-β signaling in myeloid cells is required for tumor metastasis. Cancer Discovery, 3(8), 936–951.
Sun, R., et al. (2014). Neutrophils with protumor potential could efficiently suppress tumor growth after cytokine priming and in presence of normal NK cells. Oncotarget, 5(24), 12621–12634.
Andzinski, L., et al. (2016). Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. International Journal of Cancer, 138(8), 1982–1993.
Shrestha, S., et al., Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonist attenuate tumor growth via polarization of neutrophils toward an antitumor phenotype. Oncoimmunology, 2016. 5(1): p. e1067744.
Teijeira, Á., et al. (2020). CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity, 52(5), 856-871.e8.
Sandhu, J.K., et al., Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. 2000. 156(2): p. 509–518.
V Butin-Israeli et al 2019 Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. 129 2 712 726
Guglietta, S., et al., Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. 2016. 7(1): p. 1–14.
Awaji, M., et al., CXCR2 signaling promotes secretory cancer‐associated fibroblasts in pancreatic ductal adenocarcinoma. 2020. 34(7): p. 9405–9418.
Zhang, X., et al., Inflammation‐induced S100A8 activates I d3 and promotes colorectal tumorigenesis. 2015. 137(12): p. 2803–2814.
C Yan et al 2015 Stimulation of hepatocarcinogenesis by neutrophils upon induction of oncogenic kras expression in transgenic zebrafish. 63 2 420 428
Houghton, A.M., et al., Neutrophil elastase–mediated degradation of IRS-1 accelerates lung tumor growth. 2010. 16(2): p. 219–223.
Liang, J., et al., Neutrophils promote the malignant glioma phenotype through S100A4. 2014. 20(1): p. 187–198.
Cheng, S.P., et al., Overexpression of chitinase‐3‐like protein 1 is associated with structural recurrence in patients with differentiated thyroid cancer. 2020. 252(2): p. 114–124.
Yang, R., et al., Neutrophil elastase enhances the proliferation and decreases apoptosis of leukemia cells via activation of PI3K/Akt signaling. 2016. 13(5): p. 4175–4182.
Powell, D., et al., Cxcr1 mediates recruitment of neutrophils and supports proliferation of tumor-initiating astrocytes in vivo. 2018. 8(1): p. 1–12.
J Cools-Lartigue et al 2013 Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. 123 8 3446 3458
Yang, L., et al., DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. 2020. 583(7814): p. 133–138.
L-Y Yang et al 2020 Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. 13 1 1 15
Kwon, C.H., et al., S100A8 and S100A9 promotes invasion and migration through p38 mitogen-activated protein kinase-dependent NF-κB activation in gastric cancer cells. 2013. 35(3): p. 226–234.
Liu, Y., et al., Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. 2016. 30(2): p. 243–256.
Wang, Z., et al., Tumor-derived HMGB1 induces CD62Ldim neutrophil polarization and promotes lung metastasis in triple-negative breast cancer. 2020. 9(9): p. 1–17.
Kolonin, M.G., et al., Interaction between tumor cell surface receptor RAGE and proteinase 3 mediates prostate cancer metastasis to bone. 2017. 77(12): p. 3144–3150.
Deryugina, E., et al., Neutrophil elastase facilitates tumor cell intravasation and early metastatic events. 2020. 23(12): p. 101799.
Wilson, T.J., et al., Cathepsin G-mediated enhanced TGF-β signaling promotes angiogenesis via upregulation of VEGF and MCP-1. 2010. 288(2): p. 162–169.
E Pieterse et al 2017 Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. 37 7 1371 1379
Deryugina, E.I., et al., Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. 2014. 16(10): p. 771–788.
N Feldmeyer et al 2012 Arginine deficiency leads to impaired cofilindephosphorylation in activated human T lymphocytes. 24 5 303 313
Malmberg, K.-J., et al., Inhibition of activated/memory (CD45RO+) T cells by oxidative stress associated with block of NF-κB activation. 2001. 167(5): p. 2595–2601.
Yang, T.-H., et al., Membrane-associated proteinase 3 on granulocytes and acute myeloid leukemia inhibits T cell proliferation. 2018. 201(5): p. 1389–1399.
Spiegel, A., et al., Neutrophils suppress intraluminal NK cell–mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. 2016. 6(6): p. 630–649.
He, G., et al., Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. 2015. 34(1): p. 1–11.
Michaeli, J., et al., Tumor-associated neutrophils induce apoptosis of non-activated CD8 T-cells in a TNFα and NO-dependent mechanism, promoting a tumor-supportive environment. 2017. 6(11): p. e1356965.
Shang, A., et al., Exosomal KRAS mutation promotes the formation of tumor-associated neutrophil extracellular traps and causes deterioration of colorectal cancer by inducing IL-8 expression. 2020. 18(1): p. 1–14.
J Cedervall et al 2015 Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. 75 13 2653 2662
Bang, O.Y., et al., Circulating DNAs, a marker of neutrophil extracellular traposis and cancer-related stroke: the OASIS-Cancer Study. 2019. 50(10): p. 2944–2947.
Wolach, O., et al., Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. 2018. 10(436): p. eaan8292.
Perego, M., et al., Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. 2020. 12(572): p. eabb5817.
Albrengues, J., et al., Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. 2018. 361(6409): p. eaao4227.
S-Y Park J-SJE Nam M Medicine 2020 The force awakens: Metastatic dormant cancer cells. 52 4 569 581
Zhou, S.-L., et al., Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. 2016. 150(7): p. 1646–1658. e17.
J Incio et al 2016 Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. 6 8 852 869
Zhang, Y., et al., Interleukin-17–induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. 2020. 217(12).
Bui, T.M., L.K. Yalom, and R.J.E.o.o.t.t. Sumagin, Tumor-associated neutrophils: orchestrating cancer pathobiology and therapeutic resistance. 2021. 25(7): p. 573–583.
Wang, Y., et al. (2014). Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis. Mucosal immunology, 7(5), 1106–1115.
Bellocq, A., et al. (1998). Neutrophil alveolitis in bronchioloalveolar carcinoma: Induction by tumor-derived interleukin-8 and relation to clinical outcome. The American journal of pathology, 152(1), 83–92.
Ibrahim, M.L., et al., Myeloid-derived suppressor cells produce IL-10 to elicit DNMT3b-dependent IRF8 silencing to promote colitis-associated colon tumorigenesis. Cell reports, 2018. 25(11): p. 3036–3046. e6.
Li, T.-J., et al. (2017). Interleukin-17–producing neutrophils link inflammatory stimuli to disease progression by promoting angiogenesis in gastric cancer. Clinical Cancer Research, 23(6), 1575–1585.
Jablonska, E., et al. (2005). VEGF, IL-18 and NO production by neutrophils and their serum levels in patients with oral cavity cancer. Cytokine, 30(3), 93–99.
Wada, Y., et al. (2007). Neutrophil elastase induces cell proliferation and migration by the release of TGF-α, PDGF and VEGF in esophageal cell lines. Oncology reports, 17(1), 161–167.
Zhang, X., & Xu, W. (2017). Neutrophils diminish T-cell immunity to foster gastric cancer progression: The role of GM-CSF/PD-L1/PD-1 signalling pathway. Gut, 66(11), 1878–1880.
Fridlender, Z. G., et al. (2009). Polarization of tumor-associated neutrophil phenotype by TGF-β:“N1” versus “N2” TAN. Cancer Cell, 16(3), 183–194.
Aoyagi, Y., et al. (2004). Overexpression of TGF-β by infiltrated granulocytes correlates with the expression of collagen mRNA in pancreatic cancer. British journal of cancer, 91(7), 1316–1326.
Dumitru, C. A., et al. (2012). A novel p38-MAPK signaling axis modulates neutrophil biology in head and neck cancer. Journal of leukocyte biology, 91(4), 591–598.
Tsuda, Y., et al., An immunosuppressive subtype of neutrophils identified in patients with hepatocellular carcinoma. Journal of clinical biochemistry and nutrition, 2012: p. 12–32.
Eruslanov, E., et al. (2012). Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. International journal of cancer, 130(5), 1109–1119.
Yan, H. H., et al. (2015). CCL9 induced by TGFβ signaling in myeloid cells enhances tumor cell survival in the premetastatic organ. Cancer research, 75(24), 5283–5298.
Mishalian, I., et al. (2014). Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17—A new mechanism of impaired antitumor immunity. International journal of cancer, 135(5), 1178–1186.
Sasaki, S., et al. (2018). Involvement of prokineticin 2–expressing neutrophil infiltration in 5-fluorouracil–induced aggravation of breast cancer metastasis to lung. Molecular cancer therapeutics, 17(7), 1515–1525.
Ibrahim, S. A., et al. (2015). Breast cancer associated a2 isoform vacuolar ATPase immunomodulates neutrophils: Potential role in tumor progression. Oncotarget, 6(32), 33033.
Dumitru, C. A., et al. (2011). Tumor-derived macrophage migration inhibitory factor modulates the biology of head and neck cancer cells via neutrophil activation. International Journal of Cancer, 129(4), 859–869.
Khan, S., et al. (2020). Role of neutrophils and myeloid-derived suppressor cells in glioma progression and treatment resistance. International journal of molecular sciences, 21(6), 1954.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahmud, Z., Rahman, A., Mishu, I.D. et al. Mechanistic insights into the interplays between neutrophils and other immune cells in cancer development and progression. Cancer Metastasis Rev 41, 405–432 (2022). https://doi.org/10.1007/s10555-022-10024-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-022-10024-8