Abstract
The skeleton is frequently a secondary growth site of disseminated cancers, often leading to painful and devastating clinical outcomes. Metastatic cancer distorts bone marrow homeostasis through tumor-derived factors, which shapes different bone tumor microenvironments depending on the tumor cells’ origin. Here, we propose a novel insight on tumor-secreted Galectin-3 (Gal-3) that controls the induction of an inflammatory cascade, differentiation of osteoblasts, osteoclasts, and bone marrow cells, resulting in bone destruction and therapeutic failure. In the approaching era of personalized medicine, the current treatment modalities targeting bone metastatic environments are provided to the patient with limited consideration of the cancer cells’ origin. Our new outlook suggests delivering individual tumor microenvironment treatments based on the expression level/activity/functionality of tumor-derived factors, rather than utilizing a commonly shared therapeutic umbrella. The notion of “Gal-3-associated bone remodeling” could be the first step toward a specific personalized therapy for each cancer type generating a different bone niche in patients afflicted with non-curable bone metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 GAL-3: a multifunctional tumor-associated protein
In the early 1980s, it was documented that simple sugars containing galactose residues inhibit the formation of tumor emboli, leading to the notion that tumor cells express galactose-binding proteins, i.e., lectin(s) [1]. However, in that era, lectins were thought to belong only to the plant kingdom; the idea that mammalian cells in general and cancer cells in particular express lectin(s) was almost sacrilegious. The field was legitimized in 1994 when the article “Galectins: a family of animal beta-galactoside-binding lectins” was published [2]. Animal galectins are a family of 15 members that bind β-galactosides through an evolutionarily conserved carbohydrate recognition domain (CRD). The identification of Galectin-3 (Gal-3) was firstly reported and also known as IgE-binding protein, MAC2, L-29, CPB-35, etc., since the names had not been organized at that time. Since then, 14 other Galectins were discovered and the family has been classified into three groups according to their structure: prototypical, tandem repeat, and chimeric. In human cells, Gal-1, Gal-2, Gal-3, Gal-4, Gal-7, Gal-8, Gal-9, Gal-10, Gal-12, and Gal-13 are prevalent (Fig. 1 inset). Along with the advancements of molecular investigation, pleiotropic-pluripotent functions of Gal-3 have been reported and are now accepted to be involved in diverse physiological and pathological processes, e.g., differentiation, fibrosis, transcriptional regulation, mRNA processing, etc. Presently, numerous studies support that Gal-3 works as a key player in different stages of cancer progression, and therefore, it has been considered a promising therapeutic target [3, 4]. In general, malignant tumors share several typical phenotypes, e.g., sustaining proliferative signaling, inducing angiogenesis, activating invasion/metastasis, and resisting cell death, etc. Gal-3 is the only protein outside the Bcl-2 family, cell death/apoptosis regulators, that contains the amino acid sequence of Asp-Trp-Gly-Arg (NWGR), an anti-death motif that inhibits drug-induced cell apoptosis [5]. Further, efforts have been focused on the intracellular functions of Gal-3 to prove cancer malignancy by molecular techniques. In summary, the common functional characteristic of Gal-3 is that it may prefer to approach/interact with other molecules rather than taking independent single action and thereafter tempt binding targets to transform into a malignant feature. For example, Gal-3 interacts with other apoptosis-associated proteins such as Nucling, Synexin, Bax, and FasR (CD95), leading to apoptosis-resistant phenotypes via different mechanisms. Furthermore, Gal-3 also plays a significant role as a modulator of major signaling pathways, such as Wnt signaling, Ras/Raf/MAPK pathway, and PI3K/AKT pathway through bindings with β-catenin, K-Ras, and AKT, respectively, which could induce dynamic changes in cellular phenotype (e.g., increased migration). Gal-3 is mainly a cytosolic protein; however, it can translocate into the nucleus by binding with Impotin, Sufu, and Nup98, wherein it controls the cell cycle through the interaction with cyclin A, cyclin D, cyclin E, p21(WAF1), and p27 (KIP1), accelerating cancer cells’ proliferation. Altogether, intracellular Gal-3 approaches and obsesses to other molecules like a devil, consequently seducing and modifying their functions, which contribute to tumor malignancy (Fig. 1).
Gal-3 interacting molecules in cancer metastasis. Gal-3 localizes to four biological compartments, i.e., nucleus, cytoplasm, extracellular space, and circulation, and plays unique roles through interaction with numerous proteins. Due to nuclear translocation, secretion, and internalization, Gal-3 can circulate among the nucleus, cytoplasm, extracellular space, and blood stream. The figure was produced using Servier Medical Art on www.servier.com with permission. Inset: protein structures of the Galectin family, which is classified into three molecular types: (1) prototypical, (2) tandem repeat, and (3) chimeric structure. Gal-3 is the only chimera protein, and its monomers are linked through their N-terminal domain, establishing pentameric structures. This complex of multivalent interactions modulates the extracellular function of Gal-3 in the tumor microenvironment. After exposure to proteolytic enzymes (MMP and/or PSA), intact Gal-3 is cleaved at the site of collagen α-like sequence, which leads to the disruption of pentameric structures and the production of cleaved Gal-3
Ample evidence has shown the presence of Gal-3 on the cell surface, in serum and other body fluids/compartments leading to the investigation of the extracellular function for many years. Initially, aggregation of cancer cells on the cell surface was studied, followed by matrix-cancer cell interaction during metastasis progression. Secretion of Gal-3 has been well established in cancer cells, e.g., breast, prostate, lung, and thyroid cancer although their capacities to secrete Gal-3 widely differ. As a result of cancer cells’ secretion, higher Gal-3 serum concentrations were reported in patients with breast, prostate, liver, thyroid, pancreatic, and bladder cancer. Thus, secreted Gal-3 closely correlates with cancer progression, and its function(s) was further investigated using molecular approaches, which enabled the identification of various molecules interacting with Gal-3. Considering the relatively small molecular structure capable of various molecular interactions, Gal-3 binding with other proteins primarily depends on glycoconjugates via CRD, not protein-protein interaction in most cases. In contrast to conserved CRD among the Galectin family, only Gal-3 has a beneficial chimeric structure in the NH2-terminal domain, which leads to the transition from a monomeric into a pentameric configuration. This event allows Gal-3 to bridge and bind effectively with receptors and form adhesive networks on the cell surface. The chimeric protein structure originates from its genomic sequence; i.e., Gal-3 is encoded by the human LGALS3 gene at 14 q21-q22 and a chimeric gene fusion product of the 5′-end of Gal-1 with the internal domain of the collagen alpha gene. Consequently, it translates into a unique protein form consisting of three domains: a short NH2-terminal domain of 12 amino acids (AA) having serine phosphorylation sites (Ser6, Ser12) that regulate cellular targeting, a repeated collagen-like sequence of about 100 AA that is rich in Gly-Tyr- Pro residues, and a COOH-terminal domain of ∼130 AA encompassing a single CRD. The chimeric structure of Gal-3 may explain its closest relationship with cancer progression among the Galectin family members. Then, a question arose whether the collagen-like sequence and N-terminal domain also contain significant meaning for its biological activity. Thus, enzymatic cleavage on the sites during cancer progression was the next focus. Gal-3 can be cleaved by proteolytic enzymes such as matrix metalloproteases (MMPs) and prostate-specific antigen (PSA) at the amino acid sequences of Gly32-Ala33, Ala62-Tyr63, and Tyr107-Gly108 of the collagen-like sequence. Therefore, in the tumor microenvironment, Gal-3 exists as two major forms: intact and cleaved. Since this enzymatic modification affects the tumor microenvironment, it is important to consider the activity of PSA and MMP in order to interpret the functions of extracellular Gal-3 (Fig. 1 inset). This proteolytic modification of secretory factors is named the degradome-peptidome. In humans, more than 500 proteases such as MMPs and PSA and their substrates, including Gal-3 categorized in this manner, could significantly affect the tumor microenvironment.
As for the secretory mechanism, Gal-3 lacks the classical secretion signal sequence and therefore is not secreted by the common endoplasmic reticulum (ER)/Golgi pathway. Instead, Gal-3 is transported via an unknown non-classical pathway into the extracellular milieu. In order to resolve the issue of how Gal-3 is secreted, several patterns/mechanisms of Gal-3 secretion have been examined, i.e., (1) vesicular release, (2) exosomal secretion, and (3) traverse lipid bilayer membrane. Thus, cells secrete Gal-3 both passively and actively. Among them, the two different secretory patterns, i.e., exosomal or soluble, may be important since exosomal Gal-3 secretion could affect systemic/distant organs whereas soluble secretion may primarily affect the local microenvironment surrounding Gal-3 expressing tumor cells. In addition, further studies showed that special external conditions induce Gal-3 secretion, such as (1) mechanotransduction, (2) specific protein-mediated release (e.g., fetuin), and (3) chemotherapy-induced secretion (e.g., doxorubicin) [4]. Thereafter, once Gal-3 is exported extracellularly, it interacts with its binding partners. While Gal-3 is not a bona fide cytokine, it is a potent pro- inflammatory protein and a key driver of tumor development and progression. For example, it collaborates with epidermal growth factor receptor (EGFR) and/or transforming growth factor-beta receptor (TGFβR) stimulating cell growth, with p-glycoprotein for drug resistance, with mucin-1 for adhesion, with vascular endothelial growth factor receptor-2 (VEGFR-2) for angiogenesis, and CD66 with for inflammatory induction in the tumor microenvironment. In addition to externalization above, others have shown Gal-3 internalization into mammary carcinoma cells, uterine cervix carcinoma cells, endothelial cells, macrophages, and fibroblasts through interaction with β1-integrin and/or CD44 in a carbohydrate-dependent manner [6]. Thus, Gal-3 circulates among the serum, extracellular space, cytoplasm, and nucleus, which could have a considerable impact on the tumor microenvironmental structure (Fig. 1).
2 GAL-3 in bone tumor microenvironment
2.1 Secreted Gal-3 disrupts bone cells’ homeostasis
Gal-3 has been implicated as a secreted factor that modulates the tumor microenvironment and correlates with metastasis. In the framework of cancer metastasis, the skeleton is frequently a secondary growth site. However, the functional role(s) of Gal-3 in bone has not been reviewed. Bone cells consist of three cell populations: osteoblasts (bone-forming cells), osteocytes (bone-maintaining cells), and osteoclasts (bone-degrading cells). The balance of appropriate bone production and resorption by these cells is necessary to maintain the structure and functional integrity for healthy bone since bone is not a static organ but dynamically metabolic. Osteoblasts produce bone matrix, composed of type I collagen, osteocalcin and other extracellular proteins, and calcium phosphate in the form of hydroxyapatite. Along with bone formation, a mature subset of osteoblasts becomes osteocytes, which embed in the bone matrix and form an extensive network with each other in order to maintain bone metabolism. In normal bone homeostasis, hedgehog, Wnt, bone morphogenetic protein (BMP), and fibroblast growth factor (FGF) signal pathways positively regulate osteoblast differentiation. In contrast, Notch signaling pathway inhibits osteoblast differentiation [7]. Similarly, modulation of osteoblast differentiation by tumor cells is controlled by parathyroid hormone-related protein (PTHrP), TGF-β, BMP, FGF, and Wnt acting as activators, whereas DKK-1 and IL-3 act as suppressors. Adding to these lists, tumor-secreted Gal-3 was identified as a suppressor of osteoblast differentiation through cleavage of Notch intracellular domain (NICD) and activation of Notch signaling, an inhibitory pathway of osteoblastogenesis [8], whereas cytoplasmic Gal-3 upregulates along with osteoblast differentiation [9]. In an in vivo finding, the phenotype of Gal-3(−/−) mice shows increased bone matrix inside the bone marrow cavity compared to wild type [10], suggesting that Gal-3 shifts to a reduction of bone matrix formation as a net result. Thus, Gal-3 potentially regulates the differentiation of osteoblasts and bone formation in both an intracellular and extracellular manner.
Osteoclasts are able to degrade hard bone in the skeletal system and are essential to physiological bone resorption as well as pathological bone destruction. The bone resorption and destruction are promoted by acidification and matrix-degrading proteases such as cathepsin K, MMP, and tartrate-resistant acid phosphatase. In general, tumor-induced osteolysis is not caused by the direct effects of tumor cells on the bone, but rather by osteoclast activation. Therefore, further investigations were made to prove how Gal-3 affects osteoclasts. During osteoclastogenesis, hematopoietic stem cells mature into osteoclast precursors and then differentiate into mature osteoclasts through a unique passage: cell fusion of mononuclear precursors through CD200, DC-STAMP, and E-cadherin. Thereafter, mature osteoclasts become large, multinucleated cells and locate on the bone surface. Physiologically, receptor activator of nuclear factor kappa-B ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) are the major regulators controlling osteoclast differentiation in bone homeostasis. Most importantly, RANKL is known as a pivotal regulator of osteoclast differentiation and is produced by osteoblasts, cancer cells, and activated immune cells in the bone marrow. RANKL binds with its receptor RANK on immature osteoclast precursors and then activates (1) NF-κB via recruitment of TNF receptor-associated factors (TRAFs) and/or (2) MAPK pathway via c-Fos. These events contribute to the transcriptional activations of NFATc1, AP-1, and NF-κB, leading to increased osteoclastogenesis and bone resorption [11].
Considering the fact that multiple osteoclast-stimulating factors regulate the step-by-step processes of osteoclastogenesis, M-CSF also plays a crucial role in the maturation of the monocyte/macrophage lineage and differentiation into osteoclast precursors. M-CSF stimulation induces cytoplasmic Gal-3 expression of osteoclast in an in vivo model, suggesting a role of Gal-3 in osteoclastogenesis [12]. Similarly, in pathologic conditions, e.g., bone tumor microenvironment, Gal-3 expression was found in osteoclasts and/or their precursors on human patients’ samples of giant cell tumors (primary benign bone tumor), osteosarcoma (primary malignant bone tumor), and bone metastasis [13]. These tumor cells also release osteoclastogenic factors extracellularly. Along with these, cancer-secreted Gal-3 mediates cellular fusion of osteoclast precursors through binding with Myosin-2A, a modulator for osteoclast differentiation, which leads to enhanced osteoclastogenesis. During the fusion process, the Gal-3/Myosin-2A interaction may affect transcriptional activities of osteoclast differentiation markers and enhance the downstream pathway of RANKL/RANK [13]. In addition, Gal-3 also interacts with Integrin αM (CD11b) and Integrin β2 (CD18) on the cell surface. Thus, Gal-3-activating integrins may also induce a signaling and cross-talk to RANKL-mediated signaling pathways and transcription. Altogether, these findings provide evidence of Gal-3 roles on osteoclast differentiation in the bone tumor microenvironment.
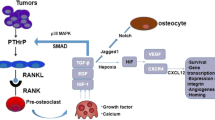
Thus, tumor cells distort the normal differentiation process of osteoblast and osteoclast cells through their secretory factors. Among them, tumor-secreted Gal-3 exhibits dual properties: (1) an enhancer for osteoclast fusion and (2) a suppressor for osteoblast differentiation, effectively leading to osteolytic bone remodeling (Fig. 2).
Roles of Gal-3 in the bone microenvironment. In breast cancer bone metastasis, intact Gal-3 is the predominant form. Cancer-secreted Gal-3 exhibits dual properties in bone metastasis: (1) secreted Gal-3 mediates osteoclast fusion and (2) suppresses osteoblast differentiation, leading effectively to osteolytic bone remodeling. Consequently, in breast cancer bone metastasis, Gal-3 drives osteolytic bone remodeling along with other osteoclast stimulators such as PTHrP and IL-8, inducing intracellular signaling for osteoclast differentiation/maturation, e.g. cFOS, NF-κB, and NFATc1. On the other hand, in prostate cancer bone metastasis, cleaved Gal-3 is the major form, which reduces the potent function of intact Gal-3 and gives priority to other secretory factors controlling the bone tumor microenvironment such as osteoblast stimulators, BMP, Wnt, and FGF. In prostate cancer bone metastasis, osteoblast stimulation is generally predominant in the context of complex tumor/environment-derived factors, leading to osteoblastic signal activation such as SMAD (in BMP signaling), β-catenin, δPKC (in Wnt signaling), and MAPK (in FGF signaling), which induce osteoblast differentiation and bone matrix production. Consequently, these events result in osteosclerotic bone remodeling. The figure was produced using Servier Medical Art on www.servier.com with permission
2.2 Secreted Gal-3 ignites cytokine reservoir of bone marrow
Bone marrow plays an essential role as a hematopoietic organ that produces immune cells and contains various cytokines that foster immature immune cells. Therefore, bone marrow is a huge reservoir of bioactive substances/inflammatory mediators that are critical for successful cancer growth. A close link has long been recognized between cancer and inflammation. In immunology, Gal-3 is known as a pro-inflammatory mediator extracellularly, so the question is how Gal-3 affects immune cells in bone marrow during tumor progression. Gal-3 was previously named macrophage-2 antigen (“Mac-2 antigen”); its expression and secretion have been well recognized in the study of macrophage/monocyte lineage as well as cancer cells. The Gal-3 level is positively correlated with the maturation status of macrophages [14]. Matured/activated macrophages secrete Gal-3 and concurrently present Gal-3 binding receptors on the cell surface, e.g., CD11b/CD18 (also known as Mac-1 antigen or integrin αMβ2), LAMP-1/-2 (lysosomal membrane glycoproteins), CD107b (also known as Mac-3 antigen), and CD98. Secreted Gal-3 causes a release of superoxide, a reactive oxygen species, from macrophages and thereafter induces inflammation. Thus, considering the fact that macrophages express both Gal-3 and its binding receptor, these secretions may be a feedback loop which drives inflammatory induction. Further, Gal-3-mediated inflammatory amplification expands to neutrophils as well. Extracellular Gal-3 enhances degranulation of inflammatory granules and/or superoxide production in a dose-dependent manner by binding with CD66a/b on the cell surface of neutrophils. Taken together, secreted Gal-3 enhances the inflammatory response in an autocrine or paracrine fashion through the activation of macrophages and neutrophils.
Activated immune cells are able to cross talk with bone cells directly, and the mechanisms of their interaction were termed “osteo-immunology.” For example, immune-related factors influencing bone loss have been identified in diseases of chronic inflammation such as rheumatoid arthritis, periodontitis, osteomyelitis (bacterial infection of bone), and peri-prosthetic wear caused by implant loosening [15]. In these pathological bone conditions, IL-17 and/or RANKL-producing lymphocytes primarily contribute to enhanced osteoclastogenesis [16, 17]. Likewise, activated lymphocytes produce other interleukins such as TNF-α, TGF-β, and granulocyte-macrophage colony-stimulating factor (GM-CSF), leading to osteoclastogenesis [16]. Thus, several cytokines participate in inflammation-induced bone destruction and a question then arises on how the role of Gal-3 in the process is. An osteoarthritis model using Gal-3(−/−) mice helped provide an answer. The mice showed reduced numbers of IL-17-producing cells and the decreased concentration of other bone-degrading cytokines, which leads to suppression of the inflammatory response and bone destruction. Thus, the phenotype proved that Gal-3-associated immune activation plays a central role in augmenting osteoclastogenesis [18]. In addition to Gal-3, cancer-producing immune activators, e.g., IL-1β, IL-6, and TNF-α, induce inflammatory conditions in the bone metastatic niche. Activated neutrophils, macrophages/monocytes, and lymphocytes trigger further production of inflammatory mediators, comprising a chronic/continuous inflammatory cycle [19]. Additionally, bone metastatic cancer cells produce PTHrP, IL-7, and IL-8 that can recruit or activate lymphocytes, leading to osteoclastic resorption [20]. Taken together, the interface between the skeletal and immune systems plays a crucial role in the bone microenvironment; an axis of “cancer cells-immune cells-bone remodeling” destroys the bone homeostasis, and therefore, Gal-3/cytokine-induced inflammation is an indispensable part of bone tumor progression.
2.3 Secreted Gal-3 delays the differentiation of myeloid lineage cells
Bone cells and immune cells participate in bone remodeling as described above. Looking back at their origin in the bone marrow, there are many multipotent progenitor cells which have the potential to differentiate into multiple cell types; e.g., mesenchymal stem cells in bone marrow can differentiate into adipocytes, myocytes, chondrocytes, fibroblasts, and osteoblasts. On the other hand, hematopoietic stem cells are a common ancestor of all blood cells and can differentiate into several types of blood cell types such as lymphocytes, monocytes, neutrophils, eosinophils, basophils, macrophages, and osteoclasts. Therefore, the family tree-like cell production and differentiation of bone marrow brought up a question as to how tumor cells and their secretory factors exert influence on stem cells and undifferentiated marrow cells since the events may determine the fate of their differentiation and/or the predominant cellular population in bone marrow. As an answer, prostate cancer cells compete with hematopoietic stem/progenitor cells in the bone marrow and reduce the number of hematopoietic stem cells by driving their terminal differentiation in the bone metastatic niche [21]. The onerous effect of disseminated cancer cells expands to their descendants. The additional study shows that hematopoietic progenitor cells interacting with prostate cancer transform stromal cells into an osteoblastic phenotype, whereas hematopoietic stem cells interacting with prostate cancer transform themselves into osteoclasts via cytokine-mediated pathways [22]. Thus, tumor cell/hematopoietic stem cell interactions come into the spotlight since they may affect the population of all marrow cells and result in destructive bone remodeling. As a tumor-derived factor, extracellular Gal-3 delays the differentiation of the stem cell population since it nullifies the effect of GM-CSF, a potent secretory factor regulating the differentiation of bone marrow cells at an early stage [23]. Consistently, the bone marrow of Gal-3(−/−) mice shows the increased undifferentiated hematopoietic progenitors, whereas differentiated mature cell populations are reduced, suggesting that Gal-3 potentially regulates the differentiation of bone marrow cells [10]. Thus, cancer-secreted Gal-3 may seriously damage the bone microenvironment.
Although the cytokines are now thought to be major players for bone environmental control, the findings summarized here proved that a member of the lectin family, Gal-3, is a new player orchestrating the bone tumor microenvironment by affecting osteoblasts, osteoclasts, immune cells, and myeloid cells.
3 Bone tumor microenvironment: a different landscape of tumor/GAL-3-induced bone remodeling
Gal-3 functions in the bone tumor microenvironment were outlined above. The findings lead to make a profile of Gal-3 expression status in bone metastasis. Gal-3 was expressed at a higher percentage (76 %) in breast bone metastasis, whereas it was expressed at a lower percentage (13 %) in prostate bone metastasis while cleaved Gal-3 was prevalent (74 %) instead due to enzymatic cleavage, e.g., PSA or MMP [13]. Thus, the staining patterns confirmed that the bone tumor microenvironment of skeletal metastasis, at least, Gal-3 expression and its cleavage status, differs depending on the cancer cells’ origin. The results subsequently brought further questions of whether the other tumor-derived factors differently affect the bone tumor microenvironment and whether bone destruction processes are different among the bone-related tumors. Currently, there is no answer to these questions in the literature. Therefore, in this section, we compare the pathological patterns of bone tumor microenvironment underlying bone destruction of the major bone-related tumors while considering cooperative/uncooperative relationships among the tumor-derived factors (Fig. 3).
Comparative pathology of the bone tumor microenvironment in bone-related tumors. Tumor cells control their bone microenvironment differently, depending on the tumor type and the tumor-derived factors. Consequently, bone niches respond with different signal activations. The figure was produced using Servier Medical Art on www.servier.com with permission. a Breast cancer bone metastasis. b Prostate cancer bone metastasis. c Osteosarcoma. d Multiple myeloma. e Oral squamous cell carcinoma. f Giant cell tumor of the bone
3.1 Breast cancer bone metastasis
In breast cancer bone metastases, osteolytic lesions are more frequently found than osteoblastic lesions [24]. This means that osteolytic factors are predominant compared to osteosclerotic factors in breast cancer bone metastasis. The osteolytic mechanisms of breast cancer bone metastasis are classified into two interactions, (1) tumor-derived factors directly enhance osteoclastogenesis, or (2) tumor-derived factors induce RANKL expression on osteoblasts, which indirectly enhance osteoclastogenesis. Direct enhancement can be induced by PTHrP [25], IL-8 [26, 27], VEGF [28], syndecan-1 [29], heparanase [30], and GM-CSF [31]. Among them, tumor-derived GM-CSF activates the NF-κB pathway, a pivotal signaling of osteoclast differentiation, leading to osteolytic bone remodeling [31]. On the other hand, secretory factors acting indirectly may include PTHrP, COX-2, IL-1, TGF-β, and PDGF [32]. In addition to secretory factors, direct contact on the cell surface between cancer cells and osteoclast precursors plays a crucial role in the development of osteolytic lesions. For example, vascular cell adhesion molecule-1 (VCAM-1) recruits monocytic osteoclast progenitors and elevates osteoclast activity by interacting with integrin α4β1 in breast cancer bone metastasis [33]. Similarly on the cell surface, tumor-derived Jagged-1 engages Notch signaling in both osteoclasts and osteoblasts. Activation of Notch signaling induces osteoclast differentiation and inhibits osteoblast differentiation, which promotes osteolytic bone metastasis of breast cancer [34]. Cancer-secreted Gal-3 also has a bifunctional effect on osteoblasts and osteoclasts; Gal-3 inhibits osteoblast differentiation through the further activation of Notch signaling [8] and mediates osteoclast fusion by binding with Myosin-2A, a modulator of osteoclast differentiation, leading to enhanced osteolytic bone remodeling [13]. Thus, tumor-derived Gal-3 drives bone destruction in the complex bone microenvironment of metastatic breast cancer (Fig. 3a).
3.2 Prostate cancer bone metastasis
In prostate cancer patients with bone metastasis, osteosclerotic lesions account for 80–90 % [35]. This means that osteoblastic factors are predominant compared to osteolytic factors in prostate cancer bone metastasis. In osteosclerotic lesions, bone production is promoted by cancer-activated osteoblasts. Specifically, prostate cancer cells secrete endothelin-1 (ET-1) [36], Wnts [37, 38], urokinase-type plasminogen activator (uPA) [39, 40], BMP [41, 42], FGF [43], and PTHrP [44], enhancing osteoblast differentiation through the activation of multiple signaling pathways. Considering the fact that in physiological bone homeostasis, Wnt, BMP, and FGF are essential stimulators of osteoblast differentiation, cancer-induced bone production is similar, in part, to physiological regulation, but at a higher level. As another mechanism, prostate cancer cells inhibit osteoclastogenesis by releasing osteoprotegerin (OPG), which is a potent inhibitor of RANKL [45, 46]. On the other hand, osteoclastic factors also play an important role in prostate cancer bone metastasis; a clinical study confirmed the significance [47]. For example, prostate cancer cells directly activate osteoclasts through the secretion of RANKL, TGF-β, and PTHrP [44, 45, 48]. In addition, tumor-derived MMP cleaves membrane-bound RANKL on the osteoblast surface, which promotes the release of soluble RANKL into the bone microenvironment, consequently leading to osteolytic remodeling [49]. In contrast to RANKL cleavage, Gal-3 cleavage is a cause of osteosclerosis. As depicted, intact Gal-3 demonstrates an osteolytic effect; however, the cleaved form of Gal-3 is more abundant in prostate cancer bone metastases, and consequently, the shift to cleaved Gal-3 attenuates the enhancement of osteoclast differentiation [13]. Of note, during the cancer dissemination process, Gal-3 enhances prostate cancer cell spreading to the bone marrow. In an in vitro assay, prostate cancer cells preferentially adhere to human bone marrow endothelium through Gal-3 interaction when compared with endothelium derived from other sources [50]. Consistently, an in vivo model using Gal-3 antibody/lactulose-l-leucine, a specific inhibitor of Gal-3, showed that preferable metastatic adhesion to skeletal organs is mediated by Gal-3 [51, 52]. These studies indicate that Gal-3 is essential during the bone dissemination of prostate cancer, and therefore, Gal-3 therapeutic targeting may preclude malignant cell lodging in bone (Fig. 3b).
The bone destructive mechanisms of other cancer bone metastases remain unclear. However, in a comparison between breast and prostate cancer bone metastases, here, we have elucidated that the bone remodeling patterns vary depending on the cancer cells’ origin and cancer-derived factors, which induce different bone remodeling signaling in osteoblasts and osteoclasts.
3.3 Additional bone-destructive tumors
In addition to bone-seeking tumors, further investigations were made to unveil the mechanism of tumor-induced bone destruction among other bone-related tumors. Firstly, osteosarcoma is a bone-originating tumor and known as a major primary bone sarcoma. Recent studies reveal evidence that mesenchymal stem cells carrying mutations produce a variety of sarcoma phenotypes. Among them, mesenchymal stem cell-derived osteogenic progenitors having a gene mutation are reported to be an origin of osteosarcoma [53]. Indeed, similarly to osteoblasts, osteosarcoma cells independently produce RANKL [54], which is likely to be responsible for osteoclastogenesis among the various osteosarcoma-secreting factors [55]. In addition, osteosarcoma cells also produce M-CSF or other stimulators, leading to osteoclast formation [55]. In particular, osteosarcoma-derived PTHrP exposes to osteoblasts, leading them to present M-CSF and RANKL, which results in enhanced osteoclast differentiation [56]. These findings imply a highly activated downstream signaling of RANKL in the osteosarcoma bone microenvironment. Thus, the balance of bone homeostasis is disturbed by osteosarcoma-derived factors and the shift toward increasing osteoclast activity may be associated with the aggressiveness of the osteosarcoma [57]. Simultaneously, osteosarcoma highly produces bone matrix, and BMP plays an important role in the augmentation of bone formation [58]. Consequently, most lesions show mixed pattern of bone-degrading and bone-producing remodeling, and Gal-3 may participate in the degrading process. A higher expression of Gal-3 is reported in patients with osteosarcoma, which is positively correlated with advanced stage [59] since cytoplasmic Gal-3 enhances the malignant phenotype of osteosarcoma [60, 61]. Osteosarcoma cells secrete Gal-3 [62], which possibly resembles the osteolytic effects of bone metastasis. In addition to tumor cells, Gal-3-positive osteoclast precursors appear to congregate near the matured osteoclasts in the osteosarcoma microenvironment [13]. Thus, Gal-3 enhances the progression of osteosarcoma and osteoclastogenesis and therefore is associated with bone destruction (Fig. 3c).
Secondly, multiple myeloma is characterized by infiltration of malignant plasma cells in the bone marrow, producing a high level of immunoglobulin in the blood (monoclonal gammopathy). In a clinical diagnosis, multiple myeloma is also known as an osteolytic tumor spreading throughout the whole body. In the bone marrow, multiple myeloma cells secrete macrophage inflammatory protein-1α (MIP1α), inducing osteoclastogenesis through interaction with CCR1, a chemokine receptor on the osteoclast cells [63]. On the other hand, osteoblast differentiation is inhibited by the secretion of Dickkopf 1 (DKK1) and IL-3 secreted by multiple myeloma cells. DKK1 suppresses Wnt signaling, a pathway for inducing osteoblast differentiation [64, 65], whereas IL-3 invalidates the effect of BMP, a potent inducer of osteoblast differentiation [66]. Thus, in the tumor microenvironment of multiple myeloma, Wnt and BMP signalings are down-regulated. Altogether, stimulation of osteoclastogenesis and inhibition of osteoblastogenesis promote osteolysis in multiple myeloma. Multiple myeloma cells express Gal-3, regulating their proliferation and apoptosis during chemotherapeutic treatment [67, 68]. Once Gal-3 is released extracellularly from the myeloma cells, osteolytic bone remodeling may be enhanced (Fig. 3d).
Thirdly, oral squamous cell carcinoma is a bone-invading malignant tumor that often lyses the jaw bone with an erosive, mixed, or infiltrative pattern, weakening the bone and causing pain, which are major clinical concerns. Tumor-derived factors such as IL-6 and PTHrP induce RANKL expression on osteoblasts, which lead to osteoclastogenesis [69]. Squamous cell carcinoma cells also directly enhance osteoclastogenesis via secretion of RANKL, IL-6, CXCL12 (SDF-1), CXCL13, TNF-α, prostaglandin E2/F2, and PTHrP [70–77]. Altogether, these events result in osteolytic bone remodeling. Since the activated downstream pathways of tumor-affected osteoclasts and osteoblasts have not been examined, the key factor/pathway remains to be elucidated. Gal-3 is often expressed in squamous cell carcinoma, inducing malignant transformation of oral mucosa cells, and a higher concentration was reported in patients’ serum [78–80], implying Gal-3 secretion. Given that Gal-3 is released from the cancer cells, secreted Gal-3 initiates bone remodeling and favors cancer cells to invade into the surrounding bone (Fig. 3e).
Fourthly, giant cell tumor is a benign osteoclast-producing tumor, characterized by osteoclastogenic stromal cells and giant cells, which are excessively multinucleated osteoclast cells. The forming process of giant cells is initiated by stromal cell-derived factor-1 (SDF-1) and monocyte chemoattractant protein-1 (MCP-1), enhancing monocyte recruitment from blood components. Migrated monocytes (giant cell precursors) expressing CXCR4 and Gal-3 are further nourished by stromal cells releasing RANKL, IL-6, IL-34, TNF-α, and M-CSF [13, 81, 82]. Consequently, these secretory factors stimulate the expression of NFATc1 and C/EBPβ, which are transcriptional factors for osteoclast differentiation of the giant cells [83, 84]. These events drive excessive osteoclastogenesis, thereafter resulting in bone loss. Thus, Gal-3 plays a role in the osteolytic lesion of giant cell tumor (Fig. 3f).
Altogether, we showed that the tumor-derived factors affecting bone tumor microenvironment vary widely. Accordingly, bone remodeling mechanisms also differ depending on tumor cells’ origin. Of note, even in the group of bone metastatic lesions, bone tumor microenvironments are different between breast and prostate cancers.
4 Tumor GAL-3 in the bone metastatic niche: a therapeutic target
The idea of different bone metastatic niches hints at an individual management based on metastatic cancer cells’ origin to prevent cancer-induced bone destruction. Clinically, bone metastases are a common clinical outcome of many solid tumors; the incidences of bone metastases are 65–75 % in prostate, 65–75 % in breast, 30–40 % in lung, 40–60 % in thyroid, 20–35 % in renal, 40 % in bladder, 14–45 % in malignant melanoma, and 5 % in gastrointestinal cancer [85, 86] and often arise in multiple lesions (Fig. 4a). In any cancers, the bone metastasis patients frequently experience hypercalcemia, severe pain, spinal compression, and pathological bone fractures due to either destructive osteolytic lesions or intrinsically low strength of new bone overgrowth in osteosclerotic lesions (Fig. 4b, c). These symptoms are referred to as “skeletal-related events (also known as SRE),” which are one of the main focuses during treatment. So far, clinical trials have been directed in an attempt to halt the skeletal-related events and bone metastatic cancer progression, and consequently, treatment guidelines were established [85, 87, 88]. As a consensus, treatment strategies are mainly directed at palliation and include radiation, surgery, ablation, chemotherapy, and hormone therapy, which are aimed at reducing/removing cancer growth/mass while considering individual cancer’s biological features (e.g., most prostate cancers are sensitive and activated by androgen; therefore, hormone therapy is chosen first). With respect to the bone microenvironment control, osteoclast targeting therapies were recommended in order to alleviate cancer-induced bone destruction, e.g., zoledronic acid, a specific apoptosis inducer of osteoclasts and denosumab (anti-RANKL antibody), a suppresser for osteoclast differentiation (Fig. 5). Currently, the shared therapeutic approaches are offered for bone metastatic environments (Table 1) [89–97]. Considering the different bone tumor microenvironments depicted in this review, we suggest that bone metastatic lesions should be treated differently in a more personalized manner centering on the expressional/secretory/functional status of tumor-derived factors, e.g., Gal-3 cleavage status. As a detailed example, single-nucleotide polymorphism (SNP) analysis of human Gal-3 gene, LGALS3, showed that Gal-3 cleavage status is determined by a mutation (polymorphism) in the position 191 (rs4644) of the Gal-3 gene causing an allelic variation which translates into proline or histidine at position 64 in the amino acid sequence where MMP cleaves [98]. Therefore, the genetic information may be influential in using anti-Gal-3 therapy in bone metastatic patients and even represent a first prototype.
Clinical presentation of bone metastases. a A full-body bone scan in a breast cancer patient using technetium-99m shows multiple bone metastatic lesions in the spine, pelvis, and femur (red circles). b CT hip images demonstrate distinct differences in bone metastasis patterns based on the origin of cancer cells. Osteolytic remodeling is seen in a breast cancer bone metastasis (left, green arrowhead), whereas osteosclerotic remodeling is seen in prostate cancer bone metastases (right, green arrowheads). c A gross specimen shows an example of osteolytic remodeling in renal cell carcinoma with cortical erosion and loss of cancellous bone (left, white arrowheads). Osteosclerotic remodeling in prostate cancer (right, asterisks) is marked by bone production in the lesser trochanter (right, white arrowhead) and a pathologic femoral neck fracture (right, red arrow). Clinical images were approved to present in this article by the Institutional Review Board in Gunma University Hospital on October 7, 2015 (Registration no. 15-58)
Current therapeutic concepts for bone metastasis. In bone marrow, metastatic tumor cells proliferate and disturb the normal homeostasis and cross talk among the niches of bone cells, immune cells, stem cells, and hematopoietic cells. These interactions lead to abnormal bone remodeling and enhance tumor progression. Hence, current therapeutic concepts to treat patients with bone metastasis are classified into two categories, (1) treatments against cancer cells and (2) treatments targeting bone tumor microenvironments. As for treatment against cancer cells, the therapeutic modalities include radiation, surgery/ablation, chemotherapy, hormone therapy, and radiopharmaceuticals. In the clinical setting, the treatment options are selected based on cancer characteristics, e.g., detected number and location of bone metastatic lesions, hormone sensitivity, chemotherapeutic sensitivity, and radiation sensitivity. With respect to treatment targeting bone tumor microenvironments, the therapeutic modalities include bisphosphonates and anti-RANKL therapy for the purpose of suppression of activated osteoclasts. Although bone tumor microenvironment contains various cells, specific therapeutic approaches are clinically not established, except for osteoclasts. In the future, personalized approaches may be necessary based on different statuses of tumor-derived factors affecting the bone tumor microenvironments
Bone metastasis is considered a terminal stage of disease, and the therapeutic approaches often fail to cure. Considering the devastating circumstances, we hypothesize how anti-Gal-3 therapy may contribute clinically. In the framework of bone metastatic dissemination, cancer cells utilize the fertile “soil” of bone marrow to proliferate and disturb the peaceful bone marrow society and normal bone homeostasis. Once the tumor cells build a nest in the bone marrow, it is not easy to eradicate them because of the surrounding hard bone and the transferrable bloody liquid scaffold with exportable vessels used as escape routes to the systemic circulation. After colonization in the bone marrow, cancer cells stimulate the osteoclastogenesis, and then, activated osteoclasts invade the bone matrix whereby TGF-β, insulin-like growth factors (IGF), and calcium are released. These factors promote proliferation and survival of cancer cells. Thus, cancer growth and bone destruction are indeed bi-directional events in multiple cancer foci. This repetitive pathology underlying bone metastatic lesions has been referred to as a “vicious cycle.” In order to halt it, the simultaneous suppression by targeting a common and specific molecule organizing both cancer cells and bone microenvironment may be necessary while sparing normal host cell functions in the context of the complex bone metastatic niche. Thus, considering Gal-3 orchestration in the bone tumor microenvironment to be a vicious cycle rotator, anti-Gal-3 therapy may promise multiple clinical benefits to halt systemic bone metastasis. The notion of “Gal-3-associated bone remodeling” in the tumor microenvironment may provide a novel outlook in the coming era of personalized treatment for the patients suffering from cancer bone metastasis, whereby tumor origin should be considered.
References
Raz, A., Bucana, C., McLellan, W., & Fidler, I. J. (1980). Distribution of membrane anionic sites on B16 melanoma variants with differing lung colonising potential. Nature, 284(5754), 363–364.
Raz, A., & Lotan, R. (1981). Lectin-like activities associated with human and murine neoplastic cells. Cancer Research, 41(9 Pt 1), 3642–3647.
Liu, F. T., & Rabinovich, G. A. (2005). Galectins as modulators of tumour progression. Nature Review. Cancer, 5(1), 29–41.
Nangia-Makker, P., Balan, V., & Raz, A. (2008). Regulation of tumor progression by extracellular galectin-3. Cancer Microenvironment, 1(1), 43–51.
Harazono, Y., Nakajima, K., & Raz, A. (2014). Why anti-Bcl-2 clinical trials fail: a solution. Cancer and Metastasis Reviews, 33(1), 285–294.
Lakshminarayan, R., Wunder, C., Becken, U., Howes, M. T., Benzing, C., Arumugam, S., et al. (2014). Galectin-3 drives glycosphingolipid-dependent biogenesis of clathrin-independent carriers. Nature Cell Biology, 16(6), 595–606.
Long, F. (2012). Building strong bones: molecular regulation of the osteoblast lineage. Nature Reviews. Molecular Cell Biology, 13(1), 27–38.
Nakajima, K., Kho, D. H., Yanagawa, T., Harazono, Y., Gao, X., Hogan, V., et al. (2014). Galectin-3 inhibits osteoblast differentiation through notch signaling. Neoplasia, 16(11), 939–949.
Stock, M., Schäfer, H., Stricker, S., Gross, G., Mundlos, S., & Otto, F. (2003). Expression of galectin-3 in skeletal tissues is controlled by Runx2. The Journal of Biological Chemistry, 278(19), 17360–17367.
Brand, C., Oliveira, F. L., Ricon, L., Fermino, M. L., Boldrini, L. C., Hsu, D. K., et al. (2011). The bone marrow compartment is modified in the absence of galectin-3. Cell and Tissue Research, 346(3), 427–437.
Edwards, J. R., & Mundy, G. R. (2011). Advances in osteoclast biology: old findings and new insights from mouse models. Nature Reviews. Rheumatology, 7(4), 235–243.
Niida, S., Amizuka, N., Hara, F., Ozawa, H., & Kodama, H. (1994). Expression of Mac-2 antigen in the preosteoclast and osteoclast identified in the op/op mouse injected with macrophage colony-stimulating factor. Journal of Bone and Mineral Research, 9(6), 873–881.
Nakajima, K., Kho, D. H., Yanagawa, T., Harazono, Y., Hogan, V., Chen, W., et al. (2016). Galectin-3 cleavage alters bone remodeling: different outcomes in breast and prostate cancer skeletal metastasis. Cancer Research, 76(6), 1391–1402.
Leenen, P. J., Jansen, A. M., & van Ewijk, W. (1986). Murine macrophage cell lines can be ordered in a linear differentiation sequence. Differentiation, 32(2), 157–164.
Crotti, T. N., Dharmapatni, A. A., Alias, E., & Haynes, D. R. (2015). Osteoimmunology: major and costimulatory pathway expression associated with chronic inflammatory induced bone loss. Journal of Immunology Research, 2015(2015), 281287.
Takayanagi, H. (2009). Osteoimmunology and the effects of the immune system on bone. Nature Reviews. Rheumatology, 5(12), 667–676.
Kong, Y. Y., Feige, U., Sarosi, I., Bolon, B., Tafuri, A., & Morony, S. (1999). Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature, 402(6759), 304–309.
Forsman, H., Islander, U., Andréasson, E., Andersson, A., Onnheim, K., Karlström, A., et al. (2011). Galectin 3 aggravates joint inflammation and destruction in antigen-induced arthritis. Arthritis and Rheumatism, 63(2), 445–454.
Roca, H., & McCauley, L. K. (2015). Inflammation and skeletal metastasis. Bonekey Reports, 4, 706.
Fournier, P. G., Chirgwin, J. M., & Guise, T. A. (2006). New insights into the role of T cells in the vicious cycle of bone metastases. Current Opinion in Rheumatology, 18(4), 396–404.
Shiozawa, Y., Pedersen, E. A., Havens, A. M., Jung, Y., Mishra, A., Joseph, J., et al. (2011). Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. The Journal of Clinical Investigation, 121(4), 1298–1312.
Joseph, J., Shiozawa, Y., Jung, Y., Kim, J. K., Pedersen, E., Mishra, A., et al. (2012). Disseminated prostate cancer cells can instruct hematopoietic stem and progenitor cells to regulate bone phenotype. Molecular Cancer Research, 10(3), 282–292.
Krugluger, W., Frigeri, L. G., Lucas, T., Schmer, M., Förster, O., Liu, F. T., et al. (1997). Galectin-3 inhibits granulocyte-macrophage colony-stimulating factor (GM-CSF)-driven rat bone marrow cell proliferation and GM-CSF-induced gene transcription. Immunobiology, 197(1), 97–109.
Du, Y., Cullum, I., Illidge, T. M., & Ell, P. J. (2007). Fusion of metabolic function and morphology: sequential [18F]fluorodeoxyglucose positron-emission tomography/computed tomography studies yield new insights into the natural history of bone metastases in breast cancer. Journal of Clinical Oncology, 25(23), 3440–3447.
Guise, T. A., Yin, J. J., Taylor, S. D., Kumagai, Y., Dallas, M., Boyce, B. F., et al. (1996). Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. The Journal of Clinical Investigation, 98(7), 1544–1549.
Bendre, M. S., Margulies, A. G., Walser, B., Akel, N. S., Bhattacharrya, S., Skinner, R. A., et al. (2005). Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Research, 65(23), 11001–11009.
Bendre, M. S., Montague, D. C., Peery, T., Akel, N. S., Gaddy, D., & Suva, L. J. (2003). Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone, 33(1), 28–37.
Aldridge, S. E., Lennard, T. W., Williams, J. R., & Birch, M. A. (2005). Vascular endothelial growth factor acts as an osteolytic factor in breast cancer metastases to bone. British Journal of Cancer, 92(8), 1531–1537.
Kelly, T., Suva, L. J., Nicks, K. M., MacLeod, V., & Sanderson, R. D. (2010). Tumor-derived syndecan-1 mediates distal cross-talk with bone that enhances osteoclastogenesis. Journal of Bone and Mineral Research, 25(6), 1295–1304.
Kelly, T., Suva, L. J., Huang, Y., Macleod, V., Miao, H. Q., Walker, R. C., et al. (2005). Expression of heparanase by primary breast tumors promotes bone resorption in the absence of detectable bone metastases. Cancer Research, 65(13), 5778–5784.
Park, B. K., Zhang, H., Zeng, Q., Dai, J., Keller, E. T., Giordano, T., et al. (2007). NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nature Medicine, 13(1), 62–69.
Chen, Y. C., Sosnoski, D. M., & Mastro, A. M. (2010). Breast cancer metastasis to the bone: mechanisms of bone loss. Breast Cancer Research, 12(6), 215.
Lu, X., Mu, E., Wei, Y., Riethdorf, S., Yang, Q., Yuan, M., et al. (2011). VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell, 20(6), 701–714.
Sethi, N., Dai, X., Winter, C. G., & Kang, Y. (2011). Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell, 19(2), 192–205.
Ceci, F., Castellucci, P., Graziani, T., Schiavina, R., Chondrogiannis, S., Bonfiglioli, R., et al. (2015). 11C- choline PET/CT identifies osteoblastic and osteolytic lesions in patients with metastatic prostate cancer. Clinical Nuclear Medicine, 40(5), e265–e270.
Nelson, J. B., Hedican, S. P., George, D. J., Reddi, A. H., Piantadosi, S., Eisenberger, M. A., et al. (1995). Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nature Medicine, 1(9), 944–949.
Hall, C. L., Bafico, A., Dai, J., Aaronson, S. A., & Keller, E. T. (2005). Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Research, 65(17), 7554–7560.
Dai, J., Hall, C. L., Escara-Wilke, J., Mizokami, A., Keller, J. M., & Keller, E. T. (2008). Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Research, 68(14), 5785–5794.
Rabbani, S. A., Desjardins, J., Bell, A. W., Banville, D., Mazar, A., Henkin, J., et al. (1990). An amino- terminal fragment of urokinase isolated from a prostate cancer cell line (PC-3) is mitogenic for osteoblast- like cells. Biochemical and Biophysical Research Communications, 173(3), 1058–1064.
Achbarou, A., Kaiser, S., Tremblay, G., Ste-Marie, L. G., Brodt, P., Goltzman, D., et al. (1994). Urokinase overproduction results in increased skeletal metastasis by prostate cancer cells in vivo. Cancer Research, 54(9), 2372–2377.
Feeley, B. T., Gamradt, S. C., Hsu, W. K., Liu, N., Krenek, L., Robbins, P., et al. (2005). Influence of BMPs on the formation of osteoblastic lesions in metastatic prostate cancer. Journal of Bone and Mineral Research, 20(12), 2189–2199.
Nishimori, H., Ehata, S., Suzuki, H. I., Katsuno, Y., & Miyazono, K. (2012). Prostate cancer cells and bone stromal cells mutually interact with each other through bone morphogenetic protein-mediated signals. The Journal of Biological Chemistry, 287(24), 20037–20046.
Valta, M. P., Hentunen, T., Qu, Q., Valve, E. M., Harjula, A., Seppänen, J. A., et al. (2006). Regulation of osteoblast differentiation: a novel function for fibroblast growth factor 8. Endocrinology, 147(5), 2171–2182.
Liao, J., Li, X., Koh, A. J., Berry, J. E., Thudi, N., Rosol, T. J., et al. (2008). Tumor expressed PTHrP facilitates prostate cancer-induced osteoblastic lesions. International Journal of Cancer, 123(10), 2267–2278.
Zhang, J., Dai, J., Qi, Y., Lin, D. L., Smith, P., Strayhorn, C., et al. (2001). Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. Journal of Clinical Investigation, 107(10), 1235–1244.
Corey, E., Brown, L. G., Kiefer, J. A., Quinn, J. E., Pitts, T. E., Blair, J. M., et al. (2005). Osteoprotegerin in prostate cancer bone metastasis. Cancer Research, 65(5), 1710–1718.
Smith, M. R., Saad, F., Coleman, R., Shore, N., Fizazi, K., Tombal, B., et al. (2012). Denosumab and bone- metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet, 379(9810), 39–46.
Jin, J. K., Dayyani, F., & Gallick, G. E. (2011). Steps in prostate cancer progression that lead to bone metastasis. International Journal of Cancer, 128(11), 2545–2561.
Lynch, C. C., Hikosaka, A., Acuff, H. B., Martin, M. D., Kawai, N., Singh, R. K., et al. (2005). MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell, 7(5), 485–496.
Lehr, J. E., & Pienta, K. J. (1998). Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. Journal of the National Cancer Institute, 90(2), 118–123.
Glinskii, O. V., Huxley, V. H., Glinsky, G. V., Pienta, K. J., Raz, A., & Glinsky, V. V. (2005). Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia, 7(5), 522–527.
Glinskii, O. V., Sud, S., Mossine, V. V., Mawhinney, T. P., Anthony, D. C., Glinsky, G. V., et al. (2012). Inhibition of prostate cancer bone metastasis by synthetic TF antigen mimic/galectin-3 inhibitor lactulose-L-leucine. Neoplasia, 14(1), 65–73.
Rubio, R., Gutierrez-Aranda, I., Sáez-Castillo, A. I., Labarga, A., Rosu-Myles, M., Gonzalez-Garcia, S., et al. (2013). The differentiation stage of p53-Rb-deficient bone marrow mesenchymal stem cells imposes the phenotype of in vivo sarcoma development. Oncogene, 32(41), 4970–4980.
Kinpara, K., Mogi, M., Kuzushima, M., & Togari, A. (2000). Osteoclast differentiation factor in human osteosarcoma cell line. Journal of Immunoassay, 21(4), 327–340.
Miyamoto, N., Higuchi, Y., Mori, K., Ito, M., Tsurudome, M., Nishio, M., et al. (2002). Human osteosarcoma-derived cell lines produce soluble factor(s) that induces differentiation of blood monocytes to osteoclast-like cells. International Immunopharmacology, 2(1), 25–38.
Itoh, K., Udagawa, N., Matsuzaki, K., Takami, M., Amano, H., Shinki, T., et al. (2000). Importance of membrane- or matrix-associated forms of M-CSF and RANKL/ODF in osteoclastogenesis supported by SaOS-4/3 cells expressing recombinant PTH/PTHrP receptors. Journal of Bone and Mineral Research, 15(9), 1766–1775.
Avnet, S., Longhi, A., Salerno, M., Halleen, J. M., Perut, F., Granchi, D., et al. (2008). Increased osteoclast activity is associated with aggressiveness of osteosarcoma. International Journal of Oncology, 33(6), 1231–1238.
Urist, M. R., Nakata, N., Felser, J. M., Nogami, H., Hanamura, H., Miki, T., et al. (1977). An osteosarcoma cell and matrix retained morphogen for normal bone formation. Clinical Orthopaedics and Related Research, 124, 251–266.
Zhou, X., Jing, J., Peng, J., Mao, W., Zheng, Y., Wang, D., et al. (2014). Expression and clinical significance of galectin-3 in osteosarcoma. Gene, 546(2), 403–407.
Park, G. B., Kim, D. J., Kim, Y. S., Lee, H. K., Kim, C. W., & Hur, D. Y. (2015). Silencing of galectin-3 represses osteosarcoma cell migration and invasion through inhibition of FAK/Src/Lyn activation and β-catenin expression and increases susceptibility to chemotherapeutic agents. International Journal of Oncology, 46(1), 185–194.
Lei, P., He, H., Hu, Y., & Liao, Z. (2015). Small interfering RNA-induced silencing of galectin-3 inhibits the malignant phenotypes of osteosarcoma in vitro. Molecular Medicine Reports, 12(4), 6316–6322.
Mercer, N., Ahmed, H., McCarthy, A. D., Etcheverry, S. B., Vasta, G. R., & Cortizo, A. M. (2004). AGE- R3/galectin-3 expression in osteoblast-like cells: regulation by AGEs. Molecular and Cellular Biology, 266(1-2), 17–24.
Choi, S. J., Oba, Y., Gazitt, Y., Alsina, M., Cruz, J., Anderson, J., et al. (2001). Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease. Journal of Clinical Investigation, 108(12), 1833–1841.
Tian, E., Zhan, F., Walker, R., Rasmussen, E., Ma, Y., Barlogie, B., et al. (2003). The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. The New England Journal of Medicine, 349(26), 2483–2494.
Yaccoby, S., Ling, W., Zhan, F., Walker, R., Barlogie, B., & Shaughnessy, J. D. J. (2007). Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood, 109(5), 2106–2111.
Ehrlich, L. A., Chung, H. Y., Ghobrial, I., Choi, S. J., Morandi, F., Colla, S., et al. (2005). IL-3 is a potential inhibitor of osteoblast differentiation in multiple myeloma. Blood, 106(4), 1407–1414.
Chauhan, D., Li, G., Podar, K., Hideshima, T., Neri, P., He, D., et al. (2005). A novel carbohydrate-based therapeutic GCS-100 overcomes bortezomib resistance and enhances dexamethasone-induced apoptosis in multiple myeloma cells. Cancer Research, 65(18), 8350–8358.
Streetly, M. J., Maharaj, L., Joel, S., Schey, S. A., Gribben, J. G., & Cotter, F. E. (2010). GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death. Blood, 115(19), 3939–3948.
Jimi, E., Furuta, H., Matsuo, K., Tominaga, K., Takahashi, T., & Nakanishi, O. (2011). The cellular and molecular mechanisms of bone invasion by oral squamous cell carcinoma. Oral Diseases, 17(5), 462–468.
Zhang, X., Junior, C. R., Liu, M., Li, F., D’Silva, N. J., & Kirkwood, K. L. (2013). Oral squamous carcinoma cells secrete RANKL directly supporting osteolytic bone loss. Oral Oncology, 49(2), 119–128.
Carter, R. L., Tsao, S. W., Burman, J. F., Pittam, M. R., Clifford, P., & Shaw, H. J. (1983). Patterns and mechanisms of bone invasion by squamous carcinomas of the head and neck. American Journal of Surgery, 146(4), 451–455.
Shibahara, T., Nomura, T., Cui, N. H., & Noma, H. (2005). A study of osteoclast-related cytokines in mandibular invasion by squamous cell carcinoma. International Journal of Oral and Maxillofacial Surgery, 34(7), 789–793.
Tada, T., Jimi, E., Okamoto, M., Ozeki, S., & Okabe, K. (2005). Oral squamous cell carcinoma cells induce osteoclast differentiation by suppression of osteoprotegerin expression in osteoblasts. International Journal of Cancer, 116(2), 253–262.
Okamoto, M., Hiura, K., Ohe, G., Ohba, Y., Terai, K., Oshikawa, T., et al. (2000). Mechanism for bone invasion of oral cancer cells mediated by interleukin-6 in vitro and in vivo. Cancer, 89(9), 1966–1975.
Takayama, Y., Mori, T., Nomura, T., Shibahara, T., & Sakamoto, M. (2010). Parathyroid-related protein plays a critical role in bone invasion by oral squamous cell carcinoma. International Journal of Oncology, 36(6), 1387–1394.
Tang, C. H., Chuang, J. Y., Fong, Y. C., Maa, M. C., Way, T. D., & Hung, C. H. (2008). Bone-derived SDF-1 stimulates IL-6 release via CXCR4, ERK and NF-kappaB pathways and promotes osteoclastogenesis in human oral cancer cells. Carcinogenesis, 29(8), 483–492.
Pandruvada, S. N., Yuvaraj, S., Liu, X., Sundaram, K., Shanmugarajan, S., Ries, W. L., et al. (2010). Role of CXC chemokine ligand 13 in oral squamous cell carcinoma associated osteolysis in athymic mice. International Journal of Cancer, 126(10), 2319–2329.
Gillenwater, A., Xu, X. C., El-Naggar, A. K., Clayman, G. L., & Lotan, R. (1996). Expression of galectins in head and neck squamous cell carcinoma. Head & Neck, 18(5), 422–432.
Hossaka, T. A., Ribeiro, D. A., Focchi, G., André, S., Fernandes, M., Lopes Carapeto, F. C., et al. (2014). Expression of Galectins 1, 3 and 9 in normal oral epithelium, oral squamous papilloma, and oral squamous cell carcinoma. Dental Research Journal, 11(4), 508–512.
Aggarwal, S., Sharma, S. C., & Das, S. N. (2015). Galectin-1 and galectin-3: plausible tumour markers for oral squamous cell carcinoma and suitable targets for screening high-risk population. Clinica Chimica Acta, 442, 13–21.
Atkins, G. J., Haynes, D. R., Graves, S. E., Evdokiou, A., Hay, S., Bouralexis, S., et al. (2000). Expression of osteoclast differentiation signals by stromal elements of giant cell tumors. Journal of Bone and Mineral Research, 15(4), 640–649.
Baud’huin, M., Renault, R., Charrier, C., Riet, A., Moreau, A., Brion, R., et al. (2010). Interleukin-34 is expressed by giant cell tumours of bone and plays a key role in RANKL-induced osteoclastogenesis. The Journal of Pathology, 221(1), 77–86.
Skubitz, K. M., Cheng, E. Y., Clohisy, D. R., Thompson, R. C., & Skubitz, A. P. (2004). Gene expression in giant-cell tumors. The Journal of Laboratory and Clinical Medicine, 144(4), 193–200.
Smink, J. J., Tunn, P. U., & Leutz, A. (2012). Rapamycin inhibits osteoclast formation in giant cell tumor of bone through the C/EBPβ - MafB axis. Journal of Molecular Medicine (Berl), 90(1), 25–30.
Japanese Society of Medical Oncology. Comprehensive guidelines on the diagnosis and treatment of bone metastases [in Japanese] (2015).
Coleman, R. E. (2006). Clinical features of metastatic bone disease and risk of skeletal morbidity. Clinical Cancer Research, 12(20 Pt 2), 6243s–6249s.
Gralow, J. R., Sybil Biermann, J., Farooki, A., Fornier, M. N., Gagel, R. F., Kumar, R., et al. (2013). NCCN Task Force Report: bone health in cancer care. Journal of the National Comprehensive Cancer Network, 11(3), S1–S51.
Coleman, R., Body, J. J., Aapro, M., Hadji, P., Herrstedt, J., & Group., E. G. W. (2014). Bone health in cancer patients: ESMO Clinical Practice Guidelines. Annals of Oncology, 25(3), 124–137.
Fizazi, K., Carducci, M., Smith, M., Damião, R., Brown, J., Karsh, L., et al. (2011). Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet, 377(9768), 813–822.
Stopeck, A. T., Lipton, A., Body, J. J., Steger, G. G., Tonkin, K., de Boer, R. H., et al. (2010). Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. Journal of Clinical Oncology, 28(35), 5132–5139.
Scagliotti, G. V., Hirsh, V., Siena, S., Henry, D. H., Woll, P. J., Manegold, C., et al. (2012). Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. Journal of Thoracic Oncology, 7(12), 1823–1829.
Henry, D. H., Costa, L., Goldwasser, F., Hirsh, V., Hungria, V., Prausova, J., et al. (2011). Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. Journal of Clinical Oncology, 29(9), 1125–1132.
Saad, F., & Eastham, J. A. (2010). Zoledronic acid use in patients with bone metastases from renal cell carcinoma or bladder cancer. Seminars in Oncology, Suppl 1, S38–S44.
Orita, Y., Sugitani, I., Toda, K., Manabe, J., & Fujimoto, Y. (2011). Zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Thyroid, 21(1), 31–35.
Santini, D., Pantano, F., Riccardi, F., Di Costanzo, G. G., Addeo, R., Guida, F. M., et al. (2014). Natural history of malignant bone disease in hepatocellular carcinoma: final results of a multicenter bone metastasis survey. PloS One, 9(8), e105268.
Silvestris, N., Pantano, F., Ibrahim, T., Gamucci, T., De Vita, F., Di Palma, T., et al. (2013). Natural history of malignant bone disease in gastric cancer: final results of a multicenter bone metastasis survey. PloS One, 8(10), e74402.
Santini, D., Tampellini, M., Vincenzi, B., Ibrahim, T., Ortega, C., Virzi, V., et al. (2012). Natural history of bone metastasis in colorectal cancer: final results of a large Italian bone metastases study. Annals of Oncology, 23(8), 2072–2077.
Balan, V., Nangia-Makker, P., Schwartz, A. G., Jung, Y. S., Tait, L., Hogan, V., et al. (2008). Racial disparity in breast cancer and functional germ line mutation in galectin-3 (rs4644): a pilot study. Cancer Research, 68(24), 10045–10050.
Acknowledgments
The authors thank Jayne Bissonette (Barbara Ann Karmanos Cancer Institute, Wayne State University) for editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Financial support
This work was supported by NIH/National Cancer Institute R37CA46120 (A. Raz).
Rights and permissions
About this article
Cite this article
Nakajima, K., Kho, D.H., Yanagawa, T. et al. Galectin-3 in bone tumor microenvironment: a beacon for individual skeletal metastasis management. Cancer Metastasis Rev 35, 333–346 (2016). https://doi.org/10.1007/s10555-016-9622-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-016-9622-4