Abstract
Purpose
Recently, the estimated total atrial conduction time measured using tissue Doppler imaging (PA-TDI duration) has been reported as a more accurate predictor of atrial fibrillation (AF) recurrence after catheter ablation than left atrial volume index (LAVI). The PA-TDI duration is considered to reflect electrical and structural remodeling in the right atrium (RA) and left atrium (LA). We sought to investigate the association between AF recurrence and PA-TDI duration after AF ablation.
Methods
We studied 209 patients who underwent radiofrequency ablation for paroxysmal AF and 75 patients who underwent second ablation for AF recurrence. We assessed the duration from the onset of the P wave on the surface electrocardiogram to the atrial electrogram in distal coronary sinus (CS) (PA-CSd duration) indicating electrical remodeling of the atrium, the PA-CS proximal duration (PA-CSp duration) representing electrical remodeling of RA, and the conduction time in CS (proximal to distal) (CSp-CSd duration) reflecting electrical remodeling of LA. We also measured LAVI as a marker of structural remodeling of LA.
Results
The PA-TDI duration had a positive correlation with PA-CSd duration. In the patients with AF recurrence, PA-TDI duration, PA-CSd duration, and CSp-CSd duration in the second ablation were significantly longer than those in the first (p < 0.01, respectively), whereas there was no significant difference in LAVI and PA-CSp duration between the first and second ablation sessions.
Conclusion
A prolonged PA-TDI duration after AF ablation may indicate advanced electrical remodeling of LA, and may predict AF recurrence after ablation in patients with paroxysmal AF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Catheter ablation is an effective approach for the management of supraventricular tachycardia [1], but it has been reported that catheter ablation for paroxysmal atrial fibrillation (AF) has a higher recurrence rate than other supraventricular tachycardias [1,2,3]. It has been widely reported that the prevalence of AF increases with age and is associated with high mortality and morbidity [4]. Therefore, the number of catheter ablation procedures for paroxysmal AF has been increasing every year [5], and various studies have reported a reduction in the recurrence rate after catheter ablation for paroxysmal AF in recent years [6, 7]. It has been reported that the predictors of AF recurrence after catheter ablation are the type of AF, flow velocity in the left atrial appendage, left atrial volume (LAV), left atrial dimension (LAD), brain natriuretic peptide (BNP) level, and concomitant hypertension [8,9,10]. In particular, the left atrial (LA) size has been demonstrated to be the most important predictor of recurrence after AF ablation in a previous report [11] and to be useful for patient selection for AF ablation.
Recently, Den Uijl et al. [12] reported that total atrial conduction time measured using tissue Doppler imaging (PA-TDI duration) was a stronger predictor of AF recurrence after catheter ablation than the LA size and a marker of atrial remodeling in patients with AF. Merckx et al. [13] reported that the time interval from the onset of the P wave on electrocardiogram (ECG) to the peak A’ wave on tissue Doppler imaging (TDI) tracings of the left atrial lateral wall (PA-TDI duration) provided a good estimation of the total atrial conduction time. It has been reported that the PA-TDI duration reflects not only structural atrial remodeling but also electrical atrial remodeling [14]. Moreover, electrical remodeling has been known to appear in an earlier phase than structural remodeling after the initiation of AF [15, 16]. However, there was no significant difference in the PA-TDI duration between patients with and without AF recurrence in our previous study [17]. In addition, the PA-TDI duration obtained before ablation could not predict AF recurrence after ablation in patients with paroxysmal AF. Nevertheless, our study showed that a longer PA-TDI duration several months after catheter ablation may indicate AF recurrence after ablation for paroxysmal AF [17]. To the best of our knowledge, all relevant reports have considered the PA-TDI duration as a composite marker of the electrical remodeling and structural remodeling of the atrium. In addition, it has not been previously reported that the PA-TDI duration after AF ablation is influenced by electrical atrial remodeling and structural atrial remodeling. The purpose of this study was to investigate the association between AF recurrence after radiofrequency ablation and the time course of the PA-TDI duration after AF ablation. In addition, we investigated the impact of electrical atrial remodeling and structural atrial remodeling on the PA-TDI duration after AF ablation.
Study population
We studied 209 patients who underwent a first radiofrequency ablation (complex fractionated atrial electrogram [CFAE] ablation or CFAE ablation + pulmonary vein isolation [PVI]) for paroxysmal AF between October 2008 and October 2016. Paroxysmal AF was defined as recurrent AF (two or more episodes) that terminated spontaneously within 7 days, according to the 2012 Heart Rhythm Society Expert Consensus Statement [18].
Materials and methods
All patients underwent transthoracic echocardiography (TTE) within 1 week before and 1 day after the ablation with ultrasound systems using a sector-type probe. Each patient was scheduled for an outpatient visit at 6 months after the ablation and underwent TTE. The devices used were the Philips iE33 (Philips Healthcare, Bothell, WA, USA), Vivid E9 (GE Healthcare, Horten, Norway), and Toshiba Artida (Toshiba Medical Systems, Tokyo, Japan). During the paroxysmal AF ablation, the electrograms were collected and measured with the EP-WorkMate (St. Jude Medical Inc., St. Paul, MN, USA) and CARTO3 (Biosense Webster, CA, USA) systems.
Measurement of left atrial volume index (LAVI)
All patients underwent standard two-dimensional TTE including M-mode and Doppler echocardiography in the left lateral decubitus position. The LAV was obtained using the biplane area–length formula as follows: (0.85 × [LA area in the apical 4-chamber view] × [LA area in the apical 2-chamber view])/shortest length from the mitral annulus midplane to the superior border of the LA in the 4- and 2-chamber views. The LAVI was calculated by dividing the LAV by the body surface area of the patient.
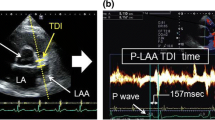
Measurement of PA-TDI duration
Using color-coded tissue Doppler imaging, the sample volume was placed on the lateral wall of the LA just above the mitral annulus in the apical 4-chamber view. The PA-TDI duration was assessed by measuring the time interval between the onset of the P wave in lead II of the surface ECG to the peak of the A’ wave on atrial tissue Doppler. The PA-TDI duration was measured over three cycles and calculated as the mean value. In addition, the PA-TDI duration was measured at a sweep speed of 100 mm/s for an accurate measurement (Fig. 1). We preliminarily assessed the reliability of the PA-TDI duration measurements. Fifteen patients were randomly selected to determine the intra-class correlation coefficients (ICC) for the PA-TDI duration measurements. For the intra-observer reliability, each measurement was performed by one examiner. For the inter-observer reliability, the first measurement was performed by one observer and the second measurement by another observer.
Measurement of PA-TDI duration using tissue Doppler imaging with transthoracic echocardiography (TTE). The sample volume was placed on the lateral wall of the left atrium (LA) just above the mitral annulus in the apical 4-chamber view. The PA-TDI duration was defined as the time interval between the onset of the P wave in lead II of the surface electrocardiogram (ECG) to the peak of the A’ wave on atrial tissue Doppler
Measurement of intra-cardiac electrogram data by electrode catheter
All patients underwent catheter ablation in the supine position. The coronary sinus (CS) was cannulated with a decapolar catheter (Dynamic XT Decapolar Steerable-Cath; C.R. Bard, Murray Hill, NJ) that was placed via the right femoral vein. The CS catheter was inserted as far distally as possible in the CS for recording the electrogram. The PA-CS distal duration was assessed by measuring the time interval between the onset of the P wave in lead II of the surface ECG and the onset of the most distal CS bipolar intra-cardiac electrogram potential before the AF ablation. The CS proximal–distal duration was obtained by subtracting the PA-CS proximal from the distal. We hypothesized that the RA electrical remodeling was indicated by the PA-CS proximal duration, the LA electrical remodeling by the CS proximal–distal duration, and the LA and RA electrical remodeling by the PA-CS distal duration (Fig. 2).
Measurement of PA-coronary sinus (CS) distal duration. The CS catheter was inserted as far distally as possible in the CS for recording with reference to the CARTO system and fluoroscopic image. The PA-CS distal duration was defined as the time interval between the onset of the P wave in lead II of the surface electrocardiogram (ECG) to the atrial wave in CS 1–2 of the intra-cardiac electrogram at a sweep speed of 200 mm/s. PA-CS proximal duration was measured with the same measurement method as that for PA-CS distal duration. The CS proximal–distal duration was obtained by subtracting the PA-CS proximal duration from the distal duration (a). A representative case of PA-TDI duration and intra-cardiac electrogram data between the first and second sessions in the recurrence group is shown (b). LA left atrial, RA right atrial, CS coronary sinus
Mapping and catheter ablation procedure
The CFAE-guided AF ablation technique described by Nademanee et al. [19, 20] was modified and utilized for this study as reported in our previous reports [21, 22]. In brief, the LA was accessed using a single transseptal puncture assisted by an intra-cardiac ultrasound catheter (AcuNav; Biosense Webster, Diamond Bar, CA). After the CS was cannulated with a decapolar catheter (Dynamic XT Decapolar Steerable-Cath; C.R. Bard, Murray Hill, NJ) for recording and induction of AF, patients underwent non-fluoroscopic electroanatomical mapping with the CARTO3 system. A 3.5-mm NaviStar ThermoCool Smart Touch catheter (Biosense Webster) was used for ablation in all patients. Heparin was administered to keep the activated clotting time > 300 s during the procedure. Electroanatomical maps were created and displayed as a shortest complex interval map on the CARTO3 system, and the CFAE areas were also identified. The CFAE parameters on the CARTO3 system used modified settings (voltage range of 0.05–0.30 mV and interval range of 40–70 ms) [23]. The primary endpoints during ablation were either the complete elimination of the CFAE areas or conversion of AF to sinus rhythm (SR) with or without an intravenous injection of nifekalant (0.3 mg/kg over 10 min, once or twice). If the atrial arrhythmias were not successfully terminated, internal cardioversion was performed.
In patients who underwent a PVI combined with a CFAE ablation, the PVI was performed during sinus rhythm after the CFAE ablation. The PVI was performed to encircle the left- and right-sided PVs in pairs 1–2 cm from their ostia, as defined by fluoroscopy and three-dimensional maps [2]. At the anterior aspect of the left PV, ablation was performed along the ridge between the left atrial appendage and PV ostia. Pulmonary vein potentials were recorded using a circular catheter (Lasso 2515 NAV Variable Decapolar catheter—20 poles, Biosense Webster) before and after ablation to confirm the success of the PVI. Radiofrequency applications were used with a maximal power output of 35 watts and irrigation rates of 30 mL/minute (3.5-mm Navistar ThermoCool SmartTouch catheter, Biosense Webster). The esophageal temperature was monitored continuously in real time throughout the procedure [24]. The power was reduced to 15 watts in the posterior LA and CS, due to the close proximity with the esophagus [25].
Definition of AF recurrence after ablation
Patients continued anticoagulation with warfarin for a minimum of 3 months. Early AF recurrence within a blanking period of 3 months after ablation was excluded from AF recurrence. Evaluation of AF recurrence started 6 months after the AF ablation. Surface ECG and 24-h Holter recording were performed routinely in all patients at each of the follow-up visits. External loop recorders were used in some patients to confirm the rhythm for any patient-reported suspicious symptoms outside of the routine follow-ups. Recurrences were based upon patient reporting, loop recordings, Holter devices, and/or surface ECG detection.
Statistical analysis
First, the data obtained before the ablation were examined between the recurrence and no-recurrence groups. Second, all data mentioned in Table 1 were included in the univariable analysis. A multicollinearity analysis using Spearman’s correlation test (r > 0.9) was performed to identify the collinearity between the variables before multivariate logistic regression analysis. Data with a p value < 0.05 in the univariate analysis were included in the multivariate logistic regression analysis. A multivariate analysis was performed using a backward stepwise conditional approach. The receiver operator characteristics (ROC) curve was calculated to evaluate the performance of the possible independent predictors of AF recurrence after catheter ablation obtained by multivariate analysis. The time-dependent changes in PA-TDI duration and LAVI were observed to investigate the predictors of AF recurrence after AF ablation. The data are presented as the mean ± standard deviation or median (interquartile range). All data were tested for a normal distribution by means of the Kolmogorov–Smirnov test. We used the Student’s t test, Welch’s t test, or Wilcoxon signed-rank test to analyze the data. Categorical data were evaluated using the χ2 test. A p < 0.05 was considered statistically significant. All statistical analyses were performed with IBM SPSS software (version 24 for Windows; IBM Corp., Armonk, NY, USA).
Results
Outcomes of AF ablation
Our study included 209 patients with paroxysmal AF who underwent AF ablation (CFAE ablation or CFAE ablation + PVI) for the first time. After the first ablation, 105 patients (50.2%) maintained sinus rhythm and 104 (49.8%) experienced AF recurrence. The mean follow-up period after the first AF ablation was 49.1 ± 32.8 months. We explored independent predictors of AF recurrence after ablation, except for in patients with AF during TTE and the first ablation procedure (recurrence group [n = 78], no-recurrence group [n = 66]). In addition, we compared PA-TDI duration and LAVI between those before and 6 months after the AF ablation, except for in patients with AF during TTE (recurrence group [n = 70], no-recurrence group [n = 73]).
Among the recurrence group, 27 patients continued medications, two underwent AF ablation at another hospital, and the other 75 underwent re-ablation (CFAE ablation or CFAE ablation + PVI or CTI ablation or PVI). We compared the LAVI, PA-TDI duration, and intra-cardiac electrogram data during the first ablation with those during the second ablation (n = 32) (Fig. 3).
We studied 209 patients who underwent atrial fibrillation (AF) ablation in the first session for paroxysmal AF. First, we explored independent predictors of AF recurrence after ablation. Second, we observed PA-TDI duration and LAVI 6 months after the first ablation and divided patients into the recurrence and no-recurrence groups. Finally, we compared the PA-TDI duration, LAVI, and intra-cardiac electrogram data between the first and second ablation sessions in the recurrence group
In the first ablation session, both the PA-TDI duration and intra-cardiac electrogram data could be measured in 144 of 209 (68.9%) patients. In the second ablation session, both the PA-TDI duration and intra-cardiac electrogram data could be measured in 36 of 75 (48.0%) patients. We investigated the association of PA-TDI duration and intra-cardiac electrogram data between the first and second ablation sessions (Fig. 3).
Investigation of predictors of AF recurrence after radiofrequency ablation
The patient characteristics in the no-recurrence group and recurrence group are shown in Table 1. The LAV and LAVI were significantly larger in the recurrence group than in the no-recurrence group (LAV: 64.8 ± 18.4 ml vs. 58.4 ± 18.3 ml, p = 0.021; LAVI: 36.7 ± 10.0 ml/m2 vs. 33.6 ± 10.1 ml/m2, p = 0.038). There was no significant difference in the other variables between the two groups. A multicollinearity analysis using Spearman’s correlation test was performed to identify the collinearity between the variables before the multivariate logistic regression analysis. We found that the “LAV” and “LAVI” were highly correlated, as indicated by Spearman’s correlation test (r > 0.9). Based on these findings, we excluded the LAV from the multivariate logistic regression analysis.
The univariate and multivariate logistic regression analyses were performed to identify the predictors of AF recurrence after radiofrequency ablation (Table 2). In the multivariate logistic regression analysis, the LAVI and LVEF were suggested to be independent predictors of AF recurrence after catheter ablation (LAVI: OR 1.039, 95% CI 1.003–1.075, p = 0.031; LVEF: OR 1.051, 95% CI 1.004–1.100, p = 0.032). However, the ROC curve analyses demonstrated that the LAVI and LVEF had a low accuracy of predicting an AF recurrence after radiofrequency ablation (LAVI: area under the curve (AUC) 0.601, 95% CI 0.507–0.695, p = 0.038; LVEF: AUC 0.579, 95% CI 0.485–0.673, p = 0.104) (Fig. 4).
Comparison of PA-TDI duration and LAVI in the first session between that before and 6 months after AF ablation
There was no significant difference in PA-TDI duration between that before and 6 months after ablation in the no-recurrence group (144 [121–156] ms vs. 133 [121–148] ms, p = 0.056). In the recurrence group, the PA-TDI duration 6 months after ablation was significantly longer than that before ablation (140 [125–153] ms vs. 154 [138–170] ms, p < 0.01) (Fig. 5). However, there was no significant difference in LAVI between that before and 6 months after ablation in both the recurrence and no-recurrence groups (recurrence group: 34.8 [30.4–42.7] ml/m2 vs. 35.7 [28.7–40.8] ml/m2, p = 0.545; no-recurrence group: 31.8 [25.8–37.7] ml/m2 vs. 32.8 [26.1–37.6] ml/m2, p = 0.768) (Fig. 5).
Relationship between PA-TDI duration and electrical atrial remodeling and structural atrial remodeling
The PA-TDI duration was significantly longer than the PA-CS distal duration in the first and second ablation sessions (first ablation: 140 [122–155] ms vs. 83 [76–93] ms, p < 0.01; second ablation: 153 [140–166] ms vs. 90 [81–97] ms, p < 0.01). The PA-TDI duration had a strong positive correlation with the PA-CS distal duration in the first and second ablation sessions (first ablation: R = 0.513, p < 0.01; second ablation: R = 0.617, p < 0.01).
In addition, the PA-TDI duration also had a weak positive correlation with the PA-CS proximal duration (first ablation: R = 0.350, p < 0.01; second ablation: R = 0.369, p = 0.027), CS proximal–distal duration (first ablation: R = 0.258, p < 0.01; second ablation: R = 0.452, p < 0.01), and LAVI (first ablation: R = 0.410, p < 0.01; second ablation: R = 0.385, p = 0.02). However, the PA-TDI duration had no correlation with RAVI in the first and second ablation sessions (first ablation: R = 0.102, p = 0.224; second ablation: R = 0.042, p = 0.809) (Fig. 6).
This shows the relationship between the preprocedural PA-TDI duration, PA-coronary sinus (CS) distal duration (a, f), PA-CS proximal duration (b, g), CS proximal distal duration (c, h), RAVI (d, i), and LAVI (e, j) in the first and second AF ablation sessions. CS coronary sinus, RAVI right atrial volume index, LAVI left atrial volume index
Comparison of PA-TDI duration, RAVI, LAVI, and intra-cardiac electrogram data between the first and second ablation sessions in the recurrence group
In the second ablation session, the PA-CS distal duration and CS proximal–distal duration were significantly longer than in the first ablation session (PA-CS distal duration: 83 [76–90] ms vs. 90 [81–96] ms, p < 0.01; CS proximal distal duration: 32 [22–35] ms vs. 38 [30–42] ms, p < 0.01). However, there was no significant difference in PA-CS proximal duration between the first and second ablation sessions (54 [46–62] ms vs. 53 [46–60] ms, p = 0.596) (Fig. 7).
In the second ablation session, the PA-TDI duration was significantly longer than in the first session (140 [119–150] ms vs. 153 [140–166] ms, p < 0.01). However, there were no significant differences in RAVI and LAVI between the first and second ablation sessions (RAVI: 18.1 [15.4–22.2] ml/m2 vs. 18.8 [12.7–25.6] ml/m2, p = 0.926; LAVI: 34.9 [30.7–43.0] ml/m2 vs. 33.3 [28.5–41.2] ml/m2, p = 0.746) (Fig. 7).
Evaluation of intra- and inter-observer reliability
Fifteen patients were randomly selected to determine the intra-class ICC for the PA-TDI duration measurements. The ICC (2,1) for the intra-observer reliability of PA-TDI duration was 0.974 (95% CI 0.851–0.993). The ICC (1,2) for the inter-observer reliability of PA-TDI duration was 0.918 (95% CI 0.763–0.972) and 0.932 (95% CI 0.804–0.977). The intra- and inter-observer reliabilities were excellent for PA-TDI durations with ICCs > 0.9.
Discussion
Comparison of patient characteristics before AF ablation between the recurrence and no-recurrence groups
In the univariate and multivariate logistic regression analyses, LAVI was suggested to be an independent predictor of AF recurrence after catheter ablation in this study. However, the ROC curve analysis demonstrated that LAVI had a low accuracy of predicting AF recurrence after catheter ablation. In addition, there was no significant difference in PA-TDI duration between the recurrence and no-recurrence groups in this study. In our study, PA-TDI duration also had a weak positive correlation with LAVI (R = 0.410, p < 0.01). Furthermore, we investigated PA-TDI duration and LA function. PA-TDI duration had a weak negative correlation with septal tissue Doppler imaging atrial filling velocity (septal TDI A) (R = − 0,217, p = 0.009). However, we could not find a significant correlation between other factors and PA-TDI duration (transmitral flow velocity pattern atrial filling velocity [TMF A]: R = − 0.037, p = 0.660; lateral TDI A: R = − 0.062, p = 0.464) (data not shown). Despite all subjects in this study having paroxysmal AF, more than half of the patients (n = 74, 51.4%) had a larger LA size than those with a moderate LAVI (≧34 ml/m2). Based on these findings, we could suggest that prolongation of the PA-TDI duration before AF ablation did not significantly differ between the recurrence and no-recurrence groups. In our study, the pre-ablation data including PA-TDI duration and LAVI could not accurately predict AF recurrence after radiofrequency ablation in paroxysmal AF patients with relatively large LA.
Relationship between electrical and structural atrial remodeling and AF recurrence after radiofrequency ablation
PA-TDI duration has been reported to provide an estimation of the total atrial conduction time and has been proposed as a marker of atrial remodeling. In addition, it was also demonstrated that PA-TDI duration reflected both atrial electrical remodeling and atrial structural remodeling, and that LAVI reflected the extent of the atrial structural remodeling in the LA [12]. In our study, the PA-TDI duration at 6 months after ablation in the recurrence group was significantly longer than that before ablation. However, there was no significant difference in LAVI in the recurrence group between that before and 6 months after ablation. Therefore, despite the fact that PA-TDI duration and LAVI before ablation had a weak positive correlation, PA-TDI duration and LAVI 6 months after ablation did not show a positive correlation in our study.
In the recurrence group, PA-TDI duration, PA-CS distal duration, and CS proximal–distal duration in the patients who underwent a second ablation were significantly longer in the second ablation session than in the first session. However, there was no significant difference in PA-CS proximal duration, RAVI, and LAVI between the first and second ablation sessions (Fig. 7). Our data indicated that the PA-TDI duration before ablation could not predict AF recurrence after radiofrequency ablation in paroxysmal AF patients with relatively large LA, but a longer PA-TDI duration at both 6 months after the first ablation and second ablation sessions may have been caused by AF recurrence after AF ablation.
In addition, PA-TDI duration had a strong positive correlation with PA-CS distal duration in the first and second ablation sessions. Our data were consistent with those in the previous reports showing that PA-TDI duration reflected both atrial electrical remodeling and atrial structural remodeling in the LA [13, 14]. During the first and second ablation sessions, PA-TDI duration had a positive correlation with PA-CS distal duration, PA-CS proximal duration, and CS proximal–distal duration, indicating electrical remodeling of the LA and RA. From these findings, we could suggest that the PA-TDI duration may reflect atrial electrical remodeling rather than atrial structural remodeling. These data suggested that the estimated total atrial conduction time, especially the left atrial conduction time, may be associated with an increased recurrence of AF after radiofrequency ablation. The results of this study seem to support the findings of de Vos et al. [26], i.e., that prolonged PA-TDI duration is associated with development of new-onset AF.
Furthermore, it was suggested that PA-TDI duration, which can reflect prolongation of the left atrial conduction time, may be related to AF recurrence after catheter ablation, and may become a possible indicator for deciding on a treatment strategy after AF ablation.
Study limitations
Our study had several limitations. First, this was a single-center study and the rate of AF recurrence was relatively high. Second, PA-TDI duration, PA-CS distal duration, PA-CS proximal duration, and CS proximal–distal duration could be assessed only during sinus rhythm. Therefore, there were some patients in whom we could not measure those parameters during follow-up or pre-ablation. In addition, we could not find any previous reports about validation studies of the correlation of each invasive parameter from intra-cardiac atrial mapping with both LA and RA electrical activation time. Therefore, our study may not be adequately validated in comparison to previous studies. Third, detection of AF recurrence after ablation was based on surface ECG recordings, 24-h Holter ECG recordings, and external loop recorders in some patients with any suspicious symptoms. Nevertheless, asymptomatic episodes of arrhythmias may have been missed.
Conclusion
The PA-TDI duration reflecting the estimated left atrial conduction time pre-ablation could not accurately predict AF recurrence after radiofrequency ablation in paroxysmal AF patients with relatively large LA. However, a longer PA-TDI duration at 6 months after AF ablation may be related to AF recurrence after radiofrequency ablation for paroxysmal AF.
References
Kay GN, Epstein AE, Dailey SMPV. Role of radiofrequency ablation in the management of supraventricular arrhythmias: experience in 760 consecutive patients. J Cardiovasc Electrophysiol. 1993;4:371–89.
Oral H, Scharf C, Chugh A, et al. Catheter ablation for paroxysmal atrial fibrillation:segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355–60.
Nanthakumar K, Plumb VJ, Epstein AE, et al. Resumption of electrical conduction in previously isolated pulmonary veins: rationale for a different strategy? Circulation. 2004;109:1226–9.
Inoue H, Fujiki A, Origasa H, et al. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. 2009;137:102–7.
Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005;111:1100–5.
Shurrab M, Di Biase L, Briceno DF, et al. Impact of contact force technology on atrial fibrillation ablation: a meta-analysis. J Am Heart Assoc. 2015;4:1–9.
Li J, Jiang J, Zhong G, Ke H, He Y. Comparison of empiric isolation and conventional isolation of superior vena cava in addition to pulmonary vein isolation on the outcome of paroxysmal atrial fibrillation ablation. Int Heart J. 2017;58:500–5.
Berruezo A, Tamborero D, Mont L, et al. Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J. 2007;28:836–41.
Hof I, Chilukuri K, Arbab-Zadeh A, et al. Does left atrial volume and pulmonary venous anatomy predict the outcome of catheter ablation of atrial fibrillation? J Cardiovasc Electrophysiol. 2009;20:1005–10.
Hussein AA, Saliba WI, Martin DO, et al. Plasma B-type natriuretic peptide levels and recurrent arrhythmia after successful ablation of lone atrial fibrillation. Circulation. 2011;123:2077–82.
Abecasis J, Dourado R, Ferreira A, et al. Left atrial volume calculated by multi-detector computed tomography may predict successful pulmonary vein isolation in catheter ablation of atrial fibrillation. Europace. 2009;11:1289–94.
Den Uijl DW, Gawrysiak M, Tops LF, et al. Prognostic value of total atrial conduction time estimated with tissue doppler imaging to predict the recurrence of atrial fibrillation after radiofrequency catheter ablation. Europace. 2011;13:1533–40.
Merckx KL, De Vos CB, Palmans A, et al. Atrial activation time determined by transthoracic doppler tissue imaging can be used as an estimate of the total duration of atrial electrical activation. J Am Soc Echocardiogr. 2005;18:940–4.
Ejima K, Kato K, Arai K, et al. Impact of atrial remodeling on the outcome of radiofrequency catheter ablation of paroxysmal atrial fibrillation. Circ J. 2014;78:872–7.
Ausma J, Van der Velden HMW, Lenders MH, et al. Reverse structural and gap-junctional remodeling after prolonged atrial fibrillation in the goat. Circulation. 2003;107:2051–8.
Teh AW, Kistler PM, Lee G, et al. Electroanatomic remodeling of the left atrium in paroxysmal and persistent atrial fibrillation patients without structural heart disease. J Cardiovasc Electrophysiol. 2012;23:232–8.
Maenosono R, Mizukami N, Oketani N, et al. Relationship between total atrial conduction time and recurrence of atrial fibrillation after complex fractionated atrial electrogram ablation of paroxysmal atrial fibrillation. J Soc Sonogr. 2017;42:399–409.
Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171–257.
Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–53.
Nademanee K, Schwab MC, Kosar EM, et al. Clinical outcomes of catheter substrate ablation for high-risk patients with atrial fibrillation. J Am Coll Cardiol. 2008;51:843–9.
Iriki Y, Ishida S, Oketani N, et al. Relationship between clinical outcomes and unintentional pulmonary vein isolation during substrate ablation of atrial fibrillation guided solely by complex fractionated atrial electrogram mapping. J Cardiol. 2011;58:278–86.
Ichiki H, Oketani N, Ishida S, et al. Incidence of asymptomatic cerebral microthromboembolism after atrial fibrillation ablation guided by complex fractionated atrial electrogram. J Cardiovasc Electrophysiol. 2012;23:567–73.
Namino F, Iriki Y, Maenosono R, et al. The optimal setting of complex fractionated atrial electrogram software in substrate ablation for atrial fibrillation. J Arrhythmia. 2015;31:6–11.
Singh SM, d’Avila A, Doshi SK, et al. Esophageal injury and temperature monitoring during atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2008;1:162–8.
Maenosono R, Oketani N, Ishida S, et al. Effectiveness of esophagus detection by three-dimensional electroanatomical mapping to avoid esophageal injury during ablation of atrial fibrillation. J Cardiol. 2012;60:119–25.
De Vos CB, Weijs B, Crijns HJGM, et al. Atrial tissue Doppler imaging for prediction of new-onset atrial fibrillation. Heart. 2009;95:835–40.
Acknowledgements
We thank the staff of the ultrasound examination center and cardiac catheterization room at Kagoshima University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the following: Grant-in-Aid for Encouragement of Scientists (19H00450) (R.M) and Grant-in-Aid for Early-Career Scientists (19K17608) (F.N).
Ethical statements
The study protocol was approved by the institutional ethics committee of Kagoshima University Hospital. All patients provided written informed consent for the procedure.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Maenosono, R., Mizukami, N., Ichiki, H. et al. Total atrial conduction time as a possible predictor of atrial fibrillation recurrence after catheter ablation for paroxysmal atrial fibrillation: relationship between electrical atrial remodeling and structural atrial remodeling time courses. J Med Ultrasonics 48, 295–306 (2021). https://doi.org/10.1007/s10396-021-01090-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-021-01090-6