Abstract
Identifying patients at high risk of atrial fibrillation (AF) recurrence remains challenging. This study aimed to evaluate total atrial conduction time (TACT) and left atrial (LA) asynchrony as predictors of AF recurrence. Consecutive patients after the first AF episode, terminated either spontaneously or with cardioversion, underwent transthoracic echocardiography. TACT, estimated by the time delay between the onset of P-wave and the peak A′-wave on the Tissue Doppler Imaging (PA-TDI duration), atrial volumetric and functional parameters, and biatrial strain were assessed. We calculated mean PA-TDI—the average of PA-TDI measurements in all left atrial (LA) walls—and the difference between the longest and the shortest PA interval (DLS) and the standard deviation of 4 PA intervals (SD4) to assess the LA global remodeling and asynchrony, respectively. The primary endpoint was AF recurrence. Patients with recurrent AF had significantly prolonged PA-TDI intervals in each LA wall—and thus mean PA-TDI—than those without recurrence (mean PA-TDI: 157.4 ± 17.9 vs. 110.2 ± 7.7 ms, p < 0.001). At univariate analysis, LA maximum volume index, total LA emptying fraction, right atrial maximum volume index, PA-TDI, DLS, and SD4 were predictors of AF recurrence. At multivariable analysis, PA-TDI intervals in all LA walls remained strong predictors with mean PA-TDI (odds ratio 1.04; 95% confidence interval 1.03–1.06) having an optimal cutoff of 125.8 ms in receiver operator characteristics curve analysis providing 98% sensitivity and 100% specificity for AF recurrence (area under the curve = 0.989). PA-TDI was an independent predictor of AF recurrence and outperformed established echocardiographic parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is associated with a fivefold higher risk of stroke and impairs the patients’ quality of life significantly despite substantial progress in its management [1,2,3,4,5]. Antiarrhythmic drug therapy—apart from the probable adverse drug reactions—has modest efficacy to prevent AF recurrences, while catheter ablation shows improved efficacy in freedom from AF at the expense of multiple procedures [2, 6]. Therefore, recurrent AF after initial drug therapy or even catheter ablation procedures remains a common clinical scenario and a subject of interest and investigation.
AF causes atrial electrical and structural changes, which play an important role in the perpetuation and progression of the arrhythmia. This process, known as atrial remodeling, causes dilatation, slower and asynchronous intra-atrial conduction, and thus, AF is maintained or relapses. It is commonly, albeit partially, assessed by the left atrial (LA) size, which was identified as a predictor of new-onset and recurrent AF after catheter ablation [7,8,9].
On the contrary, indices that evaluate both the atrial electrical and mechanical changes are emerging. Total atrial conduction time (TACT) is an important electrophysiological parameter, its prolongation reflects conduction slowing and atrial dilatation [10] and identifies those prone to develop AF [11]. The PA-TDI interval, a tissue Doppler imaging (TDI) parameter, has been initially correlated with P wave duration obtained from electrocardiogram (ECG) [11] and then, with TACT measurements from electrophysiological studies in healthy subjects [12]. This parameter, along with novel PA-TDI associated indices developed to assess LA asynchrony, provide a more accurate assessment of the extent of atrial remodeling and asynchrony than conventional echocardiographic markers and thus, a greater prognostic ability of new-onset AF [11, 13] and recurrence after catheter ablation [14,15,16]. This study aimed to investigate the predictive value of PA-TDI interval and its associated LA asynchrony indices in the recurrence of AF among patients who restored sinus rhythm (SR) either spontaneously or with cardioversion after the first AF episode.
Methods
Study design and patient population
The study was approved by the Institutional Review Board of AHEPA Hospital and Aristotle University Ethics Committee and was conducted according to the current version of the Declaration of Helsinki (2013). Consecutive consented patients with restored SR [self-terminated AF, or after pharmaceutical (or electrical if failed) cardioversion] after the first recorded AF episode were enrolled between July 2014 and April 2016. Patients with a history of previous AF episodes were excluded. The remaining exclusion criteria were structural heart disease, such as significant valvular heart disease and heart failure with reduced ejection fraction, congenital heart diseases, as well as a history of cardiac surgery. One patient with poor acquisition of two-dimensional (2D) data and insufficient echocardiographic tracking of LA and right atrium (RA) was also excluded.
All patients were followed on a systematic basis as outpatients. Echocardiographic examination, ECG, and 24-h Holter monitoring were performed on each visit at 1, 6, and 12 months. All patients were encouraged to obtain an ECG recording when experiencing palpitations immediately. Antiarrhythmic drugs were continued for at least 3 months and were discontinued later at the physician’s discretion. AF recurrence was defined as any recording of AF on 12-lead resting ECG or an episode lasting longer than 30 s on 24-h Holter registration.
ECG and echocardiographic examination
Within the first 8 h of restored SR, all patients underwent a 12-lead surface ECG and a comprehensive transthoracic echocardiographic examination by an independent investigator blinded to the baseline patient characteristics. The ECG was recorded at a paper speed of 25 mm/s and 1 mV/cm standardization. The P-wave duration was calculated in all 12 leads simultaneously recorded, and the maximum P-wave duration (P maximum) in any of the 12 ECG leads was used as a marker of a prolonged atrial conduction time. The standard echocardiographic examination of 2D echocardiogram, including Doppler echocardiography, was performed using a commercially available ultrasound system (Vivid 4, General Electric Vingmed) during continuous ECG monitoring. All images were stored in a cine loop format for offline analyses (EchoPAC, General Electric Medical Systems). During the examination LA, RA, and left ventricular (LV) size and function were assessed. A detailed description of the echocardiographic examination is available in the “Appendix”.
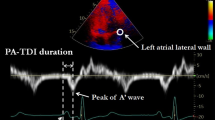
With regards to the atrial TDI, color-coded TDI-images of the LA were obtained during expiration in the apical 2-chamber, 3- and 4-chamber view, with sector size and depth optimized for the highest frame rates possible (> 115 frames/s). The region of interest was placed just above the mitral annulus in the 4-chamber view (septal and lateral wall), in the 2-chamber view (anterior and posterior wall) and the 3-chamber view (inferolateral wall), and above the tricuspid annulus in the 4-chamber view for the RA. The PA-TDI duration was assessed by measuring the time delay between the P-wave onset in lead II of the surface ECG and the peak A′-wave on the tissue Doppler tracing (Fig. 1a, b). In the 4-chamber view, PA-TDI was estimated in the LA septal and the lateral walls, and the free wall of RA. LA anterior and posterior wall PA-TDI intervals were assessed in the 2-chamber view, while the inferolateral LA wall interval was assessed in the 3-chamber view [15]. All intervals were measured in at least three cardiac cycles and averaged only in sinus beats, and P waves of different morphology were discarded. Mean PA-TDI was calculated as the average of the PA-TDI measurements in LA septal, lateral, anterior, posterior, and inferolateral walls, representing a surrogate and representative marker of the LA global remodeling indicative of changes in the electrical and mechanical activation. To assess the LA asynchrony, the difference between the longest and the shortest PA interval (DLS PA) measured at the septal, lateral, anterior, and posterior wall and the standard deviation of the abovementioned 4 PA intervals (SD4 PA) were calculated.
Examples of PA-TDI measurement. PA-TDI is defined as the time interval between the onset of electrocardiographic P wave in lead II and the top of the A′-wave on the atrial tissue Doppler velocity curve from the left atrial lateral wall. a Patient without a recurrence of AF. b Patient with recurrent AF
Statistical analysis
All variables were tested for normal distribution using means of the Kolmogorov–Smirnov test. Normally distributed data are presented as mean ± standard deviation, whereas non-normally distributed data are presented as median and interquartile range. Means were compared by Student’s t test or one-way analysis of variance (ANOVA) for normally distributed variables. Skewed variables were assessed with the Mann–Whitney or Kruskal–Wallis non-parametric tests, as appropriate. Categorical variables are presented as number (percentage) and were compared by the Chi square test. Multivariate Cox regression was used to assess and compare the impact of parameters on survival free from primary endpoint occurrence. DLS PA and SD4 PA were not included in the multivariate model due to multicollinearity.
Receiver operating characteristics (ROC) curves were generated, and the area under the curve (AUC) [and its 95% confidence interval (95% CI)] was calculated to determine the best discriminating level of mean PA-TDI for predicting recurrence of AF. We assessed the consistency of PA-TDI prolongation among three prespecified subgroups, and we also performed a sensitivity analysis in the subset of patients with self-terminated AF in order to eliminate any potential effect of the use of antiarrhythmic drugs for cardioversion on the PA-TDI interval. The significance level was set to a p value < 0.05 and two-tailed. Data analysis was performed using the IBM/Statistical Package for Social Sciences (SPSS version 24, IBM, Chicago, IL, USA) program.
Results
Patient characteristics
A total of 60 patients (mean age 67 ± 12 years, 31 male) were included (Table 1). Forty-six patients (76.7%) had palpitations by the time that visited the emergency department. Thirty-nine patients (65%) presented with paroxysmal and 21 (35%) with persistent AF. In terms of AF termination, 20 (33%) patients restored SR spontaneously, 20 (33%) with the use of antiarrhythmic drugs (16 with propafenone and 4 with amiodarone), and 20 (33%) with synchronized continuous electrical current.
At 3-year follow-up, 42 (70%) patients had at least one recurrent AF episode, while 18 (30%) maintained stable SR. Of those who relapsed, 4 (10%) had an early recurrence within the first 2 weeks. An AF recurrence occurred in 12 (60%) patients of self-terminated AF at baseline, in 12 (60%) patients from the group of pharmacological cardioversion and in 18 (90%) patients that required electrical cardioversion (HR 2.42; 95% CI 1.16 to 5.07, p = 0.02). Concerning the AF pattern, persistent AF at baseline was associated with an increased risk of recurrent AF during follow-up compared to paroxysmal AF (HR 2.51; 95% CI 1.34 to 4.69, p = 0.003).
With regards to ECG measurements, the mean P maximum was 136.2 ± 21.5 ms, and there was a significant positive linear correlation (Pearson r = 0.49; 95% CI 0.23 to 0.64, p < 0.001) between P maximum and PA-TDI duration (Fig. 2). The former was particularly increased in patients with AF recurrence compared to non-recurrent patients (141.1 ± 22.7 vs. 124.5 ±12.8 p < 0.001), and it was an independent predictor of AF recurrence (odds ratio 1.05; 95% CI 1.01 to 1.08, p = 0.01).
Left and right atrial characteristics
LA and RA echocardiographic parameters of patients with maintained SR compared to those with recurrent AF are shown in Table 2. Noteworthy, the latter patients had larger LA and RA maximum volumes (p = 0.02 and p < 0.001, respectively). On the contrary, patients with AF recurrence had reduced LA total emptying fraction (p = 0.01) and late diastole LA strain (p = 0.03), measured in the 2D speckle tracking echocardiography (Table 2).
Total atrial conduction time
Mean PA-TDI duration for the overall population was 143.0 ± 26.7 ms, particularly prolonged in patients with persistent AF compared to those with paroxysmal AF (153.7 ± 22.5 vs. 137.7 ± 27.4 ms, p=0.02). Patients with recurrent AF had significantly longer PA-TDI intervals, measured at all LA walls and free wall of RA compared to non-recurrent AF (mean PA-TDI, 157.4 ± 17.9 vs. 110.2 ± 7.7 ms, p < 0.001) (Table 3). Findings were similar in all prespecified subgroups of patients, namely patients aged > 65 years, on beta-blockers, or renin-angiotensin-aldosterone inhibitors (all p values< 0.001). Interestingly, a sensitivity analysis of patients with exclusively self-terminated AF at baseline showed very similar results between recurrent and non-recurrent AF groups (mean PA-TDI, 150.3 ± 13.6 vs. 112.4 ± 6.5 ms, p < 0.001).
Concerning the LA asynchrony, in the recurrent AF group were observed significantly higher DLS PA (30.9 ± 14.6 vs. 20.8 ± 12.4 ms, p = 0.01) and SD4 PA (14.1 ± 6.8 vs. 9.4 ± 5.6, p = 0.01) than in the non-recurrent group.
Univariate, multivariate analysis, and receiver operating characteristic curve
At univariate analysis, LA and RA maximum volume indices, LA total emptying fraction, DLS PA, SD4 PA, and PA-TDI interval in each LA wall -and thus mean- had prognostic value in AF recurrence. In contrast, at multivariable analysis, only PA-TDI intervals remained independently associated with recurrence AF-free survival in the predefined Cox regression model [mean PA-TDI adjusted odds ratio 1.04 (95% CI 1.03 to 1.06)] (Table 4). Adding the type of cardioversion and pattern of arrhythmia to the model demonstrated prognostic value for persistent AF with a marginal statistical significance (p = 0.048) and did not change the PA-TDI prognostic value in any significant way (Appendix Tables 6, 7, 8, 9, and 10).
Based on the ROC and the AUC values of the variables included in the multivariate model, the PA-TDI interval measured at each wall (including mean)—with an AUC ranging between 0.975 and 0.994—outperformed all other variables (Table 5). The optimal cutoff value of 125.8 ms for mean PA-TDI duration provided 98% sensitivity and 100% specificity for AF recurrence. The AUC of PA-TDI measured at all LA walls in the patients with paroxysmal AF at baseline are presented in Appendix (Table 11).
Discussion
We demonstrated that TACT estimated by PA-TDI duration is an independent predictor of AF recurrence in patients who restored SR after the first AF episode, irrespective of the type of cardioversion and arrhythmia pattern, while it outperforms all the established echocardiographic parameters such as LA volume. Findings were alike in a sensitivity analysis of patients with solely self-terminated AF at baseline. In addition, patients with AF recurrence showed greater LA asynchrony while patients who had maintained stable SR for three years had overall indirect markers of lesser extent of atrial remodeling and fibrosis than the patients with recurrent AF.
The prolongation of P-wave duration is thought to be an established marker of an interatrial conduction disturbance that may occur regardless of the atrial enlargement. Consequently, prolonged P-wave duration is commonly used for the prediction of AF [17]. We found that maximum P-wave duration was a predictor of AF recurrence and had a linear relationship with PA-TDI duration and, thus, a moderate association with the TDI-based detection of interatrial conduction disturbances. Those findings are in line with previous reports about the predictive value of this simple marker and support the ECG as an easily accessible and adequate tool to assess the disturbances in atrial conduction time [18, 19].
Atrial enlargement and slowing atrial conduction velocity can result in a large number of re-entrant wavelets inside the atria that favors the development and perpetuation of AF. TACT is the time elapsed between the initiation of atrial depolarization (usually in the region of the sinus node) and the last depolarization (usually in the lateral LA wall) of the same activation front and is related to the atrial dimensions and conduction speed. It is prolonged in case of increased atrial size and/or decreased conduction velocity. Therefore, theoretically, prolonged PA-TDI duration should correlate with vulnerability for AF. Contrary to LA size and ejection fraction that reflect the amount of structural change of the LA tissue caused by atrial remodeling [20], PA-TDI reflects both subclinical structural and electrical changes providing a more comprehensive estimation of the amount of atrial remodeling.
Limited studies have investigated the role of TACT in the prediction of recurrent and new-onset AF. However, our findings are in line with previous studies that underlined the high prognostic value of PA-TDI to predict AF recurrence within the first week and at 1-year after electrical cardioversion [21, 22]. On the contrary, multiple clinical and echocardiographic parameters have been identified as risk factors for recurrence after radiofrequency catheter ablation of AF [23,24,25,26,27], which either cause or reflect the presence and the extent of atrial remodeling. Noteworthy, TACT estimated with TDI had superior accuracy in predicting AF recurrence after ablation compared to the LA volume index [15]. Apart from recurrence, this predictive ability has also been proven for new-onset AF [13, 28].
Previous studies had shown that LA asynchrony, quantified by the DLS PA as measured at LA septal, lateral, anterior, and posterior wall as well as the SD4 PA, could be useful to identify patients with more advanced LA remodeling and at higher risk for AF recurrence after ablation [14, 16]. Our results are comparable with the ones previously reported and highlight the role of LA asynchrony indices and electrocardiographic markers in AF recurrence.
Our findings might impact AF management in everyday clinical practice extending the informative value of routine echocardiography of first diagnosed AF patients, as those with prolonged PA-TDI duration might be candidates for more aggressive treatment with long-term rhythm control drug therapy or catheter ablation. Moreover, in patients with embolic stroke of undetermined source (ESUS), PA-TDI duration has predicted future AF detection [29]. Therefore, ESUS patients eligible for anticoagulation may be identified based on prolonged TACT measured by PA-TDI, as the approach of anticoagulation in all ESUS patients has been failed and there is persuasive evidence that covert AF seems to be a less important ESUS etiology [30, 31].
This study has a long-term follow-up and a single-center study design, which has the advantage of integrity and homogeneity in patients’ data and laboratory values. Although we demonstrated very high accuracy of PA-TDI to predict AF recurrence, this is consistent with previous studies that reported sensitivity and specificity above 90%. To our knowledge, this is the first study that calculated mean PA-TDI as the average of PA-TDI in each LA wall. This approach could better reflect the global process of remodeling in LA and increase the accuracy of the calculated measurements, but it may not be so practical. However, PA-TDI intervals in all LA walls had similar prognostic values for AF recurrence. Some limitations of the current study should be acknowledged, and especially the relatively small sample size. Moreover, the atrial stunning post-cardioversion could have affected the baseline measurements in our cohort, so follow-up echocardiogram examinations at later time points or a non-AF control group could be useful. Therefore, our results correspond to a short post-cardioversion period and cannot be extrapolated in different time points in the progression of AF. In addition, asymptomatic episodes may have been missed, although the patients were encouraged to obtain an ECG registration when experiencing palpitations in order to confirm AF. Finally, the use of antiarrhythmic drugs for cardioversion that slow intra-atrial conduction and atrioventricular conduction may increase TACT and act as a confounding factor. However, our results remained unchanged in the sensitivity analysis of patients with self-terminated AF, consistent with previous studies that PA interval remained the best discriminator for AF patients after excluding patients pre-treated with amiodarone [16].
Conclusion
TACT is an important electrophysiological parameter which can be assessed by PA-TDI interval, a non-invasive, easy-to-use echocardiographic index. Patients with AF recurrence had prolonged PA-TDI and greater LA asynchrony. Prolonged PA-TDI outperformed the established markers for predicting AF recurrence in patients who restored SR after the first AF episode. Its potential role in predicting AF complications and thus tailored antiarrhythmic medication or catheter ablation remains to be elucidated in future studies.
Code availability
R code available upon request.
Data availability
Data available upon request
References
Conen D (2018) Epidemiology of atrial fibrillation. Eur Heart J 39(16):1323–1324. https://doi.org/10.1093/eurheartj/ehy171
Kirchhof P, Benussi S, Kotecha D et al (2016) 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37(38):2893–2962. https://doi.org/10.1093/eurheartj/ehw210
Haim M, Hoshen M, Reges O, Rabi Y, Balicer R, Leibowitz M (2015) Prospective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with incident non-valvular atrial fibrillation. J Am Heart Assoc. 4(1):1–12. https://doi.org/10.1161/JAHA.114.001486
Wolf PA, Abbott RD, Kannel WB (1991) Atrial fibrillation as an independent risk factor for stroke: the framingham study. Stroke. https://doi.org/10.1161/01.STR.22.8.983
White H, Boden-Albala B, Wang C et al (2005) Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the northern Manhattan study. Circulation 111(10):1327–1331. https://doi.org/10.1161/01.CIR.0000157736.19739.D0
Ganesan AN, Shipp NJ, Brooks AG et al (2013) Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2(2):e004549. https://doi.org/10.1161/JAHA.112.004549
Hirose T, Kawasaki M, Tanaka R et al (2012) Left atrial function assessed by speckle tracking echocardiography as a predictor of new-onset non-valvular atrial fibrillation: results from a prospective study in 580 adults. Eur Heart J Cardiovasc Imaging. 13(3):243–250. https://doi.org/10.1093/ejechocard/jer251
Blume GG, McLeod CJ, Barnes ME et al (2011) Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr 12(6):421–430. https://doi.org/10.1093/ejechocard/jeq175
Abecasis J, Dourado R, Ferreira A et al (2009) Left atrial volume calculated by multi-detector computed tomography may predict successful pulmonary vein isolation in catheter ablation of atrial fibrillation. Europace 11(10):1289–1294. https://doi.org/10.1093/europace/eup198
Weijs B, De Vos CB, Tieleman RG et al (2011) Clinical and echocardiographic correlates of intra-atrial conduction delay. Europace 13(12):1681–1687. https://doi.org/10.1093/europace/eur261
Merckx KL, De Vos CB, Palmans A et al (2005) Atrial activation time determined by transthoracic doppler tissue imaging can be used as an estimate of the total duration of atrial electrical activation. J Am Soc Echocardiogr 18(9):940–944. https://doi.org/10.1016/j.echo.2005.03.022
Erdem FH, Erdem A, Özlü F et al (2016) Electrophysiological validation of total atrial conduction time measurement by tissue doppler echocardiography according to age and sex in healthy adults. J Arrhythmia 32(2):127–132. https://doi.org/10.1016/j.joa.2015.11.006
De Vos CB, Weijs B, Crijns HJGM et al (2009) Atrial tissue Doppler imaging for prediction of new-onset atrial fibrillation. Heart 95(10):835–840. https://doi.org/10.1136/hrt.2008.148528
Löbe S, Knopp H, Le TV et al (2018) Left atrial asynchrony measured by pulsed-wave tissue doppler is associated with abnormal atrial voltage and recurrences of atrial fibrillation after catheter ablation. JACC Clin Electrophysiol 4(12):1640–1641. https://doi.org/10.1016/j.jacep.2018.08.017
Den Uijl DW, Gawrysiak M, Tops LF et al (2011) Prognostic value of total atrial conduction time estimated with tissue Doppler imaging to predict the recurrence of atrial fibrillation after radiofrequency catheter ablation. Europace 13(11):1533–1540. https://doi.org/10.1093/europace/eur186
Dinov B, Knopp H, Löbe S et al (2016) Patterns of left atrial activation and evaluation of atrial dyssynchrony in patients with atrial fibrillation and normal controls: factors beyond the left atrial dimensions. Hear Rhythm. 13(9):1829–1836. https://doi.org/10.1016/j.hrthm.2016.06.003
Dilaveris PE, Gialafos EJ, Sideris SK et al (1998) Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J 135(5):733–738. https://doi.org/10.1016/s0002-8703(98)70030-4
Hatam N, Aljalloud A, Mischke K et al (2014) Interatrial conduction disturbance in postoperative atrial fibrillation: a comparative study of P-wave dispersion and Doppler myocardial imaging in cardiac surgery. J Cardiothorac Surg. 9(1):114. https://doi.org/10.1186/1749-8090-9-114
Abou R, Leung M, Tonsbeek AM et al (2017) Effect of aging on left atrial compliance and electromechanical properties in subjects without structural heart disease. Am J Cardiol 120(1):140–147. https://doi.org/10.1016/j.amjcard.2017.03.243
Kuppahally SS, Akoum N, Burgon NS et al (2010) Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 3(3):231–239. https://doi.org/10.1161/CIRCIMAGING.109.865683
Maffè S, Paffoni P, Dellavesa P et al (2015) Prognostic value of total atrial conduction time measured with tissue doppler imaging to predict the maintenance of sinus rhythm after external electrical cardioversion of persistent atrial fibrillation. Echocardiography. 32(3):420–427. https://doi.org/10.1111/echo.12702
Müller P, Schiedat F, Bialek A et al (2014) Total atrial conduction time assessed by tissue doppler imaging (PA-TDI interval) to predict early recurrence of persistent atrial fibrillation after successful electrical cardioversion. J Cardiovasc Electrophysiol. https://doi.org/10.1111/jce.12306
Chen MS, Marrouche NF, Khaykin Y et al (2004) Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J Am Coll Cardiol 43(6):1004–1009. https://doi.org/10.1016/j.jacc.2003.09.056
Berruezo A, Tamborero D, Mont L et al (2007) Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J 28(7):836–841. https://doi.org/10.1093/eurheartj/ehm027
Schneider C, Malisius R, Krause K et al (2008) Strain rate imaging for functional quantification of the left atrium: atrial deformation predicts the maintenance of sinus rhythm after catheter ablation of atrial fibrillation. Eur Heart J 29(11):1397–1409. https://doi.org/10.1093/eurheartj/ehn168
Vasamreddy CR, Lickfett L, Jayam VK et al (2004) Predictors of recurrence following catheter ablation of atrial fibrillation using an irrigated-tip ablation catheter. J Cardiovasc Electrophysiol. https://doi.org/10.1046/j.1540-8167.2004.03538.x
Mouselimis D, Tsarouchas AS, Pagourelias ED et al (2020) Left atrial strain, intervendor variability, and atrial fibrillation recurrence after catheter ablation: a systematic review and meta-analysis. Hell J Cardiol. https://doi.org/10.1016/j.hjc.2020.04.008
Antoni ML, Bertini M, Atary JZ et al (2010) Predictive value of total atrial conduction time estimated with tissue doppler imaging for the development of new-onset atrial fibrillation after acute myocardial infarction. Am J Cardiol 106(2):198–203. https://doi.org/10.1016/j.amjcard.2010.02.030
Müller P, Ivanov V, Kara K et al (2017) Total atrial conduction time to predict occult atrial fibrillation after cryptogenic stroke. Clin Res Cardiol 106(2):113–119. https://doi.org/10.1007/s00392-016-1029-2
Ntaios G (2020) Embolic stroke of undetermined source. J Am Coll Cardiol 75(3):333. https://doi.org/10.1016/j.jacc.2019.11.024
Kishore A, Vail A, Majid A et al (2014) Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke 45(2):520–526. https://doi.org/10.1161/STROKEAHA.113.003433
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 16(3):233–271. https://doi.org/10.1093/ehjci/jev014
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical approval
Approved by the Institutional Review Board of AHEPA Hospital and Aristotle University Ethics Committee and conducted according to the current version of the Declaration of Helsinki (2013).
Informed consent
All subjects gave their informed consent prior to their inclusion in the study. All the authors gave consent to submit for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
All patients were imaged in left lateral decubitus position and the leads were placed in as possible identical positions. A 2D echocardiogram, including M-mode, Doppler (PW, CW and color) and TDI echocardiography were obtained in the parasternal short and long axis views.
LA volumes were obtained from the apical views by disc’s method and were indexed to body surface area (BSA) [32]. They were measured at three phases of cardiac cycle: 1. LA maximum volume at the end-systolic phase (just before mitral valve opening), 2. LA minimum volume at the end-diastolic phase (just before mitral valve closure) and 3. Volume before atrial active contraction, LA preA volume obtained from the last frame before mitral valve reopening or at time of the P wave on surface electrocardiography.
The LA function was assessed based on LA volumes by calculating the following equations: 1. Total atrial emptying fraction. 2. Active atrial emptying fraction: LA active ejection fraction, which is considered an index of LA active contraction. 3. Passive atrial emptying fraction, which is considered an index of LA conduit function; and 4. Atrial expansion index: LA expansion index, which is considered an index of LA reservoir function. Left ventricular ejection fraction (LVEF) was calculated from the standard apical 2- and 4-chamber views using the Simpson’s method.
Atrial Strain: The global and regional LA and RA strain was measured by 2D speckle tracking echocardiography (2DSTE). Recordings were processed with acoustic-tracking software (EchoPAC, GE Healthcare), allowing off line semi automated speckle tracking analysis. Along the LA and RA, the endocardium lines were manually traced. An additional epicardial line was generated automatically by the software, creating a region of interest (ROI). The ROI shape was manually adjusted, and the software divided the LA and RA region into six segments and generated the longitudinal strain curve. The zero strain point was set at the beginning of the P wave. Positive peak strain was measured during ventricular systole and negative peak strain during LA systole globally for each of the 12 segments. The total strain was the difference of positive peak strain and negative peak strain. Positive peak strain rate was measured during ventricular systole while early negative peak strain rate was measured during late diastole (see Tables 6, 7, 8, 9, 10, 11).
Rights and permissions
About this article
Cite this article
Karantoumanis, I., Doundoulakis, I., Zafeiropoulos, S. et al. Atrial conduction time associated predictors of recurrent atrial fibrillation. Int J Cardiovasc Imaging 37, 1267–1277 (2021). https://doi.org/10.1007/s10554-020-02113-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-02113-y