Abstract
Twenty-five percent of ischemic strokes (IS) are cryptogenic, but it is estimated that paroxysmal atrial fibrillation (PAF) is the underlying cause in up to a third of cases. We aimed to investigate the predictive value of speckle tracking of the left atrium (LA) in diagnosing PAF in IS patients. We retrospectively studied 186 IS patients with a clinical echocardiographic examination during sinus rhythm. Outcome was PAF defined by at least one reported episode of AF following their IS. Conventional echocardiographic measures were performed. Global longitudinal strain (GLS), LA reservoir-(εs), conduit-(εe), contraction-strain (εa) and LA dyssynchrony (standard deviation of time-to-peak εs; LA SD-T2P) were obtained by left ventricular and LA speckle tracking. Of 186 patients, 28 (15%) were diagnosed with PAF. PAF-patients did not differ from non-PAF patients with regards to GLS nor SD-TPS, but atrial strain measures were significantly impaired at baseline (εs 27 vs. 35%, εe 12 vs. 16%, εa 15 vs. 18%, p < 0.02 for all, for PAF and non-PAF, respectively). However, only εs remained independently associated with PAF after adjustment for clinical and echocardiographic parameters (OR 1.13 [1.04; 1.22], p = 0.003, per 1% decrease). εs also provided the highest area under the receiver operating characteristic curve among all variables (AUC = 0.74). With a cutoff of 29%, εs had a specificity of 76% and a negative predictive value of 93%. Atrial reservoir strain is independently associated with PAF and may be used to improve the diagnosis of PAF following IS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke (IS) is one of the five leading causes of death worldwide [1] and 20–30% of the incidents are estimated to be caused by atrial fibrillation (AF) [2]. Around 25% of IS are cryptogenic, meaning no source of the incident can be found [3]. AF remains one of the possible underlying causes and continuous monitoring of cardiac rhythm has shown PAF in up to 30% [4]. Patients with PAF have a high risk of recurrent stroke, which can be greatly reduced with anticoagulant treatment [5]. Therefore, identifying these patients to initiate preventive treatment is crucial. Newest guidelines from American Heart Association/American Stroke Association recommend 30 days monitoring of IS patients without other apparent cause [6]. Studies suggest that even longer monitoring can be beneficial in detecting PAF [4, 7], but identifying the right patients for prolonged monitoring is important. Echocardiography is performed in the diagnostic process of many IS patients [8]. Echocardiographic analysis could help detect patients at risk, who could benefit from extended cardiac rhythm monitoring to detect PAF and initiate anticoagulant treatment for prevention of recurrent stroke. The analyses include measurements of the left atrial volume (LAV) which has been shown to predict AF in stroke patients [9]. Recently, studies have shown that left atrial strain correlates well with left atrial fibrosis and LV filling pressure [10, 11], and we therefore hypothesize that left atrial strain is a strong predictor of AF. We therefore aimed to explore the predictive value of atrial strain in diagnosing PAF in stroke patients compared to the currently applied measure; LAV. Furthermore, we sought to investigate whether atrial strain added predictive value over established clinical and echocardiographic predictors. Finally, we wanted to assess the predictive value of LA strain compared to more recently proposed predictors of AF; global longitudinal strain and left atrial dyssynchrony [12, 13].
Method
Population
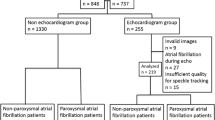
The study was retrospective and consisted of patients hospitalized at Gentofte Hospital in Copenhagen with either ischemic stroke or transient ischemic attack identified by ICD-10 codes (DI63, DI65, DI66, DG45) [4]. The study included 371 patients who had undergone an echocardiographic examination maximum 3 months prior to their index stroke and up to 6 months following. Patients with known AF were excluded and so were patients with obvious underlying reasons to their index strokes. Additional patients were excluded because of inability to perform speckle tracking examination due to poor image quality or frame rate lower than 40 frames/s (see Fig. 1). We included the remaining population for analyses (n = 186). The outcome was AF defined as the report of at least one episode of AF occurring after the index stroke. The incidence of AF, medical history and baseline demographics were obtained by reviewing electronic medical files and supplemented with notes from the echocardiographic examination.
Echocardiogram
All the echocardiographic images were digitally stored in an Image Vault (GE Vingmed Ultrasound AS, Horten, Norway) and analyzed offline with available software (EchoPac v202.34.0, GE Vingmed Ultrasound AS, Horten, Norway).
Conventional echocardiographic analyses and left ventricular (LV) strain analyses were performed by one investigator (FJO) and left atrial (LA) strain analyses were performed by another investigator (SR), both blinded to clinical data and outcome.
Conventional echocardiographic analyses were performed in the parasternal long-axis view to retrieve LV dimensions, the apical 4-chamber view to assess Doppler measures and the apical 4-chamber and 2-chamber views to measure LVEF and LAV.
Conventional echocardiography
Measurements of the LV dimensions (interventricular septal diameter, left posterior wall diameter and LV internal diameter) were performed at end diastole. LV ejection fraction (LVEF) was calculated using the modified Simpson’s biplane method. Measurement of the LAV was done by the biplane area-length method. In the apical 4-chamber view, pulsed wave Doppler at the tips of the mitral leaflets was used to measure inflow patterns of the left ventricle (E, A, E/A). The early peak tissue velocity (e′) was obtained by pulsed-wave TDI measured at the mitral annulus in the septal and lateral sites [14].
Speckle tracking echocardiography
LV speckle tracking was examined in three apical projections; apical 4-chamber, apical 2-chamber, and apical long-axis view. In the three projections, the software automatically divides each ventricular wall into three segments. With six ventricular walls and three segments each, analyses were performed in 18 segments in total. From each segment, curves for longitudinal strain were generated. From the average of the segments, GLS was calculated.
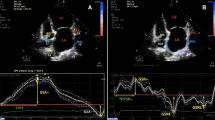
LA speckle tracking was examined in two apical projections; apical-4-chamber and apical 2-chamber. Point and click technique was used to manually trace the endocardial border of the LA. The region of interest was adjusted to fit the thickness of the atrial myocardium. In each projection, the LA was automatically divided into six segments giving longitudinal strain curves from a total of 12 segments. The QRS-complex was chosen as the baseline reference point. From the curves, LA reservoir strain (εs) corresponding to peak atrial longitudinal strain and LA contraction strain (εa) corresponding to the strain at the beginning of the P-wave during was obtained (Fig. 2). LA conduit strain (εe) was calculated as the difference between reservoir and contraction strain.
The synchrony of the LA segments (LA SD-T2P) was assessed by estimating the standard deviation of the time from the QRS complex to the positive peak strain of the 12 segments. A higher value of LA SD-T2P indicates more dyssynchrony of the LA [15].
The cine-loop view was played to ensure that the segments were properly tracked during the cardiac cycle and manually corrected if possible. Segments that remained untraceable after manual adjustments were excluded from the analysis and if more than two segments failed to be tracked the analysis of that projection was excluded.
Statistical analysis
STATA software version 12.0 (StataCorp, College Station, TX) was used for statistical analyses. Students t-test were used for comparing baseline variables displaying Gaussian distribution and are presented as mean ± SD. Variables displaying non-Gaussian distribution were tested with Wilcoxon-Rank sum test and displayed as median with interquartile ranges. Proportions were tested with Chi2-test and displayed as percentages.
Univariable logistic regression models were performed for clinical and echocardiographic parameters to identify predictors of PAF. Multivariable logistic regression models were performed for each atrial strain derived measure to control for potential confounders in three models; model 1 with adjustment for potential clinical confounders: age, hypertension, ischemic heart disease, and heart failure. Model 2 included clinical parameters and conventional echocardiographic confounders; LVEF, LAV and E/e′. Model 3 included the same variables as in model 2 + GLS. Deviation from linearity was assessed by simultaneous assessment of linear and quadratic effects. Tests for interaction with gender, age, heart failure, hypertension, and LVEF were performed.
A spline curve was constructed from a univariable logistic regression spline model to assess the probability of AF according to εs. The number of knots chosen was determined from the model which resulted in the lowest Akaike information criterion (AIC) score.
Receiver operating characteristic (ROC) curves were constructed for εs, age, CHADS2-score, LAV and GLS to calculate the area under the curve (AUC) and to determine the cutoffs with highest sensitivity and specificity for detecting PAF.
To assess the reproducibility of GLS and atrial reservoir strain, 20 patients from this cohort were selected randomly to examine inter- and intraobserver variability. We calculated coefficients of variation (CVs) and bias coefficients (mean difference SD) and visually displayed the variability with Bland–Altman plots.
Results
Baseline characteristics
Of the 186 patients, 28 (15%) developed AF. Clinical and echocardiographic characteristics for the entire population and stratified by PAF are shown in Table 1. In short, the mean age was 61 ± 13 years, 62% were males, 51% had hypertension, and mean LVEF was 52%. The PAF group was significantly older than the non-PAF group (68 ± 10 vs. 59 ± 14 years; p = 0.001). They also had a higher stroke risk (CHADS2-score: 3.4 vs 2.8, p = 0.002) as well as a higher prevalence of heart failure, ischemic heart disease, and borderline significant higher prevalence of hypertension. Otherwise, the PAF group did not differ clinically from the non-PAF group (Table 1).
For echocardiographic variables, no conventional measure including LAV differed between the two groups, with only E/e′ showing a trend towards being higher in the PAF group (Table 1).
Speckle tracking echocardiography
The mean frame rate for speckle tracking was 78 frames per second. GLS was similar among outcome groups (− 16 vs. − 17%, p = 0.26 for PAF and non-PAF, respectively). LA strain showed significantly lower values in both εs (27 vs. 35%, p < 0.001, for PAF and non-PAF group respectively), εe (12 vs. 16%, p = 0.003, for PAF and non-PAF respectively) and εa (15 vs. 18%, p = 0.015, for PAF and non-PAF, respectively). The dyssynchrony parameter LA SD-T2P showed no significant association with PAF (Table 1).
Diagnostic value
In univariable logistic regression only E/e′ was a significant predictor of PAF, whereas LAV and other conventional measure did not predict PAF. In speckle tracking, only atrial strain measures, but not LA dyssynchrony measures nor GLS were significant predictors of PAF (Table 2). Spline curves (Fig. 3) show a significant association between εs and PAF, with the risk of PAF increasing markedly after the selected cutoff of 29% (see below). In multivariable regression, E/e′ did not remain significantly associated with PAF (OR 1.50 [0.43;5.26], p = 0.52, per 1 increase). From the 3 atrial strain measures, only εs remained an independent predictor of PAF in all three models, even after adjustment for both clinical and echocardiographic covariates (Model 1: εs: OR 1.10 [1.03;1.16], p = 0.003; εe: 1.06 [0.98;1.14], p = 0.15; εa: 1.08 [1.01;1.15], p = 0.025; Model 2: εs: 1.11 [1.03;1.20], p = 0.005; εe: 1.07 [0.98;1.16], p = 0.16; εa: 1.07 [1.00;1.16], p = 0.06; Model 3: εs: 1.13 [1.04;1.22], p = 0.003; εe: 1.08 [0.98;1.18], p = 0.11; εa: 1.08 [1.00;1.16], p = 0.06, per 1% decrease) (Table 2). We found no interactions between εs and clinical risk factors. εs remained significantly associated with PAF after adjusting for CHADS2 score (εs: OR 1.10 [1.04;1.16] p = 0.001, per 1% decrease; CHADS2: 1.54 [0.99;2.38] p = 0.053, per 1 increase) and CHA2DS2-VASc score (εs: OR 1.09 [1.03;1.16] p = 0.003, per 1% decrease; CHA2DS2-VASc: 1.31 [1.0;1.71] p = 0.046, per 1 increase).
As compared to age, clinical risk score (CHADS2), GLS and LAV, the εs provided the highest AUC (AUC = 0.74), though only significantly greater than GLS and LAV (age: p = 0.50; CHADS2: p = 0.26; GLS: p < 0.001; LAV: p = 0.025) (Fig. 4). With a cutoff of 29%, εs had a sensitivity of 68%, specificity of 76%, positive predictive value of 33%, and negative predictive value of 93%.
Receiver operating characteristic curves showing the area under the curve of left atrial reservoir strain (blue line), left atrial volume (red line), age (green line), CHADS2-score (yellow line) and GLS (brown line) for determining paroxysmal atrial fibrillation. AUC, area under the curve; GLS, global longitudinal strain
Reproducibility
GLS showed a lower inter- and intraobserver variability compared to εs (GLS: intraobserver CV = 13%, interobserver CV = 14%; εs: intraobserver CV = 26%, interobserver SD = 27%). The bias coefficients for GLS were lower than for εs (GLS: intraobserver = 0.31 ± 0.79%, interobserver = − 0.77 ± 1.20%; εs: intraobserver = − 1.22 ± 3.37%, interobserver = 2.12 ± 6.51%). Neither GLS nor atrial reservoir strain were consistently over- or underestimated by the two investigators illustrated by the Bland–Altman plots (Fig. 5a–d).
Bland–Altman plot for a intraobserver analysis of global longitudinal strain (GLS), b interobserver analysis of GLS, c intraobserver analysis of atrial reservoir strain (εs) and d interobserver analysis of εs. Values along both axes are presented as percentages. Solid lines represent mean difference and dotted lines represent 95% limits of agreement
Discussion
We studied the predictive value of LA strain, dyssynchrony and GLS in diagnosing PAF in stroke patients. We did not find that LA dyssynchrony nor GLS significantly predicted PAF, but atrial reservoir strain was the only parameter independently associated with PAF after multivariable adjustments. The LA reservoir strain has previously been shown to be dependent on GLS and LAV with regards to prediction of heart failure and cardiovascular death [16], but the prognostic value of LA strain appears to be independent of both parameters for AF prediction in this study. With a cutoff of 29%, LA reservoir strain had a high negative predictive value, which could be used to discard patients for prolonged monitoring. Similarly, Leong et al. have previously found that IS patients without known PAF had lower reservoir strain compared to a healthy control group and that reservoir strain was better at predicting IS than cardiovascular risk factors alone [17]. Sanchis et al. shown that reservoir strain was reduced in stroke patients with cardioembolic cause and with unknown etiology, but not in other causes. This association might be a result of undiagnosed PAF in the group of patients with cryptogenic IS [18, 19].
Possible mechanisms of reduced reservoir strain in PAF
The causes of AF are not yet fully understood, but structural remodeling with increasing fibrosis of the LA is important in the formation of a substrate for AF. Remodeling of LA is caused by several clinical conditions like hypertension, diabetes, ischemic heart disease, and heart failure. Remodeling reduces the compliance of the LA, which leads to impairment of the atrial reservoir function [20]. Atrial reservoir strain is a measure of atrial reservoir function and compliance [21]. Hence, it is speculated that impaired reservoir strain could be a sign of remodeling of the LA. Kuppahally et al. found a relationship between fibrosis of LA detected by delayed enhancement magnetic resonance imaging (DE-MRI) and reduced atrial reservoir strain in patients diagnosed with AF. Additionally, they discovered that AF appears to contribute to further remodeling of the LA and therefore reservoir strain could be a sign of both the risk of developing AF and sign of damage from AF [10]. Atrial reservoir strain has also been shown to correlate closely to LV end-diastolic pressure, which could also partly explain the association between reservoir strain and AF since elevated end-diastolic pressure is a well-known risk factor for AF [11].
Other echocardiographic measurements of the left atrium
LAV is measured during routine echocardiographic examination and has previously been shown to correlate with PAF [22]. In our study, LAV was not associated with PAF in univariable regression analysis and had a poor diagnostic value. One could speculate that atrial reservoir strain is a more sensitive marker of atrial dysfunction since LAV is known to reflect chronically elevated filling pressure and that enlarged LAV is a sign of more extensive remodeling.
Analysis of atrial dyssynchrony by STE has shown to be associated with PAF in other studies [23], but we were not able to reproduce those results in our study. It is possible that the degree of dyssynchrony is associated with the amount of fibrosis in the LA, but atrial dyssynchrony assessed by the SD of time-to-peak reservoir strain focuses on the deformation of the atrium during the ventricular systole which is primarily a passive process [24]. Hence, this method is also largely influenced by fibrosis and heterogenic contraction pattern in the LV. Furthermore, the majority of studies in this field has been done with cardiac MRI with a low frame rate, which is a major limitation when investigating time-dependent variables such as dyssynchrony parameters.
Clinical value
AF might be the underlying cause of cryptogenic stroke, but the patients will initiate anti-platelet treatment if PAF remains undetected [6]. These patients are at high risk of an additional stroke which would be markedly reduced if they started anticoagulation treatment instead [5]. Therefore, it is very important to find these patients and initiate proper preventive treatment. A recent trial, randomizing patients with cryptogenic stroke to either anti-coagulation treatment or aspirin did not result in a lower rate of recurrent stroke [25], supporting the need of selecting patients applicable for diagnostic workup to detect PAF. The CRYSTAL AF study found that insertable cardiac monitoring had a significantly higher detection rate of PAF than conventional follow-up in cryptogenic stroke patients [4]. This is an invasive procedure and expensive, thus the determination of which patients who would benefit from longer monitoring is important. In this study, we discovered that the diagnostic value of LA strain < 29% had a high negative predictive value (93%). If a stroke patient is above this cut-off value, it is very unlikely that they suffer from undiagnosed PAF. Evidently, atrial strain analysis might contribute to the selection of patients who are not in need of prolonged monitoring, such as insertable cardiac monitoring. In this way, additional patients would be diagnosed with PAF and receive anticoagulation treatment for the prevention of re-stroke.
Limitations
Despite the strong association between LA strain and AF in the study, the method has some limitations. Atrial strain analysis is currently conducted with software programmed for the LV. The analysis of the LA is manually conducted and therefore more biased by the operator. Dedicated software might lead to more information and less interoperator variability. In this study, we used a mixed group of IS patients and it would be interesting to investigate the predictive value in a population consisting solely of cryptogenic stroke patients. Furthermore, the temporal window for the echocardiographic examination is relatively large, and an echocardiogram closer to the index stroke would reflect clinical practice better and potentially better identify reduced atrial strain in patients with PAF in close proximity to the index stroke. However, we expect that the that patients with AF near the stroke would also be the patients who had recurrent PAF later, and therefore had impaired left atrial function both prior to and following the stroke. LA strain might be influenced by loading conditions, but as measurement of blood pressure was not routinely performed at Gentofte Hospital during echocardiographic examination we could not assess the influence of blood pressure on LA strain. Finally, since this was a retrospective study, rhythm monitoring was not systematic for patients, but rather reflects the clinical interest in detecting PAF. Since patients were included before the prospective ILR studies were performed no patients had ILR for PAF detection, but rather contemporary rhythm strategies performed instead.
Conclusion
Left atrial reservoir strain is associated with PAF in ischemic stroke patients and has a high sensitivity and negative predictive value in diagnosing PAF. Atrial size, global longitudinal strain, and atrial dyssynchrony do not seem to offer predictive information on PAF risk. Speckle tracking analyses of the left atrium might offer valuable information on which patients would benefit the most from prolonged cardiac monitoring for identification of PAF.
References
Benjamin EJ, Blaha MJ, Chiuve SE et al (2017) Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 135(10):e146–e603
Kirchhof P, Benussi S, Kotecha D et al (2016) 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg 50(5):e1–e88
Hart RG, Diener HC, Coutts SB et al (2014) Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 13(4):429–438
Sanna T, Diener HC, Passman RS et al (2014) Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 370(26):2478–2486
Hart RG, Pearce LA, Aguilar MI (2007) Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 146(12):857–867
Kernan WN, Ovbiagele B, Black HR et al (2014) Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45(7):2160–2236
Dahal K, Chapagain B, Maharjan R et al (2016) Prolonged cardiac monitoring to detect atrial fibrillation after cryptogenic stroke or transient ischemic attack: a meta-analysis of randomized controlled trials. Ann Noninvasive Electrocardiol 21(4):382–388
Pepi M, Evangelista A, Nihoyannopoulos P et al (2010) Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr 11(6):461–476
Skaarup KG, Christensen H, Host N et al (2016) Diagnosing paroxysmal atrial fibrillation in patients with ischemic strokes and transient ischemic attacks using echocardiographic measurements of left atrium function. Am J Cardiol 117(1):91–99
Kuppahally SS, Akoum N, Badger TJ et al (2010) Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am Heart J 160(5):877–884
Cameli M, Sparla S, Losito M et al (2016) Correlation of left atrial strain and doppler measurements with invasive measurement of left ventricular end-diastolic pressure in patients stratified for different values of ejection fraction. Echocardiography 33(3):398–405
Saha SK, Kiotsekoglou A (2017) Speckle tracking-derived mechanical dispersion of left atrial myocardial deformation: an essential parameter in atrial fibrillation management? Echocardiography 34(2):159–161
Cameli M, Mandoli GE, Loiacono F, Sparla S, Iardino E, Mondillo S (2016) Left atrial strain: a useful index in atrial fibrillation. Int J Cardiol 220:208–213
Olsen FJ, Jorgensen PG, Mogelvang R et al (2016) Predicting paroxysmal atrial fibrillation in cerebrovascular ischemia using tissue doppler imaging and speckle tracking echocardiography. J Stroke Cerebrovasc Dis 25(2):350–359
Gan GCH, Ferkh A, Boyd A, Thomas L (2018) Left atrial function: evaluation by strain analysis. Cardiovasc Diagn Ther 8(1):29–46
Ersboll M, Andersen MJ, Valeur N et al (2013) The prognostic value of left atrial peak reservoir strain in acute myocardial infarction is dependent on left ventricular longitudinal function and left atrial size. Circ Cardiovasc Imaging 6(1):26–33
Leong DP, Joyce E, Debonnaire P et al (2017) Left atrial dysfunction in the pathogenesis of cryptogenic stroke: novel insights from speckle-tracking echocardiography. J Am Soc Echocardiogr 30(1):71–79.e71
Sanchis L, Montserrat S, Obach V et al (2016) Left atrial function is impaired in some patients with stroke of undetermined etiology: potential implications for evaluation and therapy. Rev Esp Cardiol (Engl Ed) 69(7):650–656
Pagola J, Gonzalez-Alujas T, Flores A et al (2014) Left atria strain is a surrogate marker for detection of atrial fibrillation in cryptogenic strokes. Stroke 45(8):e164–166
Schotten U, Verheule S, Kirchhof P, Goette A (2011) Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 91(1):265–325
Todaro MC, Choudhuri I, Belohlavek M et al (2012) New echocardiographic techniques for evaluation of left atrial mechanics. Eur Heart J Cardiovasc Imaging 13(12):973–984
Olshansky B, Heller EN, Mitchell LB et al (2005) Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the atrial fibrillation follow-up investigation of rhythm management (AFFIRM) study. J Am Coll Cardiol 45(12):2026–2033
Shang Z, Su D, Cong T et al (2017) Assessment of left atrial mechanical function and synchrony in paroxysmal atrial fibrillation with two-dimensional speckle tracking echocardiography. Echocardiography 34(2):176–183
Sarvari SI, Haugaa KH, Stokke TM et al (2016) Strain echocardiographic assessment of left atrial function predicts recurrence of atrial fibrillation. Eur Heart J Cardiovasc Imaging 17(6):660–667
Hart RG, Sharma M, Mundl H et al (2018) Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med 378(23):2191–2201
Funding
Sif Rasmussen received a scholarship grant from Herlev & Gentofte Hospital and the P. Carl Petersens Fond during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Biering-Sørensen has received consultant fees from Amgen and Novartis. Dr. Olsen has received a research prize from the Lundbeck Foundation, however, has no collaboration with Lundbeck. None of the companies had any participation in this study.
Rights and permissions
About this article
Cite this article
Rasmussen, S.M.A., Olsen, F.J., Jørgensen, P.G. et al. Utility of left atrial strain for predicting atrial fibrillation following ischemic stroke. Int J Cardiovasc Imaging 35, 1605–1613 (2019). https://doi.org/10.1007/s10554-019-01601-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01601-0