Abstract
Strenuous exercise results in transient but minor alterations in left ventricular diastolic function (LVDF). The aim of this study is to describe and interpret the kinetics of the well-established 2D parameters of diastolic function and the novel and very sensitive 3D parameters before/after a marathon race. LVDF was evaluated by transthoracic echocardiography (TEE) in 212 healthy male [aged 42 (36–49) years)] marathon runners (all Be-MaGIC-study) in the week prior to (V1), immediately after (V2), 24 h after (V3) and 72 h after (V4) a marathon race. Real time three-dimensional echocardiography (RT3DE) included maximal and minimal left atrium (LA) volume, total LA ejection fraction (Total-EF), total LA stroke volume (Total-SV), true ejection fraction (True-EF) and atrial stroke volume (ASV). After adjustment for possible confounders (heart rate and systolic blood pressure), 2D Parameters of left ventricular inflow (E/A-ratio) decreased from pre- to immediately post-race (− 0.3 ± 0.06, p < 0.001) and returned to baseline within 24 h. \({\text{E}}/{{\text{e}^{\prime}}}_{{{\text{mean}}}}\)-ratio remained unchanged directly post-race, but was significantly increased during follow-up of 24 and 72 h. 3D LA Vmin was increased immediately postrace and in the 24 h follow-up, LA Vmax was increased immediately post-race and in the follow-up of 24 and 72 h. During follow-up of 72 h, but not immediately postrace, TrueEF and ASV were significantly increased. Both techniques revealed acute and prolonged alterations of diastolic LV function. Considering all parameters, the recovery of diastolic LV after a marathon seems to take longer than previously assumed.
Trial registration ClinicalTrials.gov ID: NCT00933218
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prolonged exercise seems to alter diastolic function [1]. Whether these changes are predominantly triggered by altered left ventricular preload after the race is still under discussion [2]. Those prior studies used left ventricular inflow, TDI-derived measures and two-dimensional atrial volumes for assessment of diastolic function. More recent observations included 3D right and left atrial volumes; they were found to be directly affected by exercise, with a decrease in volumes during exercise and long-term increase in athletes compares to non-athletes [3]. The long-term dilatation of the left atrium is assumed to be a risk factor for atrial fibrillation in athletes [4] and for possible sudden cardiac death [5, 6].
Over the past years, new methods have been developed to measure even early stages of diastolic dysfunction. The American Society of Echocardiography and the European Association of Cardiovascular Imaging most recently recommended a cut-off of 14 for the average E/e′-ratio and advised measuring the atrial filling pressure in the first step in order to diagnose diastolic dysfunction in patients with preserved ejection fraction [7]. If only septal or lateral values are available, slightly different cutoffs apply (lateral 13 and septal 15). The E/e′ ratio as well as septal and lateral e′ are beside LA volume index (cutoff 34 mL/m2) and peak tricuspid regurgitation (TR) velocity (cutoff 2.8 m/s) the key point variables in diagnosing diastolic dysfunction [7].
Aside from left ventricular diastolic function, also systolic function has been assessed after several (ultra-) marathons. The evidence to date is controversial whether a significant reduction in systolic function can be induced by these extreme forms of endurance exercise [8,9,10].
In our study, we determined diastolic function after a marathon race for the first time both with the conventional and well-established methods of measurement (Doppler-measures of left ventricular inflow and tissue Doppler) and the more recent but validated parameters of three-dimensionally determined left atrial volume and function in marathon runners (with and without adjustment for heart rate and systolic blood pressure) [11]. We hypothesized that using this newly available technique we will be able to evaluate more sensitively the underlying mechanisms resulting in exercise-induced changes of diastolic function which were not detectable using conventional methods.
Materials and methods
The investigational protocol was approved by the ethics committee of the University Hospital Klinikum rechts der Isar, Munich, Germany (approval reference number 2384/09; ClinicalTrials.gov Id: NCT00933218). A total of 277 participants, all healthy males aged between 20 and 60 years, were included in the Be-MaGIC trial according to exclusion criteria as previously described in detail [12]. All participants gave written informed consent before enrollement into the study.
Prior publications of data derived from the Be-MaGIC trial included examinations about retinal microcirculation [13], inflammation [14] and respiratory tract illness [12], stress-induced platelet activation [15], kinetics of high sensitive Troponin T (hsTropT) [16], exercise-induced immune-modulation [17], impact of polyphenols on physiological stress and cardiac burden [18], and changes of intima-media thickness [19].
The echocardiographic data described within this manuscript were collected at the four following time points: one in the week pre-race (V1), the next within 1 h post-race (V2), and at follow-up 24 h (V3) and 72 h (V4) post-race.
For all echocardiographic examinations, patients were positioned in the left lateral decubitus position and examined by experienced echocardiographers in accordance to current recommendations (using Philips iE33 xMATRIX echocardiography system, Philips Medical Imaging, Hamburg, Germany). A transthoracic broadband S5-1 transducer (frequency transmitted 1.7 MHz, received 3.4 MHz, Philips Medical Imaging, Hamburg, Germany) was used for the two-dimensional echocardiography.
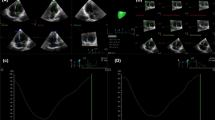
We evaluated the Doppler-derived indices of left ventricular inflow, Pulsed Wave Doppler (PWD) and Tissue Doppler Imaging (TDI), from the apical 4-chamber view in accordance to current recommendation [20] and as previously described in detail [21]. Thus, we obtained peak early (E) and late (or atrial, A) flow velocities, E/A-ratio and the deceleration time (DT) of the E-wave velocity. The TDI-derived measures included early (eʹ) and late (or atrial aʹ) diastolic mitral annular velocities. \({\text{E}}/{{\text{e}^{\prime}}}_{{{\text{med}}}}\), \({\text{E}}/{{\text{e}^{\prime}}}_{{{\text{lat}}}}\) and \({\text{E}}/{{\text{e}^{\prime}}}_{{{\text{mean}}}}\) were subsequently calculated (Fig. 1).
For the real-time three-dimensional echocardiography (RT3DE) we used a 3 to 1 MHz transthoracic matrix array transducer (X3-1, Philips Medical Imaging, Hamburg, Germany). One complete data set was constructed by the assembly of 7 R-wave-triggered sub-volumes from seven cardiac cycles during an end-expiratory breath-hold. The analysis of the 3-dimensional images was made off-line using a QLAB working station (Philips Healthcare, Hamburg, Germany). In order to cover the complete diastolic period, we used echocardiography recording with a 300 ms delay with respect to the QRS complex in simultaneously recorded ECG. Three-dimensional LA volumes were assessed by RT3DE software (4D LA Function, TomTec, Munich, Germany) as previously described [21]. Shortly, maximal volume Vmax was measured at ventricular end-systole and minimal volume Vmin at ventricular end-diastole by semiautomatic tracing of the endocardial surface in different planes. A third marker (preA) was manually set in order to subdivide the diastole in early diastolic filling E and atrial contraction A. It was assigned to the moment of the second opening of the mitral valve during left ventricular filling, defining VpreA. Atrial stroke volume ASV was calculated as VpreA − Vmin. Total Stroke Volume (Total-SV) was calculated as Vmax − Vmin, Total-EF as Total-SV/Vmax × 100%. True-EF was calculated as ASV/Vmax × 100% (Fig. 2).
The atrial volumes were indexed to the Body Surface Area (BSA), using the Mosteller formula: BSA (m2) = ([height(cm) × weight(kg)]/3600)½.
Analysis of systolic function of the left ventricle was performed offline with the novel software tool TomTec 4D LV-Analysis 3.1 (TomTec Imaging Systems, Munich, Germany) with analysis of the ejection fraction (EF) as previously described [22].
Blood samples
Fasting blood samples were taken from an antecubital vein with subjects in supine position at visit 1, 2, 3 and 4. Only the blood collection immediately after the race was not in a fasted state.
High-sensitive troponin T (hs-cTropT) was measured quantitatively with the new high-sensitive enzyme immunoassay based on electrochemiluminescence technology using the cobas e 411 analyzer (Roche Diagnostics, Penzberg, Germany) as previously described [16].
NT-proBNP was measured quantitatively with the enhanced electrochemiluminescence immunoassay system (ECLIA) method on a cobas e 411 analyser (Roche Diagnostics, Penzberg, Germany). The measuring range of this assay is 5–35,000 ng/l.
Statistical analysis
For statistical analysis, only complete data sets were considered. We used PASW Statistics 18.0.2 (SPSS Inc., Chicago IL, USA). The descriptive results were presented as mean and standard deviation if normally distributed. For non-normally distributed data, the median and interquartile range (25th/75th percentile) were reported. Shapiro–Wilk test was applied to differentiate between normally and non-normally distributed data.
To evaluate changes over the study period, we applied ANCOVA models for each parameter. In a first analysis, we analyzed the data without interfering covariates. In a second analysis, we applied selected confounders with possible influence on diastolic parameters. Arterial hypertension can lead to impaired diastolic function with especially LAmin being directly imposed to LV filling pressure [23, 24]. Diastolic function is strongly associated with the heart rate [25, 26].
A p-value < 0.05 was considered statistically significant; testing was performed two-sided.
Results
A total of 277 subjects were enrolled at the time of the screening examination. In the follow-up examinations of this echocardiographic investigation, 12 participants were excluded due to missed examinations. Furthermore 15 subjects did not finish the marathon race, 28 could not start the marathon because of infections, orthopedic injuries or other obligations. Due to insufficient image quality, analyses of ten additional subjects had to be excluded. For two-dimensional parameters, 212 participants remained for final analysis. Concerning the three-dimensional echocardiography, 61 participants could be included in the final analysis after excluding participants with insufficient or incomplete data sets. Baseline characteristics of the 212 participants are presented in Table 1.
Diastolic left ventricular function 2-dimensional
Table 2 presents changes of two-dimensional echocardiography and the comparison of visit 2, 3 and 4 to visit 1.
Directly after the marathon, E/A decreased significantly whereas DT increased significantly. In the follow-up examinations 24 and 72 h post-race both parameters returned to baseline whereas E/eʹ showed a significant increase with a maximum at 72 h post-race.
Parameters of 3-dimensional measurements of atrial function and size
Table 3 shows the descriptive results for three-dimensional echocardiography and the changes of visit 2, 3 and 4 compared to visit 1without and with adjustment.
The atrial volumes measured by RT3DE were significantly altered after the marathon. In a first analysis without adjustment, we observed smaller atrial volumes and a decline of the Total-EF, the Total-SV and the ASV immediately after the marathon race. During follow-up, Vmax, Total-SV and ASV showed increased values. After adjustment for heart rate and blood pressure, significant changes were still observable with an increase of Vmin immediately post-race and a decrease of Total-EF. During follow-up, the atrial volumes, Total-SV and ASV were increased.
Left ventricular systolic function, represented by 3-dimensionally measured EF, showed non-significant changes throughout the study period when adjusted to heart rate and blood pressure: 60.1 ± 6.3 at V1 to 58.9 ± 5.6 at V2, 60.1 ± 6.5 at V3 and 59.8 ± 6.1 at V4.
Figure 3 illustrates all 2D and 3D diastolic parameters in accordance to the visits 1–4.
Valve regurgitations and laboratory results
No clinically relevant valve regurgitations (especially regarding mitral valve) were observable neither at baseline examination nor in the follow-up examinations.
Regarding the laboratory analyses, there were no relevant associations between diastolic or systolic function and NT-proBNP or hsTropT values.
Discussion
In this study, we evaluated for the first time the diastolic left ventricular function with RT3D echocardiography parameters in healthy male runners before and after a marathon race. We were able to demonstrate that the 2D parameters displayed different alterations compared to 3D parameters and that at the end of our 72 h follow-up, signs for diastolic dysfunction were still observable within the measurement of both techniques. Left ventricular systolic function remained unchanged throughout the marathon.
Until today, it is discussed equivocally whether there is a real impairment of diastolic function after strenuous exercise or whether the observed alterations are mainly due to changes of cardiac loading conditions (pre- or afterload) or limitations of diagnostic modalities. With our design, we were able to address both considerations. Left atrial volume index can be measured using two-dimensional echocardiography via cubical method, area-length method, ellipsoid method or modified Simpson’s method [27, 28]. But especially due to the asymmetry of the left atrium, this method may provide incorrect data. Recent studies suggest three-dimensional evaluation of the left atrium as a more precise alternative [21, 21, 29,30,31]. For our analyses, both 2D and 3D techniques were combined to minimize limitations of previous studies.
Both systolic and even more diastolic duration vary with different heart rates. The diastolic proportion in the cardiac cycle is inversely correlated with subsequent heart rates [26]. Decreased diastolic proportion leads to decreased amount of time for the ventricular relaxation. As a result, the atrial contribution to LV filling gains significance and can result in increased A values compared to E values [32]. Left atrial enlargement and impaired diastolic function can be caused by increased blood pressure [33, 34]. We therefore included the described possible confounders (heart rate and systolic blood pressure) in our analysis. Regarding Doppler techniques, we observed two different alterations immediately after the race. The E/A ratio changed immediately after the marathon indicating an impaired diastolic function, whereas the E/eʹ-ratio was altered only during the latter follow-up.
Our results of the two-dimensional parameters were similar compared to prior studies [35, 36]. PWD indices (E/A and DT) were significantly altered after strenuous exercise. The TDI-derived parameters (E/e’) were also affected, but the time of the first alteration was different compared to PWD measurements. PWD parameters were altered immediately after the race. E/A-ratio decreased from 1.6 ± 0.5 to 1.1 ± 0.3 and DT increased from 0.18 ± 0.05 to 0.20 ± 0.05 s, both indicating an impairment of the diastolic function. They returned to baseline values at follow-up (24 h post-race). In contrast, E/eʹ-ratio remained unchanged immediately after the race and revealed first alterations at follow-up 24 h later. It was still significantly altered 72 h later.
E/A and DT reflect more sensitively ventricular relaxation, while E/eʹ reflects the ventricular filling pressure or atrial pressure [36, 37]. An impairment of diastolic function can be caused by either reduced ventricular relaxation or changes of the atrial pressure [38]. As E/eʹ remained unchanged after marathon, ventricular relaxation seemed to be the main reason for diastolic changes, as indicated by a decreased E/A-ratio and increased DT.
The results of our 3D analyses supported this theory and offered a more profound insight into exercise-induced changes of left ventricular diastolic and left atrial function. Immediately after the marathon race, left atrial Vmin increased and the Total-SV consecutively decreased. This observation could only be found when adjusting for heart rate and blood pressure, before the adjustment, Vmin was decreased. The reason for this observation could be the impaired ventricular relaxation as it was also displayed by the changes of the E/A-ratio and the DT. The higher values of Vmin resulted in a volume und pressure increase in the LA. In the follow-up examinations we could illustrate the result: atrial volumes Vmin and Vmax were increased along with the consecutively increased Total-SV and ASV. This was also shown in the 2D measurements by the increased E/eʹ ratio. A possible explanation for this prolonged alteration is the fact that the LA is not habituated to increased pressure, so it takes longer to achieve a complete recovery compared to the left ventricle. Even 24 h post-stress, the LA still seemed to be impaired by an increased pressure, as represented by E/eʹ, Vmax (and consecutively Total-SV and ASV).
Recent studies investigated the validity of measuring diastolic function by three-dimensional echocardiography [21, 30, 31, 39]. Although the method is still novel, the measured volumes seem to correlate well with current gold standard magnetic resonance imaging (MRI) or computed tomography (CT) scans [29, 39].
Regarding the laboratory measurements, biomarkers of cardiac damage (hsTropT and NT-proBNP) were not associated with either diastolic or systolic measures over the study period. These findings are in line with previous observations [40].
In summary, there seemed to be an impairment of the diastolic function directly after the marathon, even though E/eʹ was not altered. This might be due to the fact, that the E/eʹ ratio is a robust, but too insensitive parameter to display the distinct changes caused by prolonged and strenuous exercise. Especially in a grey zone of E/e’ (ranging from 8 to 14), it is recommended to combine the TDI parameter with e.g. the E/A ratio or the DT [7, 41].
Regarding the adjustments for heart rate and systolic blood pressure, we could draw the conclusions that the diastolic function after a marathon seems to be altered even after adjusting for these potential confounding parameters. Especially at V2 with higher heart rates after the marathon, the adjustment revealed a switch from decreased to increased Vmin.
With our study design and the two follow-up examinations until 72 h after the marathon, we were able to monitor changes during a longer recovery period. But even at 72 h, some parameters still differed from baseline values. This leads to the conclusion, that recovery of left atrium dimensions and function (as a sign of diastolic left ventricular function) after a marathon takes longer than previously assumed. It can easily be missed when only considering the parameters for ventricular relaxation (E/A-ratio and DT), which returned to baseline at V3.
Limitations
For our study, we used exclusively non-invasive measurements for evaluating the atrial filling pressures. The invasive measurements, although not practicable in the clinical setting of sports events, might show even more precise results.
Furthermore, we stopped the follow-up examinations 72 h after the marathon. By that time, not all parameters had returned to baseline values. In order to display the whole variance, a third follow-up examination of at least 5 days after the race should be pursued in future studies.
Conclusions
Both 2- and 3-dimensionally measured parameters of left ventricular diastolic function were significantly altered after completion of a marathon. Surrogate parameters of ventricular relaxation were altered immediately after the marathon, while surrogate markers for increased atrial pressure demonstrated impaired diastolic LV function with a maximum at 24 h after a marathon. Regarding all parameters of diastolic function, a longer (than previously assumed) recovery period seems to exist and the combination of 2D and 3D ultrasound can help to distinguish these alterations. Even after correcting for possible confounders, e.g. heart rate, we were able to demonstrate an impairment of diastolic function after a marathon race.
References
Neilan TG, Januzzi JL, Lee-Lewandrowski E, Ton-Nu TT, Yoerger DM, Jassal DS, Lewandrowski KB, Siegel AJ, Marshall JE, Douglas PS, Lawlor D, Picard MH, Wood MJ (2006) Myocardial injury and ventricular dysfunction related to training levels among nonelite participants in the Boston marathon. Circulation 114:2325–2333
Alarrayed S, Garadah TS, Alawdi AA (2009) The impact of left ventricular preload reduction on cardiac pulsed Doppler indices during hemodialysis and its relation to intra-dialysis hypotension: a pulsed Doppler study. Saudi J Kidney Dis Transpl 20:201–207
Schnell F, Claessen G, La Gerche A, Claus P, Bogaert J, Delcroix M, Carré F, Heidbuchel H (2017) Atrial volume and function during exercise in health and disease. J Cardiovasc Magn Reson 19:104. https://doi.org/10.1186/s12968-017-0416-9
Elliott AD, Mahajan R, Linz D, Stokes M, Verdicchio CV, Middeldorp ME, La Gerche A, Lau DH, Sanders P (2018) Atrial remodeling and ectopic burden in recreational athletes: implications for risk of atrial fibrillation. Clin Cardiol 41:843–848. https://doi.org/10.1002/clc.22967
Sharma S, Whyte G, McKenna WJ (1997) Sudden death from cardiovascular disease in young athletes: fact or fiction? Br. J. Sports Med. 31:269–276
Asif IM, Harmon KG (2017) Incidence and etiology of sudden cardiac death: new updates for athletic departments. Sports Health 9:268–279. https://doi.org/10.1177/1941738117694153
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu B, Waggoner AD (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 17:1321–1360. https://doi.org/10.1093/ehjci/jew082
Knechtle B, Nikolaidis PT (2018) Physiology and pathophysiology in ultra-marathon running. Front Physiol 9:634. https://doi.org/10.3389/fphys.2018.00634
Christensen DL, Espino D, Infante-Ramírez R, Cervantes-Borunda MS, Hernández-Torres RP, Rivera-Cisneros AE, Castillo D, Westgate K, Terzic D, Brage S, Hassager C, Goetze JP, Kjaergaard J (2017) Transient cardiac dysfunction but elevated cardiac and kidney biomarkers 24 h following an ultra-distance running event in Mexican Tarahumara. Extrem Physiol Med 6:3. https://doi.org/10.1186/s13728-017-0057-5
Hewing B, Schattke S, Spethmann S, Sanad W, Schroeckh S, Schimke I, Halleck F, Peters H, Brechtel L, Lock J, Baumann G, Dreger H, Borges AC, Knebel F (2015) Cardiac and renal function in a large cohort of amateur marathon runners. Cardiovasc Ultrasound 13:13. https://doi.org/10.1186/s12947-015-0007-6
Kuusisto JK, Järvinen VM, Sinisalo JP (2018) Validation of 3D echocardiographic volume detection of left atrium by human cadaveric casts. BMC Med Imaging 18:43. https://doi.org/10.1186/s12880-018-0282-4
Scherr J, Nieman DC, Schuster T, Habermann J, Rank M, Braun S, Pressler A, Wolfarth B, Halle M (2012) Nonalcoholic beer reduces inflammation and incidence of respiratory tract illness. Med Sci Sports Exerc 44:18–26
Pressler A, Hanssen H, Dimitrova M, Krumm M, Halle M, Scherr J (2011) Acute and chronic effects of marathon running on the retinal microcirculation. Atherosclerosis 219:864–868. https://doi.org/10.1016/j.atherosclerosis.2011.08.052
Bernecker C, Scherr J, Schinner S, Braun S, Scherbaum WA, Halle M (2013) Evidence for an exercise induced increase of TNF-α and IL-6 in marathon runners. Scand J Med Sci Sports 23:207–214. https://doi.org/10.1111/j.1600-0838.2011.01372.x
Nickel T, Lackermair K, Scherr J, Calatzis A, Vogeser M, Hanssen H, Waidhauser G, Schönermark U, Methe H, Horster S, Wilbert-Lampen U, Halle M (2016) Influence of high polyphenol beverage on stress-induced platelet activation. J Nutr Health Aging 20:586–593. https://doi.org/10.1007/s12603-016-0697-y
Scherr J, Braun S, Schuster T, Hartmann C, Moehlenkamp S, Wolfarth B, Pressler A, Halle M (2011) 72-h kinetics of high-sensitive troponin T and inflammatory markers after marathon. Med Sci Sports Exerc 43:1819–1827
Lackermair K, Scherr J, Waidhauser G, Methe H, Hoster E, Nieman DC, Hanley A, Clauss S, Halle M, Nickel T (2017) Influence of polyphenol-rich diet on exercise-induced immunomodulation in male endurance athletes. Appl Physiol Nutr Metab 42:1023–1030. https://doi.org/10.1139/apnm-2017-0063
Clauss S, Scherr J, Hanley A, Schneider J, Klier I, Lackermair K, Hoster E, Vogeser M, Nieman DC, Halle M, Nickel T (2017) Impact of polyphenols on physiological stress and cardiac burden in marathon runners—results from a substudy of the BeMaGIC study. Appl Physiol Nutr Metab 42:523–528. https://doi.org/10.1139/apnm-2016-0457
Müller J, Dahm V, Lorenz ES, Pressler A, Haller B, Grabs V, Halle M, Scherr J (2017) Changes of intima-media thickness in marathon runners: A mid-term follow-up. Eur J Prev Cardiol 24:1336–1342. https://doi.org/10.1177/2047487317713327
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18:1440–1463
Scherr J, Jung P, Schuster T, Pollmer L, Eisele G, Goss F, Schneider J, Halle M (2016) Left ventricular diastolic function is strongly correlated with active emptying of the left atrium: a novel analysis using three-dimensional echocardiography. Cardiovasc Ultrasound 14:43. https://doi.org/10.1186/s12947-016-0085-0
Hansegård J, Urheim S, Lunde K, Malm S, Rabben SI (2009) Semi-automated quantification of left ventricular volumes and ejection fraction by real-time three-dimensional echocardiography. Cardiovasc Ultrasound 7:18. https://doi.org/10.1186/1476-7120-7-18
Gradman AH, Wilson JT (2009) Hypertension and diastolic heart failure. Curr Cardiol Rep 11:422–429
Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR (2012) Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart 98:813–820
Burns AT, Connelly KA, La GA, Mooney DJ, Chan J, MacIsaac AI, Prior DL (2007) Effect of heart rate on tissue Doppler measures of diastolic function. Echocardiography 24:697–701
Bombardini T, Gemignani V, Bianchini E, Venneri L, Petersen C, Pasanisi E, Pratali L, Onso-Rodriguez D, Pianelli M, Faita F, Giannoni M, Arpesella G, Picano E (2008) Diastolic time - frequency relation in the stress echo lab: filling timing and flow at different heart rates. Cardiovasc Ultrasound 6:15
Tops LF, van der Wall EE, Schalij MJ, Bax JJ (2007) Multi-modality imaging to assess left atrial size, anatomy and function. Heart 93:1461–1470
Ujino K, Barnes ME, Cha SS, Langins AP, Bailey KR, Seward JB, Tsang TSM (2006) Two-dimensional echocardiographic methods for assessment of left atrial volume. Am J Cardiol 98:1185–1188. https://doi.org/10.1016/j.amjcard.2006.05.040
Rohner A, Brinkert M, Kawel N, Buechel RR, Leibundgut G, Grize L, Kuhne M, Bremerich J, Kaufmann BA, Zellweger MJ, Buser P, Osswald S, Handke M (2011) Functional assessment of the left atrium by real-time three-dimensional echocardiography using a novel dedicated analysis tool: initial validation studies in comparison with computed tomography. Eur J Echocardiogr 12:497–505
Vizzardi E, Aloia A, Rocco E, Lupi L, Rovetta R, Quinzani F, Bontempi L, Curnis MA, Dei CL (2012) How should we measure left atrium size and function? J Clin Ultrasound 40:155–166
Russo C, Jin Z, Homma S, Rundek T, Elkind MSV, Sacco RL, Di Tullio MR (2016) LA phasic volumes and reservoir function in the elderly by real-time 3-D echocardiography: normal values, prognostic significance, and clinical correlates. JACC Cardiovasc Imaging 10:976–985. https://doi.org/10.1016/j.jcmg.2016.07.015
Galderisi M, Benjamin EJ, Evans JC, D'Agostino RB, Fuller DL, Lehman B, Levy D (1993) Impact of heart rate and PR interval on Doppler indexes of left ventricular diastolic filling in an elderly cohort (the Framingham Heart Study). Am. J. Cardiol. 72:1183–1187
Milan A, Puglisi E, Magnino C, Naso D, Abram S, Avenatti E, Rabbia F, Mulatero P, Veglio F (2012) Left atrial enlargement in essential hypertension: role in the assessment of subclinical hypertensive heart disease. Blood Press 21:88–96
Kang S, Lim H, Choi B, Choi S, Hwang G, Yoon M, Tahk S, Shin J (2008) Longitudinal strain and torsion assessed by two-dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase-1, a marker of myocardial fibrosis, in patients with hypertension. J Am Soc Echocardiogr 21:907–911. https://doi.org/10.1016/j.echo.2008.01.015
Neilan TG, Yoerger DM, Douglas PS, Marshall JE, Halpern EF, Lawlor D, Picard MH, Wood MJ (2006) Persistent and reversible cardiac dysfunction among amateur marathon runners. Eur Heart J 27:1079–1084
George K, Oxborough D, Forster J, Whyte G, Shave R, Dawson E, Stephenson C, Dugdill L, Edwards B, Gaze D (2005) Mitral annular myocardial velocity assessment of segmental left ventricular diastolic function after prolonged exercise in humans. J Physiol 569:305–313
Tschope C, Paulus WJ (2009) Is echocardiographic evaluation of diastolic function useful in determining clinical care? Doppler echocardiography yields dubious estimates of left ventricular diastolic pressures. Circulation 120:810–820
Hees PS, Fleg JL, Dong SJ, Shapiro EP (2004) MRI and echocardiographic assessment of the diastolic dysfunction of normal aging: altered LV pressure decline or load? Am J Physiol Heart Circ Physiol 286:H782–H788
Mor-Avi V, Sugeng L, Lang RM (2009) Real-time 3-dimensional echocardiography: an integral component of the routine echocardiographic examination in adult patients? Circulation 119:314–329
Wilson M, O'Hanlon R, Prasad S, Oxborough D, Godfrey R, Alpendurada F, Smith G, Wong J, Basavarajaiah S, Sharma S, Nevill A, Gaze D, George K, Whyte G (2011) Biological markers of cardiac damage are not related to measures of cardiac systolic and diastolic function using cardiovascular magnetic resonance and echocardiography after an acute bout of prolonged endurance exercise. Br J Sports Med 45:780–784
Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De KG, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur. Heart J. 28:2539–2550
Acknowledgements
The authors would like to thank the staff and the doctoral students (Amelie Trommler, Charlotte Hartmann, Jana Habermann, Tobias Rheude, Esther Seifert) of the Department of Prevention and Sports Medicine for their assistance with the project.
Funding
Funding of the study was received by Erdinger Weissbraeu, Werner Brombach GmbH. The funders had no direct rule in the study’s design, conduct, analysis, interpretation of data and reporting beyond approval of the scientific protocol in peer review for funding. No other grants were received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors had any personal or financial conflict of interest. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roeh, A., Schuster, T., Jung, P. et al. Two dimensional and real-time three dimensional ultrasound measurements of left ventricular diastolic function after marathon running: results from a substudy of the BeMaGIC trial. Int J Cardiovasc Imaging 35, 1861–1869 (2019). https://doi.org/10.1007/s10554-019-01634-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01634-5